Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials
Abstract
:1. Introduction
1.1. Clinical Pharmacokinetics of Vitamin C
1.2. Possible Mechanisms of Anti-Tumor Effects of Vitamin C
1.3. Synergy with Chemotherapy
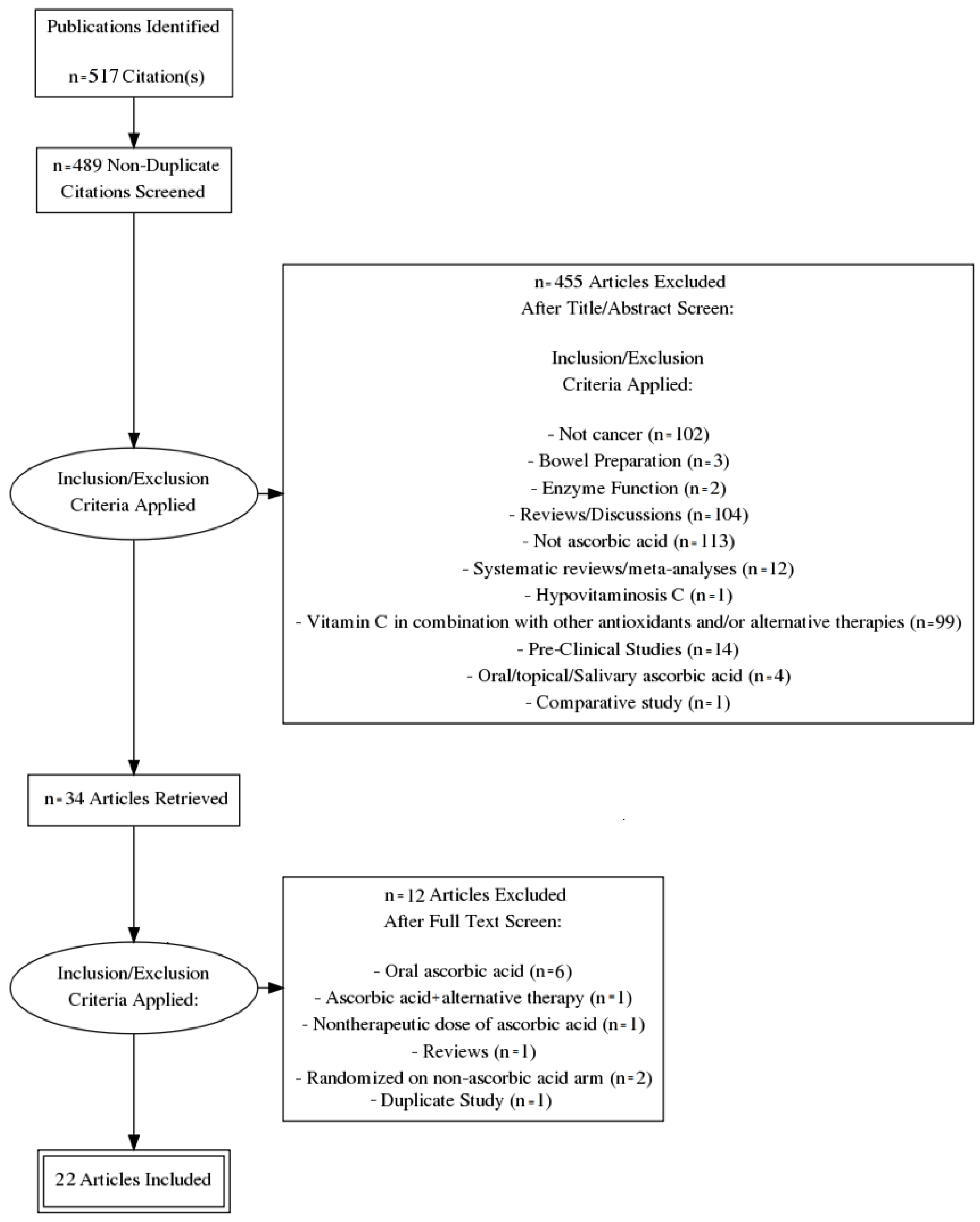
2. Materials and Methods
Study Characteristics
3. Results
3.1. Trials Evaluating Low-Dose Intravenous Ascorbic Acid in Combination with Arsenic Trioxide
3.2. Trials Evaluating High-Dose Intravenous Ascorbic Acid with Standard Chemo- and Radiotherapy Agents
3.3. IV Ascorbate Only Trials
3.4. Potential of Benefit and Current Limitations
4. Future Directions
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic Acid |
| AML | Acute Myeloid Leukemia |
| APL | Acute Promyelocytic Leukemia |
| As2O3 | Arsenic Trioxide |
| CT | Computed Tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DHA | Dehydroascorbic acid |
| DNA | Deoxyribonucleic acid |
| GBM | Glioblastoma |
| GADPH | Glyceraldehyde 3-phosphate dehydrogenase |
| GSH | Glutathione |
| HIF | Hypoxia inducible factors |
| H2O2 | Hydrogen Peroxide |
| IV | Intravenous |
| IVC | Intravenous vitamin C |
| mM | Millimolar |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PFS | Progression free survival |
| PHD | Prolyl-4-hydroxylase domain |
| ROS | Reactive oxygen species |
| TET | Ten eleven translocation |
References
- McCormick, W. Cancer: The preconditioning factor in pathogenesis; a new etiologic approach. Arch. Pediatr. 1954, 71, 313–322. [Google Scholar] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. The orthomolecular treatment of cancer. I. The role of ascorbic acid in host resistance. Chem. Biol. Interact. 1974, 9, 273–283. [Google Scholar] [CrossRef]
- Cameron, E.; Campbell, A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974, 9, 285–315. [Google Scholar] [CrossRef]
- Cameron, E.; Campbell, A.; Jack, T. The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: Double complete regression induced by high-dose ascorbic acid therapy. Chem. Biol. Interact. 1975, 11, 387–393. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayatty, S.J.; Levine, M. Reevaluation of ascorbate in cancer treatment: Emerging evidence, open minds and serendipity. J. Am. Coll. Nutr. 2000, 19, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Hutton, B.; Ng, T.; Shorr, R.; Clemons, M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 2015, 20, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Wittes, R.E. Vitamin C and cancer. N. Engl. J. Med. 1985, 312, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graumlich, J.F.; Ludden, T.M.; Conry-Cantilena, C.; Cantilena, L.R., Jr.; Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 1997, 14, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Ohno, Y.; Suzuki, N.; Soma, G.; Inoue, M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009, 29, 809–815. [Google Scholar] [PubMed]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H., Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef] [PubMed]
- Riordan, H.D.; Casciari, J.J.; Gonzalez, M.J.; Riordan, N.H.; Miranda-Massari, J.R.; Taylor, P.; Jackson, J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P. R. Health Sci. J. 2005, 24, 269–276. [Google Scholar] [PubMed]
- Duconge, J.; Miranda-Massari, J.R.; Gonzalez, M.J.; Jackson, J.A.; Warnock, W.; Riordan, N.H. Pharmacokinetics of vitamin C: Insights into the oral and intravenous administration of ascorbate. P. R. Health Sci. J. 2008, 27, 7–19. [Google Scholar] [PubMed]
- Pauling, L. Are recommended daily allowances for vitamin C adequate? Proc. Natl. Acad. Sci. USA 1974, 71, 4442–4446. [Google Scholar] [CrossRef] [PubMed]
- Langemann, H.; Torhorst, J.; Kabiersch, A.; Krenger, W.; Honegger, C.G. Quantitative determination of water- and lipid-soluble antioxidants in neoplastic and non-neoplastic human breast tissue. Int. J. Cancer 1989, 43, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Honegger, C.G.; Torhorst, J.; Langemann, H.; Kabiersch, A.; Krenger, W. Quantitative determination of water-soluble scavengers in neoplastic and non-neoplastic human breast tissue. Int. J. Cancer 1988, 41, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Vera, J.C.; Golde, D.W. Stromal cell oxidation: A mechanism by which tumors obtain vitamin C. Cancer Res. 1999, 59, 4555–4558. [Google Scholar] [PubMed]
- Violet, P.C.; Levine, M. Pharmacologic Ascorbate in Myeloma Treatment: Doses Matter. EBioMedicine 2017, 18, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Abu Hejleh, T.; et al. O2− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017, 31, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Hinshaw, D.B.; Halsey, W.A., Jr.; Schraufstatter, I.U.; Sauerheber, R.D.; Spragg, R.G.; Jackson, J.H.; Cochrane, C.G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988, 263, 1665–1675. [Google Scholar] [PubMed]
- Ahmad, I.M.; Aykin-Burns, N.; Sim, J.E.; Walsh, S.A.; Higashikubo, R.; Buettner, G.R.; Venkataraman, S.; Mackey, M.A.; Flanagan, S.W.; Oberley, L.W.; et al. Mitochondrial O2− and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J. Biol. Chem. 2005, 280, 4254–4263. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Reest, J.; Gottlieb, E. Anti-cancer effects of vitamin C. revisited. Cell Res. 2016, 26, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Roffey, D.M.; Dion, C.A.; Arab, A.; Wai, E.K. Effect of Perioperative Vitamin C Supplementation on Postoperative Pain and the Incidence of Chronic Regional Pain Syndrome: A Systematic Review and Meta-Analysis. Clin. J. Pain 2016, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martinez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoud, G.N.; Li, W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.J.; Vissers, M.C.; Bozonet, S.; Dyer, A.; Robinson, B.A.; Dachs, G.U. Restoring physiological levels of ascorbate slows tumor growth and moderates HIF-1 pathway activity in Gulo−/− mice. Cancer Med. 2015, 4, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Yu, J.; Chalmers, B.; Drisko, J.; Yang, J.; Li, B.; Chen, Q. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 2012, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, C.P. High Dose Vitamin C and Low Dose Chemo Treatment. J. Cancer Sci. 2018, 5, 4. [Google Scholar]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., 2nd; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I. clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Boccia, R.; Siegel, D.; Bozdech, M.; Bessudo, A.; Stadtmauer, E.; Talisman Pomeroy, J.; Steis, R.; Flam, M.; Lutzky, J.; et al. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: A prospective, multicentre, phase II, single-arm study. Br. J. Haematol. 2006, 135, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Abou-Jawde, R.M.; Reed, J.; Kelly, M.; Walker, E.; Andresen, S.; Baz, R.; Karam, M.A.; Hussein, M. Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients: A phase 2 trial. Med. Oncol. 2006, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Voorhees, P.M.; Kolesar, J.M.; Ahuja, H.G.; Sanchez, F.A.; Rodriguez, G.A.; Kim, K.; Werndli, J.; Bailey, H.H.; Kahl, B.S. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: A Wisconsin Oncology Network study. Hematol. Oncol. 2009, 27, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bael, T.E.; Peterson, B.L.; Gollob, J.A. Phase II trial of arsenic trioxide and ascorbic acid with temozolomide in patients with metastatic melanoma with or without central nervous system metastases. Melanoma Res. 2008, 18, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, P.R.; Lima, M.; Ardalan, B. Arsenic trioxide/ascorbic acid therapy in patients with refractory metastatic colorectal carcinoma: A clinical experience. Acta Oncol. 2007, 46, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.L.; Beksac, M.; van Droogenbroeck, J.; Amadori, S.; Zweegman, S.; Sonneveld, P. Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica 2006, 91, 1722–1723. [Google Scholar] [PubMed]
- Aldoss, I.; Mark, L.; Vrona, J.; Ramezani, L.; Weitz, I.; Mohrbacher, A.M.; Douer, D. Adding ascorbic acid to arsenic trioxide produces limited benefit in patients with acute myeloid leukemia excluding acute promyelocytic leukemia. Ann. Hematol. 2014, 93, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J.; McCafferty-Grad, J.; Jordan-McMurry, I.; Neil, J.; Reis, I.; Kharfan-Dabaja, M.; Eckman, J.; Goodman, M.; Fernandez, H.F.; Boise, L.H.; et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin. Cancer Res. 2002, 8, 3658–3668. [Google Scholar] [PubMed]
- Held, L.A.; Rizzieri, D.; Long, G.D.; Gockerman, J.P.; Diehl, L.F.; de Castro, C.M.; Moore, J.O.; Horwitz, M.E.; Chao, N.J.; Gasparetto, C. A Phase I study of arsenic trioxide (Trisenox), ascorbic acid, and bortezomib (Velcade) combination therapy in patients with relapsed/refractory multiple myeloma. Cancer Investig. 2013, 31, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Klco, J.M.; Gao, F.; Procknow, E.; Uy, G.L.; Stockerl-Goldstein, K.E.; Abboud, C.N.; Westervelt, P.; DiPersio, J.F.; Hassan, A.; et al. Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: A phase I study. Am. J. Hematol. 2011, 86, 796–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, H.; Sawanobori, M.; Tsuma-Kaneko, M.; Wasada, I.; Miyamoto, M.; Murayama, H.; Toyosaki, M.; Onizuka, M.; Tsuboi, K.; Tazume, K.; et al. Phase I Clinical Trial of Intravenous L-ascorbic Acid Following Salvage Chemotherapy for Relapsed B-cell non-Hodgkin‘s Lymphoma. Tokai J. Exp. Clin. Med. 2014, 39, 111–115. [Google Scholar] [PubMed]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase, I./IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Hojgaard, M.; Andersen, J.T.; Jorgensen, N.R.; Zerahn, B.; Kristensen, B.; Henriksen, T.; Lykkesfeldt, J.; Mikines, K.J.; Poulsen, H.E. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: A single-arm phase II trial. Transl. Androl. Urol. 2017, 6, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar] [PubMed]
- Riordan, H.D.; Hunninghake, R.B.; Riordan, N.H.; Jackson, J.J.; Meng, X.; Taylor, P.; Casciari, J.J.; Gonzalez, M.J.; Miranda-Massari, J.R.; Mora, E.M.; et al. Intravenous ascorbic acid: Protocol for its application and use. P. R. Health Sci. J. 2003, 22, 287–290. [Google Scholar] [PubMed]
- Padayatty, S.J.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Hoffer, L.J.; Levine, M. Intravenously administered vitamin C as cancer therapy: Three cases. CMAJ 2006, 174, 937–942. [Google Scholar] [CrossRef] [PubMed]
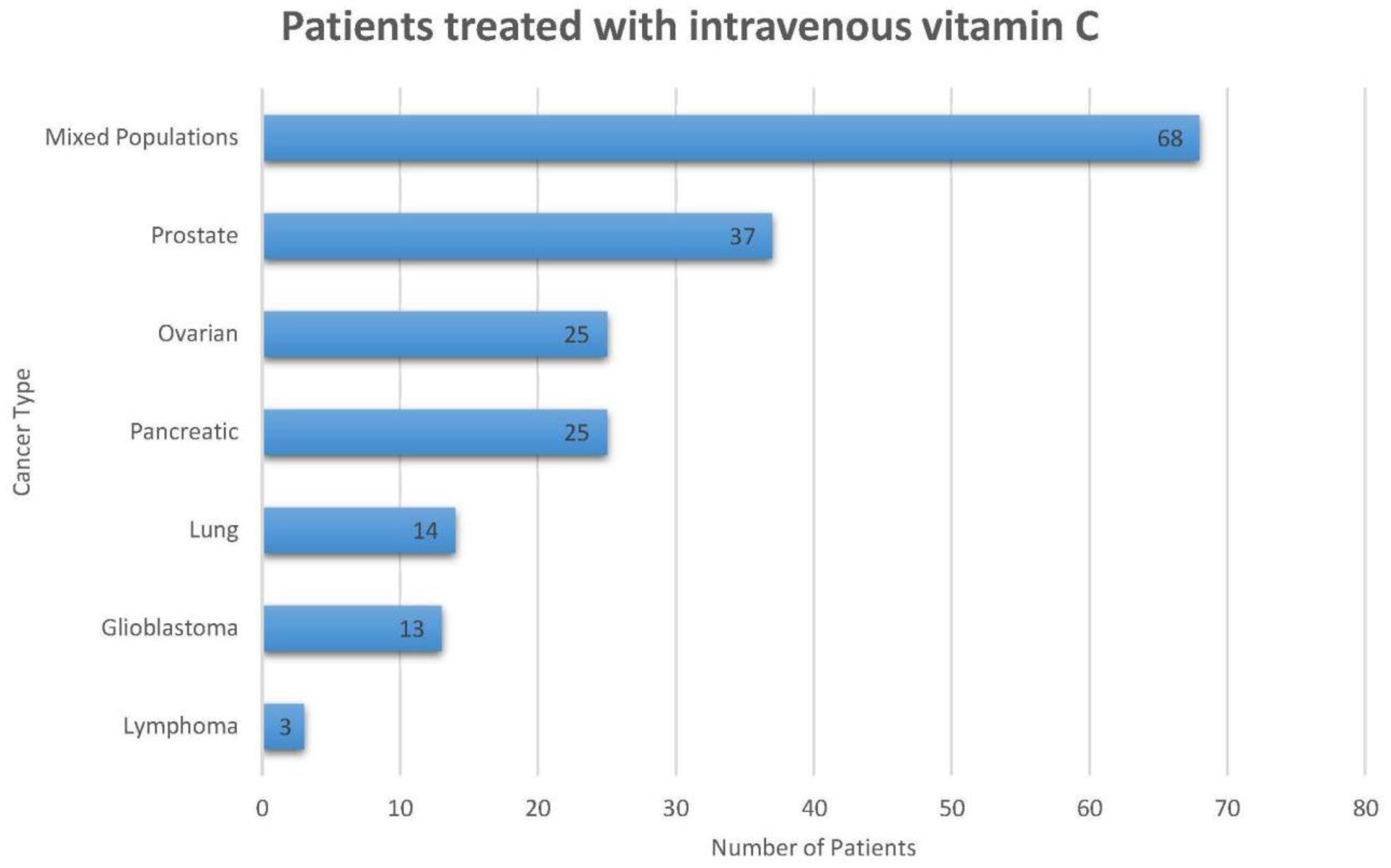
| Reference | n | Patient Diagnosis | Trial Design | IV AA Treatment Type and Frequency | Concurrent Treatment Dose | Toxicity | Reported Outcomes/Conclusions |
|---|---|---|---|---|---|---|---|
| [52] | 22 | Refractory multiple myeloma | Single Arm | 1 g on days 1, 4, 8, and 11 of a 21-day cycle for a maximum of 8 cycles | Bortezomib and Arsenic Trioxide | One occurrence of grade 4 thrombocytopenia was observed in a patient receiving high-dose bortezomib | Objective responses were observed in 27% of patients (2 partial and 4 minor). Median progression-free survival was 5 months and overall survival had not been reached. |
| [53] | 65 | Relapsed or refractory multiple myeloma | Single Arm | 1 g on days 1–4 of week 1 and twice weekly during weeks 2–5 of a 6 week cycle. | Melphalan and Arsenic Trioxide | Grade 3/4 hematological (3%) or cardiac adverse events occurred infrequently, but grade 3/4 adverse events fever/chills (15%), pain (8%), and fatigue (6%) were reported. | Objective responses occurred in 48% of patients, including complete, partial, and minor responses. Median progression-free survival and overall survival were 7 and 19 months respectively. |
| [54] | 20 | Multiple myeloma, relapsed and refractory | Single Arm | 1000 mg for 5 consecutive days during week 1, followed by twice weekly during weeks 2–12 | Dexamethasone and Arsenic Trioxide | Grade 3 events in 45% and grade 4 events in 5% | 30% complete and partial response. Overall median survival was 962 days. 10 patients developed grade 3/4 toxicity to combination treatment. |
| [55] | 17 | Lymphoid malignancies, relapsed and refractory. | Single Arm | 1000 mg for 5 days during week 1 followed by twice weekly during weeks 2–6 | Arsenic Trioxide | 1 cardiac death, multiple grade 3 and 4 events | Overall median survival was 7.6 months 6% complete and partial response. Study closed at first interim analysis. |
| [56] | 11 | Advanced melanoma | Single Arm | 1000 mg for 5 days during week 0, and then twice weekly for an 8 week cycle. | Temozolomide and Arsenic Trioxide | Multiple grade 1 and 2 events. | No responses seen in the first 10 evaluable patients leading to early closure of study. |
| [57] | 5 | Refractory metastatic colorectal carcinoma | Single Arm | 1000 mg/day for 5 days a week for 5 weeks | Arsenic Trioxide | Grade 3 nausea, vomiting, diarrhea, thrombocytopenia, and anemia | No complete or partial remission observed. CT scans showed stable or progressive disease. |
| [58] | 20 | Multiple myeloma, relapsed and refractory | Single Arm | 1 mg (one dose during the first week, twice weekly during weeks 2–4) | Dexamethasone and Arsenic Trioxide | Multiple grade 3 and 4 events | Clinical response was observed in 40% of patients (including partial and minor). Median progression free survival = 4 months and median overall survival = 11 months. Authors state that it was difficult to assess activity of each individual agent. |
| [59] | 11 | Non-acute promyelocytic leukemia; acute myeloid leukemia (non-APL AML) | Single Arm | 1 g/day for 5 days a week for 5 weeks | Arsenic Trioxide | Few grade 3 or 4 adverse effects and the most common grade 3 toxicity was infection though possibly related to the leukemia | One patient achieved a complete response; another achieved a complete remission with incomplete hematologic recovery. Authors concluded that arsenic trioxide + ascorbic acid had limited clinical meaning in non-APL AML patients. |
| [60] | 6 | Relapsed or refractory myeloma | Single Arm | 1000 mg/day for 25 days over 35 days total. | Arsenic Trioxide | One episode of grade 3 hematologic toxicity (leukopenia) was observed. | Two patients had partial responses; four had stable disease. |
| [61] | 10 | Relapsed/refractory multiple myeloma | Single Arm | 1 g daily for 3 days of week 1, then twice weekly for a 3-week cycle. | Arsenic Trioxide and Bortezomib | No dose limiting adverse effects. | 40% response rate with one patient achieving a durable partial response. |
| [62] | 13 | Myelodysplastic Syndrome and Acute Myeloid Leukemia (concurrent diagnoses) | Single Arm | 1 g for 5 days during week following each dose of IV Arsenic Trioxide and then once weekly thereafter | Decitabine and Arsenic Trioxide | Grade 3 and 4 events; two patient deaths occurred not related to treatment | One morphologic complete remission was observed. Five patients had stable disease after recovery. 0.2 mg/kg identified as maximum tolerated dose of arsenic in combination with Decitabine and Ascorbic Acid. |
| Reference | n | Patient Diagnosis | Trial Design | IV AA Treatment Type and Frequency | Concurrent Treatment Dose | Toxicity | Reported Outcomes/Conclusions |
|---|---|---|---|---|---|---|---|
| [63] | 17 | Advanced tumors | Single Arm | Five cohorts treated with 30, 50, 70, 90, and 110 g/m2 for 4 consecutive days for 4 weeks. | Multivitamin and Eicosapentaenoic acid | Grade 3 and grade 4 hyponatremia, hyperkalemia | 3 patients had stable disease, 13 had progressive disease. Recommended dose is 70–80 g/m2. This translates to approximately 125 g because the average patient has a body surface area of 1.6–1.9 m². |
| [64] | 3 | Relapsed lymphoma | Single Arm | 75 g twice weekly | Rituximab, cyclophosphamide, cytarabine, etoposide, dexamethasone | Grade 3 neutropenia, anemia, thrombocytopenia | The authors concluded that 75 g was a safe dose. |
| [51] | 11 | Advanced pancreatic adenocarcinoma | Single Arm | 15–125 g twice weekly | Gemcitabine | No dose limiting adverse effects | Mean plasma ascorbate levels were significantly higher than baseline. Mean survival time of subjects completing 8 weeks of therapy was 13 ± 2 months. |
| [21] | 14 | Pancreatic adenocarcinoma, stage IV | Single Arm | 50, 75, and 100 g per infusion (3 cohorts) thrice weekly for 8 weeks | Gemcitabine and Erlotinib | Multiple toxicities, all grades, thought to not be related to AA; grade 4 adverse event included two patients with pulmonary embolism | 50% of patients had stable disease. Survival analysis excluded 5 patients who progressed quickly (3 died). Overall mean survival was 182 days. |
| [50] | 25 | Stage 3/4 ovarian cancer | Randomized | 75 or 100 g twice weekly for 12 months (target plasma concentration 20–23 mM) | Carboplatin and paclitaxel | Ascorbate did not increase grade 3/4; grade 1 and 2 toxicities were substantially decreased | 8.75 month increase in PFS in AA-treated arm. Trend to improved OS in AA group; no numerical data reported. |
| [22] | 16 | Various cancer types (lung, rectum, colon, bladder, ovary, cervix, tonsil, breast, biliary tract) | Single Arm | 1.5 g/kg body weight infused three times (at least one day apart) on week days during weeks when chemotherapy was administered (but not on the same day as intravenous chemotherapy) and any two days at least one day apart during weeks when no chemotherapy was given. | Standard care chemotherapy. | Increased thirst and increased urinary flow; these adverse symptoms did not appear to be caused by the ascorbate molecule | Patients experienced unexpected transient stable disease, increased energy, and functional improvement. |
| [30] Phase I study | 13 | Glioblastoma | Single Arm | Radiation phase: radiation (61.2 Gy in 34 fractions), temozolomide (75 mg/m2 daily for a maximum of 49 days), ascorbate (doses ranging from 15–125 g, 3 times per week for 7 weeks) Adjuvant phase: 6 cycles of 28 days; treatment with temozolomide (1 dose-escalation to 200 mg/m2 if no toxicity in cycle 1), ascorbate (2 times per week, dose-escalation until 20 mM plasma concentration, around ~85 g infusion). | Ascorbate with radiation and temozolomide | Radiation phase toxicity: Grade 2 and 3 fatigue and nausea; grade 2 infection; grade 3 vomiting Adjuvant phase toxicity: grade 2 fatigue and nausea; grade 1 vomiting; grade 3 leukopenia; and grade 3 neutropenia. | Progression-free survival 13.3 months; average overall survival 21.5 months. |
| [30] Phase II study | 14 | Advanced stage non-small cell lung cancer | Single Arm | 1 cycle is 21 days; IV carboplatin (AUC 6, 4 cycles), IV paclitaxel (200 mg/m2, 4 cycles), IV pharmacological ascorbate (two 75 g infusions per week, up to 4 cycles) | Carboplatin, paclitaxel, and ascorbate | No grade 3 or 4 toxicities related to ascorbate | Imaging confirmed partial responses to therapy (n = 4), stable disease (n = 9), disease progression (n = 1) |
| [65] | 14 | Locally advanced or metastatic prostate cancer | Single Arm | Phase I: Escalating dose of IVC from 25 g to 100 g and gemcitabine alone at 1000 mg/m2 (week 3) with a few patients receiving reduced doses and gemcitabine with IVC (week 4) Phase IIa: no gemcitabine for 1 week and then continuous treatment of gemcitabine until disease progression or unacceptable toxicity and IVC 3 times per week | IVC and gemcitabine | Low toxicity; Increased thirst and nausea were caused by IVC | Patients experienced a mix of stable disease, partial response and disease progression. |
| Reference | n | Cancer Type | Trial Design | IV AA Treatment Type and Frequency | Toxicity | Reported Outcomes/Conclusions |
|---|---|---|---|---|---|---|
| Phase I | ||||||
| [18] | 24 | Advanced cancer or hematologic malignancy | Single Arm | 1.5 g/kg body weight three times weekly | No dose limiting adverse effects. | Two patients had unexpectedly stable disease. |
| Phase II | ||||||
| [66] | 23 | Castration-resistant prostate cancer | Single Arm | 5 g during weekly week 1, 30 g weekly during week 2, and 60 g weekly during weeks 3–12 | Multiple grade 3 events including hypertension and anemia; two patients experienced pulmonary embolism. | Adverse events were thought to be more likely related to disease progression than ascorbic acid. |
| [23] | 11 | Late stage terminal cancer patients | Single Arm | 150–710 mg/kg/day for up to eight weeks | Two Grade 3 adverse events: one patient with a history of renal calculi developed a kidney stone after thirteen days of treatment and another patient experienced hypokalemia after six weeks of treatment. | One patient had stable disease and continued the treatment for forty-eight weeks Intravenous vitamin C was deemed relatively safe so long as the patient does not have a history of kidney stone formation. |
| Phase | Trial Title | Trial Design | IV AA Treatment Type and Frequency | Interventions | Status | Enrollment | NCT Identifier |
|---|---|---|---|---|---|---|---|
| Phase I | Gemcitabine, Ascorbate, Radiation Therapy for Pancreatic Cancer | Single Arm | 50 g–100 g during radiation therapy for 5–6 weeks; escalating dose based on tolerance | Ascorbate Gemcitabine Radiation Therapy | Ongoing, closed to accrual | 16 | NCT01852890 |
| Phase I | High-Dose Ascorbate in Glioblastoma Multiforme | Single Arm | 15 g–87.5 g by IV 3×/week for 12 weeks | Ascorbate Temozolomide Radiation Therapy | Ongoing, closed to accrual | 13 | NCT01752491 |
| Phase I | High Dose Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) in Patients Who Have No Prior Therapy for Their Metastatic Pancreatic Cancer | Single Arm | No dosing information provided | Ascorbic Acid Nab-paclitaxel Cisplatin Gemcitabine | Ongoing, actively recruiting participants | 36 | NCT03410030 |
| Phase II | High-dose Ascorbate for Pancreatic Cancer (PACMAN 2.1) | Single Arm | 75 g by IV 3×/week for 4 weeks | Ascorbate Gemcitabine Nab-paclitaxel | Accrual began 28 May 2018 | 30 | NCT02905578 |
| Phase II | High-Dose Ascorbate in Stage IV Non-Small Cell Lung Cancer | Single Arm | 75 g by IV 2×/week for up to 12 weeks | Ascorbic Acid Carboplatin Paclitaxel | Ongoing, actively recruiting participants | 57 | NCT02420314 |
| Phase II | High-Dose Ascorbate in Glioblastoma Multiforme | Single Arm | 87.5 g by IV 3×/week during radiation therapy After radiation ascorbate 2×/week | Temozolomide Ascorbic Acid Radiation Therapy | Ongoing, actively recruiting participants | 90 | NCT02344355 |
| Phase II | Adding Ascorbate to Chemotherapy and Radiation Therapy for NSCLC (XACT-LUNG) | Single Arm | Concurrent phase: 75 g by IV 3×/week for up to 7 weeks Consolidation phase: 75 g by IV 2×/week for two cycles (42 days) | Paclitaxel Carboplatin Ascorbate Radiation Therapy | Ongoing, actively recruiting participants | 46 | NCT02905591 |
| Phase II | Docetaxel with or Without Ascorbic Acid in Treating Patients with Metastatic Prostate Cancer | Randomized | 1 g/kg 3× per week | Docetaxel Ascorbic Acid or Placebo | Ongoing, actively recruiting participants | 69 | NCT02516670 |
| Phase I/II | Randomized Study to Evaluate the Role of Intravenous Ascorbic Acid Supplementation to Conventional Neoadjuvant Chemotherapy in Women with Breast Cancer | Randomized | 1.5 g on day 1 followed by 0.75 g on day 2–4 at each chemotherapy cycle | Ascorbic Acid Placebo | Status Unknown | 30 | NCT03175341 |
| Phase I/II | Evaluating the Safety and Tolerability of Vitamin C in Patients with Intermediate or High Risk Myelodysplastic Syndrome with TET2 Mutations | Single Arm | 50 gm CIVI/24 h x 5 days every 4 week | Ascorbic acid | Accrual begins 26 June 2018 | 18 | NCT03433781 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nauman, G.; Gray, J.C.; Parkinson, R.; Levine, M.; Paller, C.J. Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants 2018, 7, 89. https://doi.org/10.3390/antiox7070089
Nauman G, Gray JC, Parkinson R, Levine M, Paller CJ. Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants. 2018; 7(7):89. https://doi.org/10.3390/antiox7070089
Chicago/Turabian StyleNauman, Gina, Javaughn Corey Gray, Rose Parkinson, Mark Levine, and Channing J. Paller. 2018. "Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials" Antioxidants 7, no. 7: 89. https://doi.org/10.3390/antiox7070089
APA StyleNauman, G., Gray, J. C., Parkinson, R., Levine, M., & Paller, C. J. (2018). Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants, 7(7), 89. https://doi.org/10.3390/antiox7070089





