Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint
Abstract
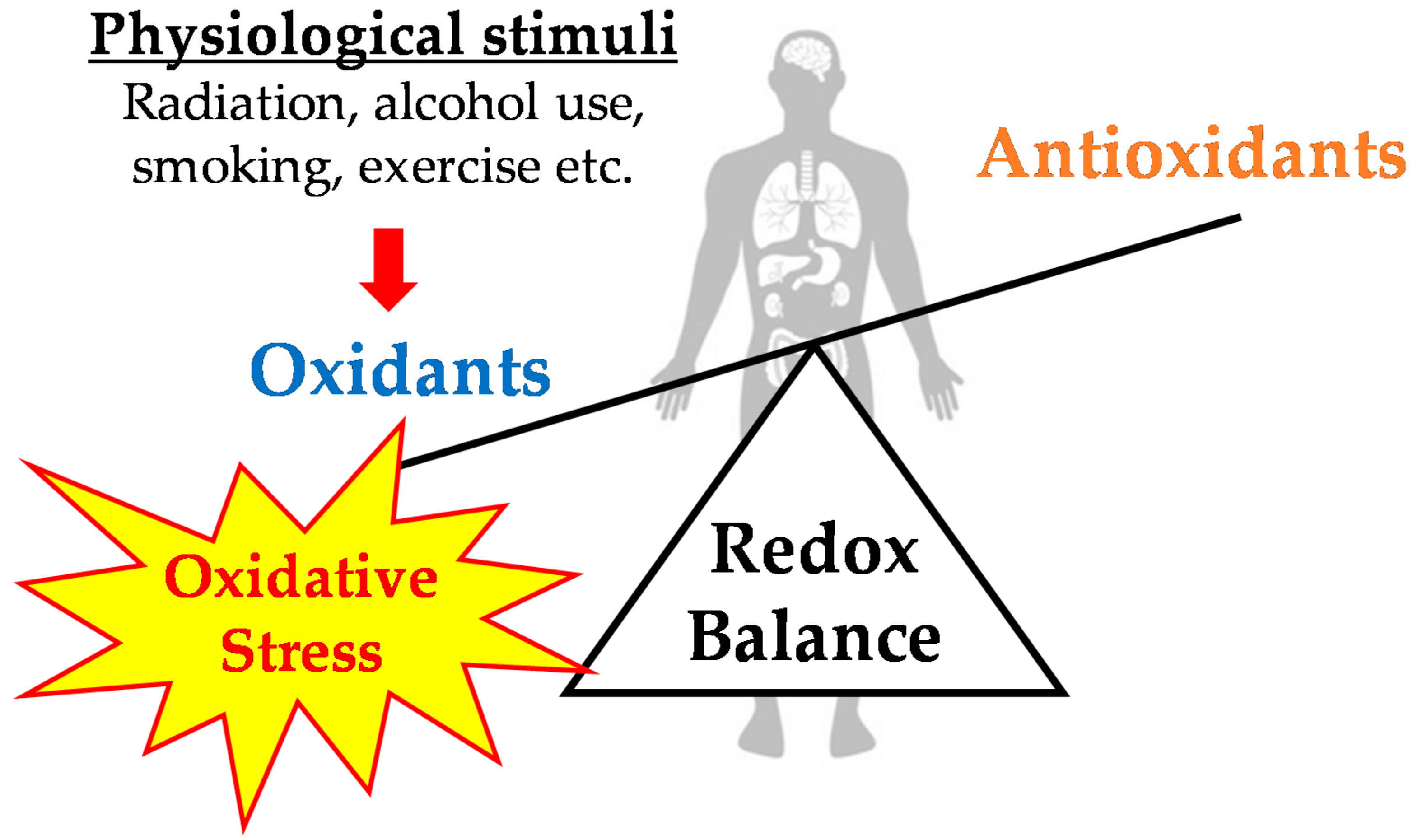
1. Introduction
2. Exercise-Induced Oxidative Stress
2.1. Exercise-Induced Oxidative Stress: Human Trials
2.2. Exercise-Induced Oxidative Stress: Animal Experiments
2.3. Advantages and Disadvantages of Human Trials and Animal Experiments
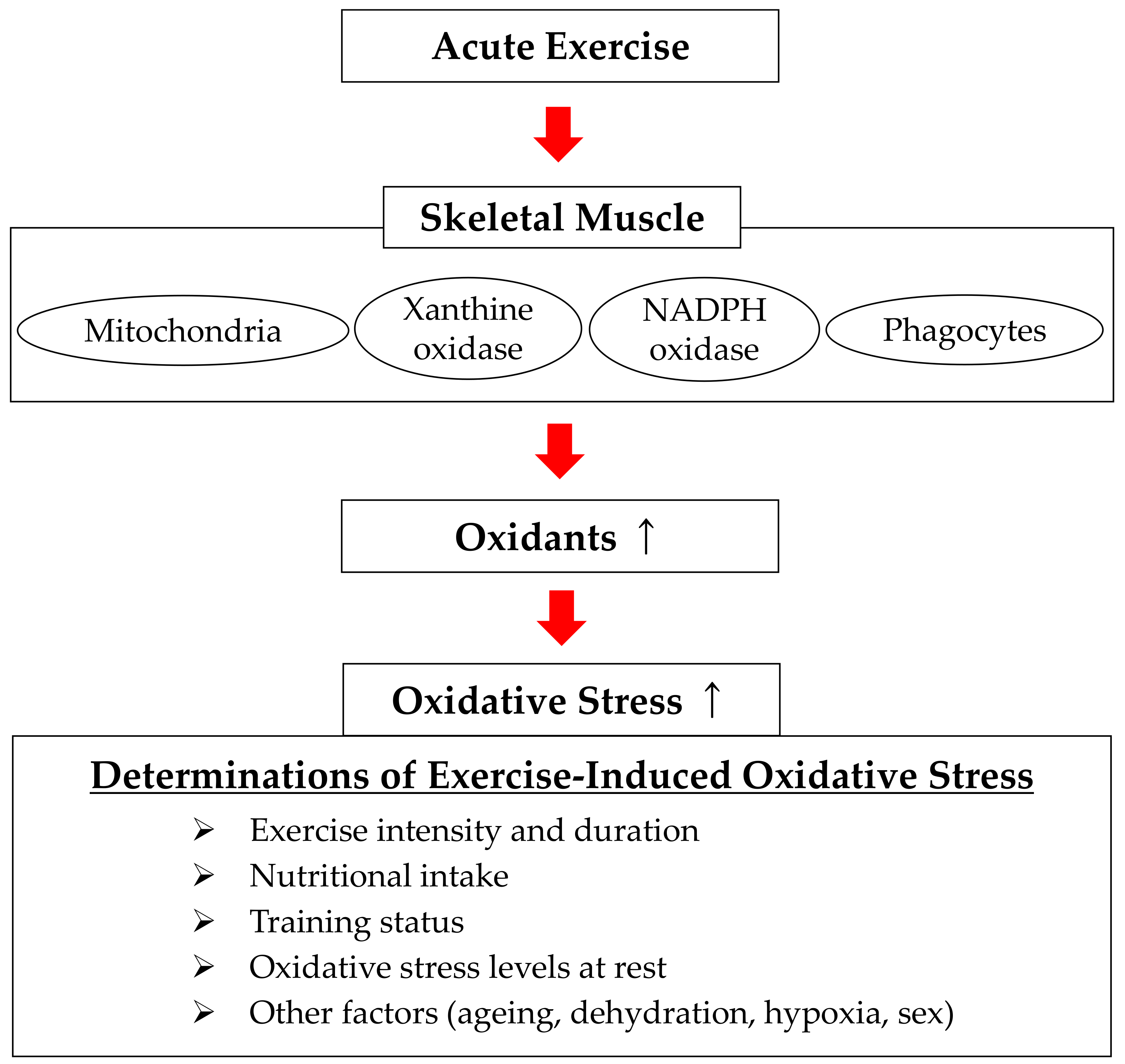
3. Determinants of Exercise-Induced Oxidative Stress and Sources of Free Radical Production
3.1. Determinants of Exercise-Induced Oxidative Stress
3.2. Sources of Free Radical Production during Exercise
4. Effects of Antioxidant Intake on Exercise-Induced Oxidative Stress
4.1. Effects of Antioxidant Intake on Exercise-Induced Oxidative Stress: Human Trials
4.2. Effects of Intake of Antioxidants on Exercise-Induced Oxidative Stress: Animal Experiments
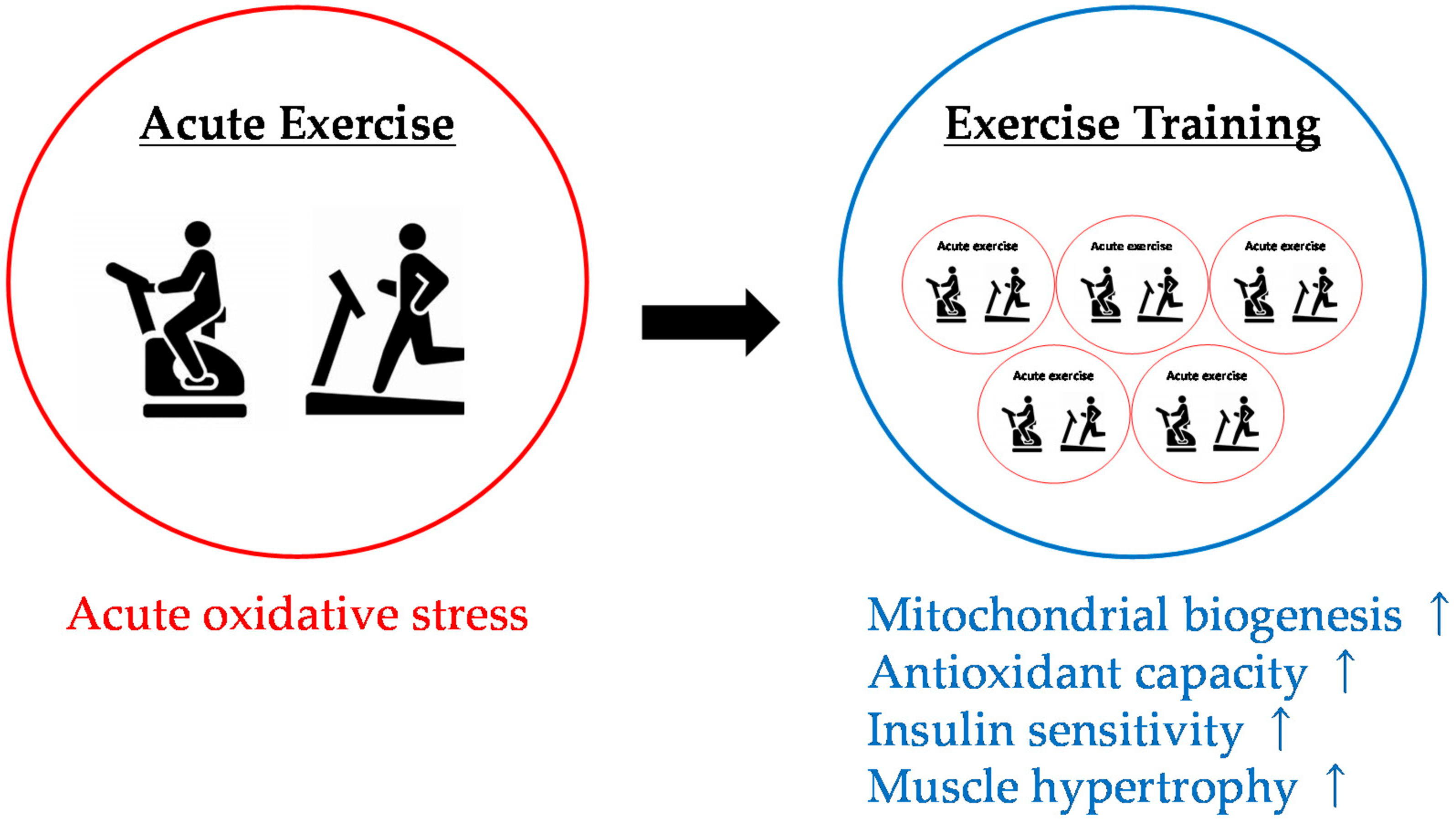
4.3. Effects of Antioxidant Intake on Physiological Adaptation to Exercise Training
5. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology & Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2007; ISBN 9780198717485. [Google Scholar]
- Commoner, B.; Townsend, J.; Pake, G.E. Free Radicals in Biological Materials. Nature 1954, 174, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Mallard, J.R.; Kent, M. Electron Spin Resonance in Surviving Rat Tissues. Nature 1966, 210, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Wyard, S.J. Electron Spin Resonance Spectroscopy of Animal Tissues. Proc. Roy. Soc. A 1968, 302, 355–360. [Google Scholar] [CrossRef]
- Morrow, J.D.; Awad, J.A.; Kato, T.; Takahashi, K.; Badr, K.F.; Roberts, L.J., 2nd; Burk, R.F. Formation of Novel Non-cyclooxygenase-Derived Prostanoids (F2-Isoprostanes) in Carbon Tetrachloride Hepatotoxicity. An Animal Model of Lipid Peroxidation. J. Clin. Investig. 1992, 90, 2502–2507. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress: Relationship with Exercise and Training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.B. Evolution and the Biosynthesis of Ascorbic Acid. Science 1973, 182, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Cadenas, E. Oxidative Stress: Damage to Intact Cells and Organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 311, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free Radical Theory of Aging: An Update. Increasing the Functional Life Span. Ann N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental Guidelines for Studies Designed to Investigate the Impact of Antioxidant Supplementation on Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; Litov, R.E.; Savin, W.M.; Dumelin, E.E.; Tappel, A.L. Effects of Exercise, Vitamin E, and Ozone on Pulmonary Function and Lipid Peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate Exercise Is an Antioxidant: Upregulation of Antioxidant Genes by Training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Modulation of Skeletal Muscle Antioxidant Defense by Exercise: Role of Redox Signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive Oxygen and Nitrogen Species as Intracellular Signals in Skeletal Muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Lovlin, R.; Cottle, W.; Pyke, I.; Kavanagh, M.; Belcastro, A.N. Are Indices of Free Radical Damage Related to Exercise Intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Viguie, C.; Stanley, W.C.; Brooks, G.A.; Packer, L. Blood Glutathione Oxidation during Human Exercise. J. Appl. Physiol. 1988, 64, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-Induced Oxidative Stress: Past, Present and Future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.M.; Nolte, L.A.; Holloszy, J.O. Effects of an Antioxidant Vitamin Mixture on Lipid Peroxidation at Rest and Postexercise. J. Appl. Physiol. 1993, 74, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Kleinman, M.T.; Hamilton, M.; Barstow, T.J. The Effect of Exercise Intensity on Lipid Peroxidation. Med. Sci. Sports Exerc. 1997, 29, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling Time Is Crucial for Measurement of Aerobic Exercise-Induced Oxidative Stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H.M.; Goldfarb, A.H.; Cao, G. Exercise-Induced Oxidative Stress before and after Vitamin C Supplementation. Int. J. Sport Nutr. 1997, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Hartmann, A.; Grunert-Fuchs, M.; Poch, B.; Speit, G. DNA Damage after Exhaustive Treadmill Running in Trained and Untrained Men. Int. J. Sports Med. 1996, 17, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Ruiz, J.; Zafrilla, P.; Almar, M.; Cuevas, M.J.; Lopez, F.J.; Abellan, P.; Villegas, J.A.; Gonzalez-Gallego, J. The Effects of an Antioxidant-Supplemented Beverage on Exercise-Induced Oxidative Stress: Results from a Placebo-Controlled Double-Blind Study in Cyclists. Eur. J. Appl. Physiol. 2005, 95, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Asensi, M.; Gasco, E.; Pallardo, F.V.; Ferrero, J.A.; Furukawa, T.; Vina, J. Exhaustive Physical Exercise Causes Oxidation of Glutathione Status In Blood: Prevention by Antioxidant Administration. Am. J. Physiol. 1992, 263, R992–R995. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Atalay, M.; Hanninen, O. Exercise-Induced Oxidative Stress: Glutathione Supplementation and Deficiency. J. Appl. Physiol. 1994, 77, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.; Rowlands, C.C.; Jones, E.; Young, I.S.; Jackson, S.K.; Davies, B.; Peters, J.R. Electron Spin Resonance Spectroscopic Detection of Oxygen-Centred Radicals in Human Serum following Exhaustive Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.; Young, I.S.; Peters, J.R.; Jones, E.; Jackson, S.K.; Davies, B.; Rowlands, C.C. Electron Spin Resonance Spectroscopy, Exercise, and Oxidative Stress: An Ascorbic Acid Intervention Study. J. Appl. Physiol. 1999, 87, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H.; McKenzie, M.J. Oxidative Stress Response to Aerobic Exercise: Comparison of Antioxidant Supplements. Med. Sci. Sports Exerc. 2006, 38, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Marzatico, F.; Pansarasa, O.; Bertorelli, L.; Somenzini, L.; Della Valle, G. Blood Free Radical Antioxidant Enzymes and Lipid Peroxides following Long-Distance and Lactacidemic Performances in Highly Trained Aerobic and Sprint Athletes. J. Sports Med. Phys. Fitness 1997, 37, 235–239. [Google Scholar] [PubMed]
- Groussard, C.; Machefer, G.; Rannou, F.; Faure, H.; Zouhal, H.; Sergent, O.; Chevanne, M.; Cillard, J.; Gratas-Delamarche, A. Physical Fitness and Plasma Non-enzymatic Antioxidant Status at Rest and after a Wingate Test. Can. J. Appl. Physiol. 2003, 28, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in Blood Lipid Peroxidation Markers and Antioxidants after a Single Sprint Anaerobic Exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic Implications of Resistive Force Selection for Oxidative Stress and Markers of Muscle Damage during 30 s of High-Intensity Exercise. Eur. J. Appl. Physiol. 2004, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Smith, W.A. Oxidative Stress in Response to Aerobic and Anaerobic Power Testing: Influence of Exercise Training and Carnitine Supplementation. Res. Sports Med. 2009, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; Kraemer, W.J.; Triplett-McBride, T.; Sebastianelli, W. Effect of Resistance Exercise on Free Radical Production. Med. Sci. Sports Exerc. 1998, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Viitala, P.E.; Newhouse, I.J.; LaVoie, N.; Gottardo, C. The Effects of Antioxidant Vitamin Supplementation on Resistance Exercise Induced Lipid Peroxidation in Trained and Untrained Participants. Lipids Health Dis. 2004, 3, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Morrow, J.D.; Utter, A.C.; Dumke, C.L. Effect of Resistance Exercise and Carbohydrate Ingestion on Oxidative Stress. Free Radic. Res. 2005, 39, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Goldfarb, A.H.; Rescino, M.H.; Hegde, S.; Patrick, S.; Apperson, K. Eccentric Exercise Effect on Blood Oxidative-Stress Markers and Delayed Onset of Muscle Soreness. Med. Sci. Sports Exerc. 2002, 34, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fry, A.C.; Falvo, M.J.; Moore, C.A. Protein Carbonyls Are Acutely Elevated following Single Set Anaerobic Exercise in Resistance Trained Men. J. Sci. Med. Sport 2007, 10, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.B.; Hosick, P.A.; McCaulley, G.O.; Schrieber, L.; Wrieden, J.; McAnulty, S.R.; Triplett, N.T.; McBride, J.M.; Quindry, J.C. The Effect of Resistance Exercise on Humoral Markers of Oxidative Stress. Med. Sci. Sports Exerc. 2008, 40, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Child, R.; Brown, S.; Day, S.; Donnelly, A.; Roper, H.; Saxton, J. Changes in Indices of Antioxidant Status, Lipid Peroxidation and Inflammation in Human Skeletal Muscle after Eccentric Muscle Actions. Clin. Sci. 1999, 96, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Pucsok, J.; Mecseki, S.; Csont, T.; Ferdinandy, P. Muscle Soreness-Induced Reduction in Force Generation Is Accompanied by Increased Nitric Oxide Content and DNA Damage in Human Skeletal Muscle. Free Radic. Biol. Med. 1999, 26, 1059–1063. [Google Scholar] [CrossRef]
- Bailey, D.M.; Lawrenson, L.; McEneny, J.; Young, I.S.; James, P.E.; Jackson, S.K.; Henry, R.R.; Mathieu-Costello, O.; McCord, J.M.; Richardson, R.S. Electron Paramagnetic Spectroscopic Evidence of Exercise-Induced Free Radical Accumulation in Human Skeletal Muscle. Free Radic. Res. 2007, 41, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Falvo, M.J.; Fry, A.C.; Schilling, B.K.; Smith, W.A.; Moore, C.A. Oxidative Stress Response in Trained Men following Repeated Squats or Sprints. Med. Sci. Sports Exerc. 2006, 38, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Im, J.; Kang, J.; Maresh, C.M.; Kraemer, W.J.; French, D.; Nioka, S.; Kime, R.; Rundell, K.W.; Ratamess, N.A.; et al. Comparison of Low- and High-Intensity Resistance Exercise on Lipid Peroxidation: Role of Muscle Oxygenation. J. Strength Cond. Res. 2007, 21, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Theodorou, A.A.; Panayiotou, G.; Zafeiridis, A.; Dipla, K.; Nikolaidis, M.G.; Vrabas, I.S. Reductive Stress after Exercise: The Issue of Redox Individuality. Redox Biol. 2014, 2, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.S.; Brady, L.J.; Ullrey, D.E. Selenium, Vitamin E and the Response to Swimming Stress in the Rat. J. Nutr. 1979, 109, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free Radicals and Tissue Damage Produced by Exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Kumar, C.T.; Reddy, V.K.; Prasad, M.; Thyagaraju, K.; Reddanna, P. Dietary Supplementation of Vitamin E Protects Heart Tissue from Exercise-Induced Oxidant Stress. Mol. Cell. Biochem. 1992, 111, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Ji, L.L. Glutathione Depletion in Rested and Exercised Mice: Biochemical Consequence and Adaptation. Arch. Biochem. Biophys. 1995, 316, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Asano, K.; Inoue, M.; Kizaki, T.; Oh-Ishi, S.; Suzuki, K.; Taniguchi, N.; Ohno, H. Superoxide Dismutase Derivative Prevents Oxidative Damage in Liver and Kidney of Rats Induced by Exhausting Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 72, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bejma, J.; Ji, L.L. Aging and Acute Exercise Enhance Free Radical Generation in Rat Skeletal Muscle. J. Appl. Physiol. 1999, 87, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Frost, S.; Most, E.; Volker, K.; Pallauf, J.; Mooren, F.C. Exercise Affects Tissue Lymphocyte Apoptosis via Redox-Sensitive and Fas-Dependent Signaling Pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1518–R1527. [Google Scholar] [CrossRef] [PubMed]
- Dalla Corte, C.L.; de Carvalho, N.R.; Amaral, G.P.; Puntel, G.O.; Silva, L.F.; Retamoso, L.T.; Royes, L.F.; Bresciani, G.B.; da Cruz, I.B.; Rocha, J.B.; et al. Antioxidant Effect of Organic Purple Grape Juice on Exhaustive Exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yeo, H.C.; Overvik-Douki, E.; Hagen, T.; Doniger, S.J.; Chyu, D.W.; Brooks, G.A.; Ames, B.N. Chronically and Acutely Exercised Rats: Biomarkers of Oxidative Stress and Endogenous Antioxidants. J. Appl. Physiol. 2000, 89, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H.M.; Goldfarb, A.H.; Cutler, R.G. MDA Content Increases in Fast- and Slow-Twitch Skeletal Muscle with Intensity of Exercise in a Rat. Am. J. Physiol. 1988, 255, C874–C877. [Google Scholar] [CrossRef] [PubMed]
- Kayatekin, B.M.; Gonenc, S.; Acikgoz, O.; Uysal, N.; Dayi, A. Effects of Sprint Exercise on Oxidative Stress in Skeletal Muscle and Liver. Eur. J. Appl. Physiol. 2002, 87, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Nakamura, A.; Nakamoto, H.; Asano, K.; Ohno, H.; Goto, S. A Period of Anaerobic Exercise Increases the Accumulation of Reactive Carbonyl Derivatives in the Lungs of Rats. Pflugers Arch. 1998, 435, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Edwards, R.H.; Symons, M.C. Electron Spin Resonance Studies of Intact Mammalian Skeletal Muscle. Biochim. Biophys. Acta 1985, 847, 185–190. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Goutianos, G.; Paschalis, V.; Margaritelis, N.V.; Tzioura, A.; Dipla, K.; Zafeiridis, A.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. The Rat Closely Mimics Oxidative Stress and Inflammation in Humans after Exercise but not after Exercise Combined with Vitamin C Administration. Eur. J. Appl. Physiol. 2016, 116, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, H.; Yamada, K. In Vivo Electron Spin Resonance-Computed Tomography/Nitroxyl Probe Technique for Non-Invasive Analysis of Oxidative Injuries. Arch. Biochem. Biophys. 2003, 416, 1–8. [Google Scholar] [CrossRef]
- Quindry, J.C.; Stone, W.L.; King, J.; Broeder, C.E. The Effects of Acute Exercise on Neutrophils and Plasma Oxidative Stress. Med. Sci. Sports Exerc. 2003, 35, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Greilberger, J.F.; Schwaberger, G.; Hofmann, P.; Oettl, K. Single Bouts of Exercise Affect Albumin Redox State and Carbonyl Groups on Plasma Protein of Trained Men in a Workload-Dependent Manner. J. Appl. Physiol. 2008, 104, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Davis, P.G.; Consitt, L.A.; Wideman, L. Plasma Protein Carbonyl Response to Increasing Exercise Duration in Aerobically Trained Men and Women. Int. J. Sports Med. 2007, 28, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Koz, M.; Erbas, D.; Bilgihan, A.; Aricioglu, A. Effects of Acute Swimming Exercise on Muscle and Erythrocyte Malondialdehyde, Serum Myoglobin, and Plasma Ascorbic Acid Concentrations. Can. J. Physiol. Pharmacol. 1992, 70, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. Oxidative Stress in Half and Full Ironman Triathletes. Med. Sci. Sports Exerc. 2007, 39, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; Rowell, B.; Dodd, S.L.; Demirel, H.A.; Naito, H.; Shanely, R.A.; Powers, S.K. Effects of Vitamin E Deficiency on Fatigue and Muscle Contractile Properties. Eur. J. Appl. Physiol. 2002, 87, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, A.T.; Packer, L. Vitamin E, physical exercise and tissue oxidative damage. Ciba Found Symp. 1983, 101, 56–69. [Google Scholar] [PubMed]
- Higuchi, M.; Cartier, L.J.; Chen, M.; Holloszy, J.O. Superoxide Dismutase and Catalase in Skeletal Muscle: Adaptive Response to Exercise. J. Gerontol. 1985, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.M.; Hamlin, R.L.; Unverferth, D.V.; Davis, H.W.; Merola, A.J. Effect of Exercise Training on Antioxidant Enzymes and Cardiotoxicity of Doxorubicin. J. Appl. Physiol. 1985, 59, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Marin, E.; Kretzschmar, M.; Hanninen, O. Skeletal Muscle and Liver Glutathione Homeostasis in Response to Training, Exercise, and Immobilization. J. Appl. Physiol. 1992, 73, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of Exercise and Fiber Type on Antioxidant Enzyme Activity in Rat Skeletal Muscle. Am. J. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Criswell, D.; Lawler, J.; Martin, D.; Ji, L.L.; Herb, R.A.; Dudley, G. Regional Training-Induced Alterations in Diaphragmatic Oxidative and Antioxidant Enzymes. Respir. Physiol. 1994, 95, 227–237. [Google Scholar] [CrossRef]
- Hammeren, J.; Powers, S.; Lawler, J.; Criswell, D.; Martin, D.; Lowenthal, D.; Pollock, M. Exercise Training-Induced Alterations in Skeletal Muscle Oxidative and Antioxidant Enzyme Activity in Senescent Rats. Int. J. Sports Med. 1992, 13, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of Glutathione Antioxidant System to Endurance Training Are Tissue and Muscle Fiber Specific. Am. J. Physiol. 1997, 272, R363–R369. [Google Scholar] [CrossRef] [PubMed]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High Intensity Training-Induced Changes in Skeletal Muscle Antioxidant Enzyme Activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Criswell, D.; Lawler, J.; Martin, D.; Lieu, F.K.; Ji, L.L.; Herb, R.A. Rigorous Exercise Training Increases Superoxide Dismutase Activity in Ventricular Myocardium. Am. J. Physiol. 1993, 265, H2094–H2098. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Fiebig, R.; Chandwaney, R.; Ji, L.L. Aging and Exercise Training in Skeletal Muscle: Responses of Glutathione and Antioxidant Enzyme Systems. Am. J. Physiol. 1994, 267, R439–R445. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Oh-ishi, S.; Ookawara, T.; Kizaki, T.; Toshinai, K.; Ha, S.; Haga, S.; Ji, L.L.; Ohno, H. Strenuous Endurance Training in Humans Reduces Oxidative Stress following Exhausting Exercise. Eur. J. Appl. Physiol. 2001, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Jamurtas, A.Z.; Villiotou, V.; Pouliopoulou, S.; Fotinakis, P.; Taxildaris, K.; Deliconstantinos, G. Oxidative Stress Responses in Older Men During Endurance Training and Detraining. Med. Sci. Sports Exerc. 2004, 36, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Connolly, D.A.; Eston, R.G.; Gleim, G.W. Exercise-Induced Muscle Damage and Potential Mechanisms for the Repeated Bout Effect. Sports Med. 1999, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Experimental Verification of Regression to the Mean in Redox Biology: Differential Responses to Exercise. Free Radic. Res. 2016, 50, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M. Estrogen and Gender Effects on Muscle Damage, Inflammation, and Oxidative Stress. Can. J. Appl. Physiol. 2000, 25, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.Y.; Jeong, M.H.; Jin, H.E.; Kim, Y.I.; Suh, A.R.; Cho, S.Y.; Roh, H.T.; Jin, C.H.; Suh, S.H. Fluid Replacement following Dehydration Reduces Oxidative Stress during Recovery. Biochem. Biophys. Res. Commun. 2009, 383, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; McEneny, J.; Mathieu-Costello, O.; Henry, R.R.; James, P.E.; McCord, J.M.; Pietri, S.; Young, I.S.; Richardson, R.S. Sedentary Aging Increases Resting and Exercise-Induced Intramuscular Free Radical Formation. J. Appl. Physiol. 2010, 109, 449–456. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, G.; Kliszczewiscz, B.; Barberio, M.; Ballmann, C.; Peters, B.; Slivka, D.; Dumke, C.; Cuddy, J.; Hailes, W.; Ruby, B.; et al. Acute Hypoxia and Exercise-Induced Blood Oxidative Stress. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Chance, B. The Mitochondrial Generation of Hydrogen Peroxide. General Properties and Effect of Hyperbaric Oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Glutathione Homeostasis in Response to Exercise Training and Nutritional Supplements. Mol. Cell. Biochem. 1999, 196, 31–42. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of Superoxide Production from Different Sites in the Mitochondrial Electron Transport Chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardo, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of Free Radical Production in Exhaustive Exercise in Humans and Rats; Role of Xanthine Oxidase and Protection by Allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of Mitochondrial and Nonmitochondrial Sources Implicate Nicotinamide Adenine Dinucleotide Phosphate Oxidase(s) in the Increased Skeletal Muscle Superoxide Generation that Occurs during Contractile Activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute Phase Response in Exercise. III. Neutrophil and IL-1 Beta Accumulation in Skeletal Muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Belcastro, A.N.; Arthur, G.D.; Albisser, T.A.; Raj, D.A. Heart, Liver, and Skeletal Muscle Myeloperoxidase Activity during Exercise. J. Appl. Physiol. 1996, 80, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Gallin, J.I. Current Concepts: Immunology. Neutrophils in Human Diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammatory Processes in Muscle Injury and Repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; MacLaren, D.P. Eccentric Exercise, Isokinetic Muscle Torque and Delayed Onset Muscle Soreness: The Role of Reactive Oxygen Species. Eur. J. Appl. Physiol. 2004, 91, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Donnelly, A.E.; Gleeson, M.; Whiting, P.H.; Walker, K.A.; Clough, P.J. Delayed-Onset Muscle Damage and Lipid Peroxidation in Man after a Downhill Run. Muscle Nerve 1989, 12, 332–336. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Paschalis, V.; Giakas, G.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D.; Jamurtas, A.Z. Decreased Blood Oxidative Stress after Repeated Muscle-Damaging Exercise. Med. Sci. Sports Exerc. 2007, 39, 1080–1089. [Google Scholar] [CrossRef]
- Meydani, M.; Evans, W.J.; Handelman, G.; Biddle, L.; Fielding, R.A.; Meydani, S.N.; Burrill, J.; Fiatarone, M.A.; Blumberg, J.B.; Cannon, J.G. Protective Effect of Vitamin E on Exercise-Induced Oxidative Damage in Young and Older Adults. Am. J. Physiol. 1993, 264, R992–R998. [Google Scholar] [CrossRef]
- Knez, W.L.; Coombes, J.S.; Jenkins, D.G. Ultra-Endurance Exercise and Oxidative Damage: Implications for Cardiovascular Health. Sports Med. 2006, 36, 429–441. [Google Scholar] [CrossRef]
- Howatson, G.; Milak, A. Exercise-Induced Muscle Damage following a Bout of Sport Specific Repeated Sprints. J. Strength Cond. Res. 2009, 23, 2419–2424. [Google Scholar] [CrossRef]
- Sumida, S.; Tanaka, K.; Kitao, H.; Nakadomo, F. Exercise-Induced Lipid Peroxidation and Leakage of Enzymes before and after Vitamin E Supplementation. Int. J. Biochem. 1989, 21, 835–838. [Google Scholar] [PubMed]
- Itoh, H.; Ohkuwa, T.; Yamazaki, Y.; Shimoda, T.; Wakayama, A.; Tamura, S.; Yamamoto, T.; Sato, Y.; Miyamura, M. Vitamin E supplementation attenuates leakage of enzymes following 6 successive days of running training. Int. J. Sports Med. 2000, 21, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.; Doi, T.; Sakurai, M.; Yoshioka, Y.; Okamura, K. Effect of a Single Bout of Exercise and Beta-Carotene Supplementation on the Urinary Excretion of 8-Hydroxy-Deoxyguanosine in Humans. Free Radic. Res. 1997, 27, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Stokic, D.S.; Koch, S.M.; Khawli, F.A.; Leis, A.A. N-acetylcysteine Inhibits Muscle Fatigue in Humans. J. Clin. Investig. 1994, 94, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Travaline, J.M.; Sudarshan, S.; Roy, B.G.; Cordova, F.; Leyenson, V.; Criner, G.J. Effect of N-acetylcysteine on Human Diaphragm Strength and Fatigability. Am. J. Respir. Crit. Care Med. 1997, 156, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X.; McKenna, M.J. N-acetylcysteine Enhances Muscle Cysteine and Glutathione Availability and Attenuates Fatigue during Prolonged Exercise in Endurance-Trained Individuals. J. Appl. Physiol. 2004, 97, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Medved, I.; Goodman, C.A.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X. N-acetylcysteine Attenuates the Decline in Muscle Na+, K+-Pump Activity and Delays Fatigue during Prolonged Exercise in Humans. J. Physiol. 2006, 576, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bryer, S.C.; Goldfarb, A.H. Effect of High Dose Vitamin C Supplementation on Muscle Soreness, Damage, Function, and Oxidative Stress to Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of Vitamin E and Eccentric Exercise on Selected Biomarkers of Oxidative Stress in Young and Elderly Men. Free Radic. Biol. Med. 2003, 34, 1575–1588. [Google Scholar] [CrossRef]
- Rokitzki, L.; Logemann, E.; Sagredos, A.N.; Murphy, M.; Wetzel-Roth, W.; Keul, J. Lipid Peroxidation and Antioxidative Vitamins under Extreme Endurance Stress. Acta Physiol. Scand. 1994, 151, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Williams, C.; McGregor, S.J.; Nicholas, C.W.; McArdle, F.; Jackson, M.J.; Powell, J.R. Prolonged Vitamin C Supplementation and Recovery from Demanding Exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.; Boal, R. An Effect of Ascorbic Acid on Delayed-Onset Muscle Soreness. Pain 1992, 50, 317–321. [Google Scholar] [CrossRef]
- Childs, A.; Jacobs, C.; Kaminski, T.; Halliwell, B.; Leeuwenburgh, C. Supplementation with Vitamin C and N-acetyl-cysteine Increases Oxidative Stress in Humans after an Acute Muscle Injury Induced by Eccentric Exercise. Free Radic. Biol. Med. 2001, 31, 745–753. [Google Scholar] [CrossRef]
- Thompson, D.; Williams, C.; Kingsley, M.; Nicholas, C.W.; Lakomy, H.K.; McArdle, F.; Jackson, M.J. Muscle Soreness and Damage Parameters after Prolonged Intermittent Shuttle-Running following Acute Vitamin C Supplementation. Int. J. Sports Med. 2001, 22, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Williams, C.; Garcia-Roves, P.; McGregor, S.J.; McArdle, F.; Jackson, M.J. Post-exercise Vitamin C Supplementation and Recovery from Demanding Exercise. Eur. J. Appl. Physiol. 2003, 89, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Bailey, D.M.; Hill, J.; Hurst, T.; Powell, J.R.; Williams, C. Prolonged Vitamin C Supplementation and Recovery from Eccentric Exercise. Eur. J. Appl. Physiol. 2004, 92, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.H.; Valente, H.F.; Casal, S.I.; Marques, A.F.; Moreira, P.A. Antioxidants Do Not Prevent Postexercise Peroxidation and May Delay Muscle Recovery. Med. Sci. Sports Exerc. 2009, 41, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Margaritelis, N.V.; Kyparos, A.; Nikolaidis, M.G. N-acetylcysteine Supplementation Increases Exercise Performance and Reduces Oxidative Stress Only in Individuals with Low Levels of Glutathione. Free Radic. Biol. Med. 2018, 115, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Witt, E.; Matsumoto, M.; Packer, L. Vitamin E Inhibits Protein Oxidation in Skeletal Muscle of Resting and Exercised Rats. Biochem. Biophys. Res. Commun. 1992, 189, 801–806. [Google Scholar] [CrossRef]
- Shindoh, C.; DiMarco, A.; Thomas, A.; Manubay, P.; Supinski, G. Effect of N-acetylcysteine on Diaphragm Fatigue. J. Appl. Physiol. 1990, 68, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.P.; Bracciotti, G.; Falsini, S. Spin-Trappers and Vitamin E Prolong Endurance to Muscle Fatigue in Mice. Free Radic. Biol. Med. 1990, 8, 9–13. [Google Scholar] [CrossRef]
- Lee, S.P.; Mar, G.Y.; Ng, L.T. Effects of Tocotrienol-Rich Fraction on Exercise Endurance Capacity and Oxidative Stress in Forced Swimming Rats. Eur. J. Appl. Physiol. 2009, 107, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin Improves Muscle Lipid Metabolism in Exercise via Inhibitory Effect of Oxidative CPT I Modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–827. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin Increases Brain and Muscle Mitochondrial Biogenesis and Exercise Tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed]
- Kyparos, A.; Sotiriadou, S.; Mougios, V.; Cheva, A.; Barbanis, S.; Karkavelas, G.; Arsos, G.; Albani, M.; Matziari, C. Effect of 5-Day Vitamin E Supplementation on Muscle Injury after Downhill Running in Rats. Eur. J. Appl. Physiol. 2011, 111, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training: Beneficial or Detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does Vitamin C and E Supplementation Impair the Favorable Adaptations of Regular Exercise? Oxid. Med. Cell. Longev. 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do Antioxidant Supplements Interfere with Skeletal Muscle Adaptation to Exercise Training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Akerstrom, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant Supplementation Does Not Alter Endurance Training Adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.H. Normal Adaptations to Exercise Despite Protection against Oxidative Stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to Endurance Training Depend on Exercise-Induced Oxidative Stress: Exploiting Redox Interindividual Variability. Acta Physiol. 2018, 222. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Fujii, R.; Li, X.; Higashida, K.; Muraoka, I. Effects of Exhaustive exercises, with different intensities, on oxidative stress markers in rat plasma and skeletal muscle. Sci. Sports 2018, 33, 169–175. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Jamurtas, A.Z. Blood as a Reactive Species Generator and Redox Status Regulator during Exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low Vitamin C Values Are Linked with Decreased Physical Performance and Increased Oxidative Stress: Reversal by Vitamin C Supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. https://doi.org/10.3390/antiox7090119
Kawamura T, Muraoka I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants. 2018; 7(9):119. https://doi.org/10.3390/antiox7090119
Chicago/Turabian StyleKawamura, Takuji, and Isao Muraoka. 2018. "Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint" Antioxidants 7, no. 9: 119. https://doi.org/10.3390/antiox7090119
APA StyleKawamura, T., & Muraoka, I. (2018). Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants, 7(9), 119. https://doi.org/10.3390/antiox7090119




