Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition
Abstract
1. Introduction
2. Chemical Composition of Evening Primrose (Oenothera biennis)
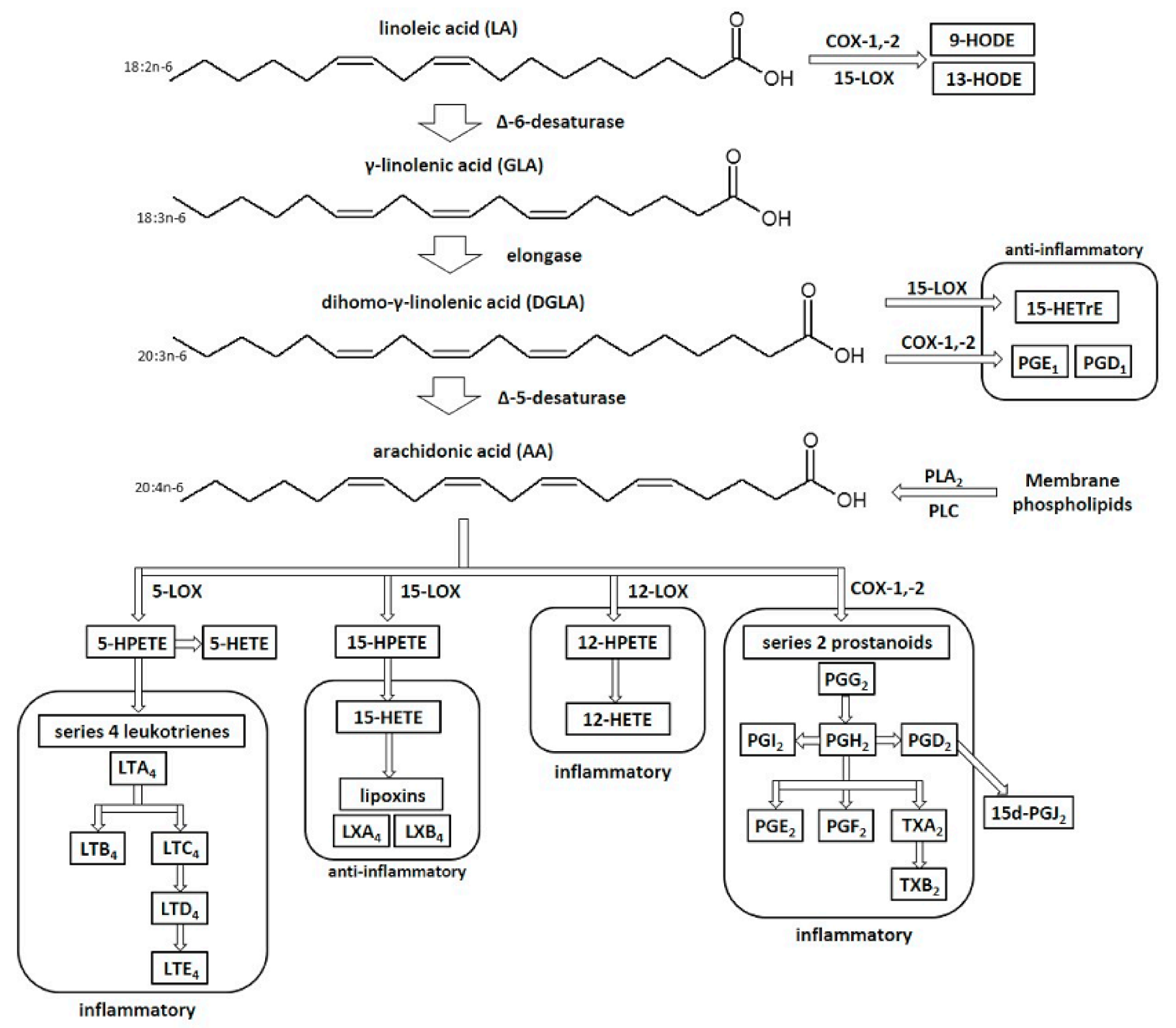
3. Biological Activity of Evening Primrose Oil (Oenothera biennis)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mihulka, S.; Pysek, P. Invasion history of Oenothera congeners in Europe: A comparative study of spreading rates in the last 200 years. J. Biogeogr. 2001, 28, 597–609. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Agrawal, A.A.; Maron, J.L.; Salminen, J.P. Heritability, covariation and natural selection on 24 traits of common evening primrose (Oenothera biennis) from a field experiment. J. Evol. Biol. 2009, 22, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, R.; Sharma, S.K. An updated review on the Oenothera genus. J. Chin. Integr. Med. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Ahmad, A.; Singh, D.K.; Fatima, K.; Tandon, S.; Luqman, S. New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Ind. Crops Prod. 2014, 58, 125–132. [Google Scholar] [CrossRef]
- Christie, W.W. The analysis of evening primrose oil. Ind. Crops Prod. 1999, 10, 73–83. [Google Scholar] [CrossRef]
- Zadernowski, R.; Polakowska-Nowak, H.; Rashed, A.A.; Kowalska, M. Lipids from evening primrose and borage seeds. Oilseed Crops 1999, 20, 581–589. [Google Scholar]
- Montserrat-de la Paz, S.; Fernandez-Arche, M.A.; Angel-Martin, M.; Garcia-Gimenez, M.D. Phytochemical characterization of potential nutraceutical ingredients from Evening Primrose oil (Oenothera biennis L.). Phytochem. Lett. 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nowak-Polakowska, H. Phenolic Acids of Borage (Borago officinalis L.) and Evening Primrose (Oenothera biennis L.). J. Am. Oil Chem. Soc. 2002, 79, 335–338. [Google Scholar] [CrossRef]
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Białek, M.; Rutkowska, J. The importance of γ-linolenic acid in the prevention and treatment. Adv. Hyg. Exp. Med. 2015, 69, 892–904. [Google Scholar] [CrossRef]
- Muggli, R. Systemic evening primrose oil improves the biophysical skin parameters of healthy adults. Int. J. Cosmet. Sci. 2005, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. Biochim. Biophys. Acta 2017, 1859, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.M.; Kummerow, F.A. Effect of magnesium deficiency on delta 6 desaturase activity and fatty acid composition of rat liver microsomes. Lipids 1989, 24, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Zietemann, V.; Kröger, J.; Enzenbach, C.; Jansen, E.; Fritche, A.; Weiker, C.; Boeing, H.; Schylze, M.B. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br. J. Nutr. 2010, 104, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Gordon, J.S.; Hsuan, C.; Stenn, K.; Prouty, S.M. Identification of the Δ-6 desaturase of human sebaceous glands: Expression and enzyme activity. J. Investig. Dermatol. 2003, 120, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. The role of fatty acid desaturases in epidermal metabolism. Dermatoendocrinol 2011, 3, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Williard, D.E.; Nwankwo, J.O.; Kaduce, T.L.; Harmon, S.D.; Irons, M.; Moser, H.W.; Raymond, G.V.; Spector, A.R. Identification of a fatty acid Δ6-desaturase deficiency in human skin fibroblasts. J. Lipid Res. 2001, 42, 501–508. [Google Scholar] [PubMed]
- Huang, S.; Liu, R.; Niu, Y.; Hasi, A. Cloning and functional characterization of a fatty acid Δ6-desaturase from Oenothera biennis: Production of γ-linolenic acid by heterologous expression in Saccharomyces cerevisiae. Russ. J. Plant Phys. 2010, 57, 568–573. [Google Scholar] [CrossRef]
- Cho, H.P.; Nakamura, M.T.; Clarke, S.D. Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J. Biol. Chem. 1999, 274, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Sabyasachi, B.; Gangopadhyay, D.N. Evening primrose oil is effective in atopic dermatitis: A randomized placebo-controlled trial. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, M.J.; Vandersall, A.; Katta, R. Diet and eczema: A review of dietary supplements for the treatment of atopic dermatitis. Dermatol. Pract. Concept 2016, 6, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 14, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama-Fujiwara, Y.; Ohmori, C.; Igarashi, O. Metabolism of γ-linolenic acid in primary cultures of rat hepatocytes and in Hep G2 cells. J. Nutr. Sci. Vitaminol. 1989, 35, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Ziboh, V.A.; Naguwa, S.; Vang, K.; Wineinger, J.; Morrissey, B.M.; Watnik, M.; Gershwin, M.E. Suppression of leukotriene B4 generation by ex-vivo neutrophils isolated from asthma patients on dietary supplementation with gammalinolenic acid-containing borage oil: Possible implication in asthma. Clin. Dev. Immunol. 2004, 11, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.J.; Hill, A. Evening primrose oil and borage oil in rheumatologic conditions. Am. J. Clin. Nutr. 2000, 71, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Luo, J.; Zang, W.; Chen, D.; Xu, H.; Shi, H.; Jing, H. Gamma-Linolenic Acid Suppresses NF-κB Signaling via CD36 in the Lipopolysaccharide-Induced Inflammatory Response in Primary Goat Mammary Gland Epithelial Cells. Inflammation 2016, 39, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E.; Koumenis, I.L.; Edens, M.B.; Tramposch, K.M.; Clayton, B.; Bowton, D.; Chilton, F.H. Inhibition of leukotriene biosynthesis by a novel dietary fatty acid formulation in patients with atopic asthma: A randomized, placebo-controlled, parallel-group, prospective trial. Clin. Ther. 2003, 25, 972–979. [Google Scholar] [CrossRef]
- Khajeh, M.; Rahbarghazi, R.; Nouri, M.; Darabi, M. Potential role of polyunsaturated fatty acids, with particular regard to the signaling pathways of arachidonic acid and its derivatives in the process of maturation of the oocytes: Contemporary review. Biomed. Pharmacother. 2017, 94, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sunberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, D.W.T.; Aarsetoey, H.; Ponitz, V.; Brugger-Andersen, T.; Staines, H.; Harris, W.S.; Grundt, H. The prognostic utility of dihomo-gamma-linolenic acid (DGLA) in patients with acute coronary heart disease. Int. J. Cardiol. 2017, 249, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef]
- Park, K.Y.; Ko, E.J.; Kim, I.S.; Li, K.; Kim, B.J.; Seo, S.J.; Kim, M.N.; Hong, C.K. The effect of evening primrose oil for the prevention of xerotic cheilitis in acne patients being treated with isotretinoin: A pilot study. Ann. Dermatol. 2014, 26, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Peter, M.; Hackler, L., Jr.; Man, I.; Szebeni, G.; Ayaydin, F.; Hideghety, K.; Vigh, L.; Kitajka, K.; Balogh, G.; et al. Lipidomic analysis reveals a radiosensitizing role of gamma-linolenic acid in glioma cells. Biochim. Biophys. Acta 2015, 1851, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Rao, K.P. Effect of γ-linolenic acid and prostaglandins E1 on gamma-radiation and chemical-induced genetic damage to the bone marrow cells of mice. Prostaglandins Leukot. Essent Fat. Acids 2006, 74, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Effect of γ-Linolenic Acid on the Transcriptional Activity of the Her-2/neu (erbB-2) Oncogene. J. Natl. Cancer Inst. 2005, 2, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Lee, J.H.; Steeg, P.S. Clinical-translational strategies for the elevation of Nm23-H1 metastasis suppressor gene expression. Mol. Cell Biochem. 2009, 329, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Hiscox, S.; Bryce, R.P.; Horrobin, D.F.; Mansel, R.E. The effects of n-6 polyunsaturated fatty acids on the expression of nm-23 in human cancer cells. Br. J. Cancer 1998, 77, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.A.; Benadiba, M.; Colquhoun, A. Gamma-linolenic acid inhibits both tumour cell cycle progression and angiogenesis in the orthotopic C6 glioma model through changes in VEGF, Flt1, ERK1/2, MMP2, cyclin D1, pRb, p53 and p27 protein expression. Lipids Health Dis. 2009, 17. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Bucolo, S.; Spinella, R.; Giuffrida, S.; Ferreri, G. Systemic Omega-6 Essential Fatty Acid Treatment and PGE1 Tear Content in Sjögren’s Syndrome Patients, Investigative Ophthalmology & Visual Science. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4474–4479. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bhattacharyya, D.K. Dietary effect of γ-linolenic acid on the lipid profile of rat fed erucic acid rich oil. J. Oleo Sci. 2007, 56, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A metaanalysis of randomized controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K. Eicosanoids and Keratinocytes in Wound Healing. Adv. Wound Care 2014, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.F.; Sales-Campos, H.; Nardini, V.; da Costa, T.A.; Fonseca, M.T.C.; Júnior, V.R.; Sorgi, C.A.; da Silva, J.S.; Chica, J.E.L.; Faccioli, L.H.; et al. The inhibition of 5-Lipoxygenase (5-LO) products leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clin. Immunol. 2018, 190, 74–83. [Google Scholar] [CrossRef] [PubMed]
| Compound Name | Contents (%) |
|---|---|
| linoleic acid | 73.88 ± 0.09 |
| γ-linolenic acid | 9.24 ± 0.05 |
| oleic acid | 6.93 ± 0.02 |
| palmitic acid | 6.31 ± 0.14 |
| stearic acid | 1.88 ± 0.02 |
| vaccenic acid | 0.81 ± 0.03 |
| eicosenoic acid | 0.55 ± 0.01 |
| eicosanoic acid | 0.31 ± 0.03 |
| behenic acid | 0.10 ± 0.01 |
| Acid Name | Included in | |||
|---|---|---|---|---|
| Free | Esters | Glycosides | Total | |
| p-hydroxyphenyl acetic | n/a | 1.03 ± 0.18 | 0.26 ± 0.05 | 1.29 ± 0.19 |
| p-hydroxybenzoic | 4.12 ± 0.25 | 0.38 ± 0.07 | 0.29 ± 0.10 | 4.79 ± 0.26 |
| 2-hydroxy-4-methoxybenzoic | 6.52 ± 0.30 | n/a | 0.83 ± 0.28 | 7.35 ± 0.41 |
| caffeic | 6.48 ± 0.29 | 0.80 ± 0.14 | n/a | 7.51 ± 0.33 |
| hydroxycaffeic | n/a | 0.77 ± 0.18 | n/a | 0.77 ± 0.18 |
| m-coumaric | 4.90 ± 0.45 | 0.83 ± 0.21 | n/a | 5.73 ± 0.50 |
| p-coumaric | 1.32 ± 0.10 | 1.96 ± 0.23 | 0.06 ± 0.06 | 3.34 ± 0.25 |
| ferulic | 4.08 ± 0.30 | 0.72 ± 0.09 | 0.22 ± 0.06 | 5.02 ± 0.32 |
| gallic | 1.87 ± 0.22 | 7.03 ± 0.82 | 5.91 ± 1.56 | 14.81 ± 1.78 |
| protocatechuic | 50.28 ± 0.77 | 10.96 ± 0.34 | 2.16 ± 2.42 | 63.40 ± 2.56 |
| vanillic | 5.22 ± 0.28 | 0.06 ± 0.02 | 0.83 ± 0.28 | 7.35 ± 0.41 |
| veratric | n/a | 0.41 ± 0.03 | 0.47 ± 0.15 | 0.88 ± 0.15 |
| homoveratric | n/a | 0.43 ± 0.06 | n/a | 0.43 ± 0.06 |
| salicylic | 1.15 ± 0.04 | 1.40 ± 0.18 | n/a | 2.55 ± 0.18 |
| Compound Name | Contents (mg/kg of Oil) |
|---|---|
| β-sitosterol | 7952.00 ± 342.25 |
| kampesterol | 883.32 ± 0.45 |
| Δ5-avenasterol | 429.65 ± 75.20 |
| sitostanol | 167.01 ± 39.77 |
| clerosterol | 120.44 ± 0.12 |
| Δ5-24-estigmastadienol | 94.60 ± 5.68 |
| Δ7-estigmasterol | 38.17 ± 14.33 |
| Δ7-avenasterol | 27.80 ± 16.07 |
| Macroelements | Contents (mg/100g of ash) |
| calcium | 1800 |
| magnesium | 530 |
| potassium | 460 |
| sodium | 18 |
| phosphorus | 410 |
| Microelements | Contents (mg/100g of ash) |
| iron | 39 |
| zinc | 7 |
| copper | 1.1 |
| manganese | 0.5 |
| Metabolite | Biological Activity | Occurrence | |
|---|---|---|---|
| anti-inflammatory | PGE1 |
| keratinocytes fibroblasts sebocyte |
| 15-HETrE |
| keratinocytes fibroblasts | |
| 13-HODE |
| keratinocytes fibroblasts | |
| 15-HETE |
| keratinocytes fibroblasts | |
| LXA4 LXB4 |
| neutrophils | |
| inflammatory | PGE2 |
| keratinocytes fibroblasts |
| 5-HETE |
| keratinocytes | |
| LTB4 |
| leukocytes keratinocytes in chronic dermatitis (psoriasis, atopic dermatitis) | |
| Cys-LT (LTC4 LTD4 LTE4) |
| leukocytes in chronic dermatitis (psoriasis, atopic dermatitis) | |
| 12-HETE |
| keratinocytes fibroblasts Langerhans cells in chronic dermatitis (psoriasis) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. https://doi.org/10.3390/antiox7080108
Timoszuk M, Bielawska K, Skrzydlewska E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants. 2018; 7(8):108. https://doi.org/10.3390/antiox7080108
Chicago/Turabian StyleTimoszuk, Magdalena, Katarzyna Bielawska, and Elżbieta Skrzydlewska. 2018. "Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition" Antioxidants 7, no. 8: 108. https://doi.org/10.3390/antiox7080108
APA StyleTimoszuk, M., Bielawska, K., & Skrzydlewska, E. (2018). Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants, 7(8), 108. https://doi.org/10.3390/antiox7080108






