Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Asparagus Roots
2.2. Extraction of Bioactive Compounds
2.3. Analysis of Asparagus Root Extracts
2.4. Total Phenolic Content
2.5. Total Flavonoid Content
2.6. Antioxidant Activity
2.6.1. Determination of DPPH Radical Scavenging Activity
2.6.2. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.6.3. Ferric Reducing Ability Power (FRAP) Assay
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction Time and Order on the Yield
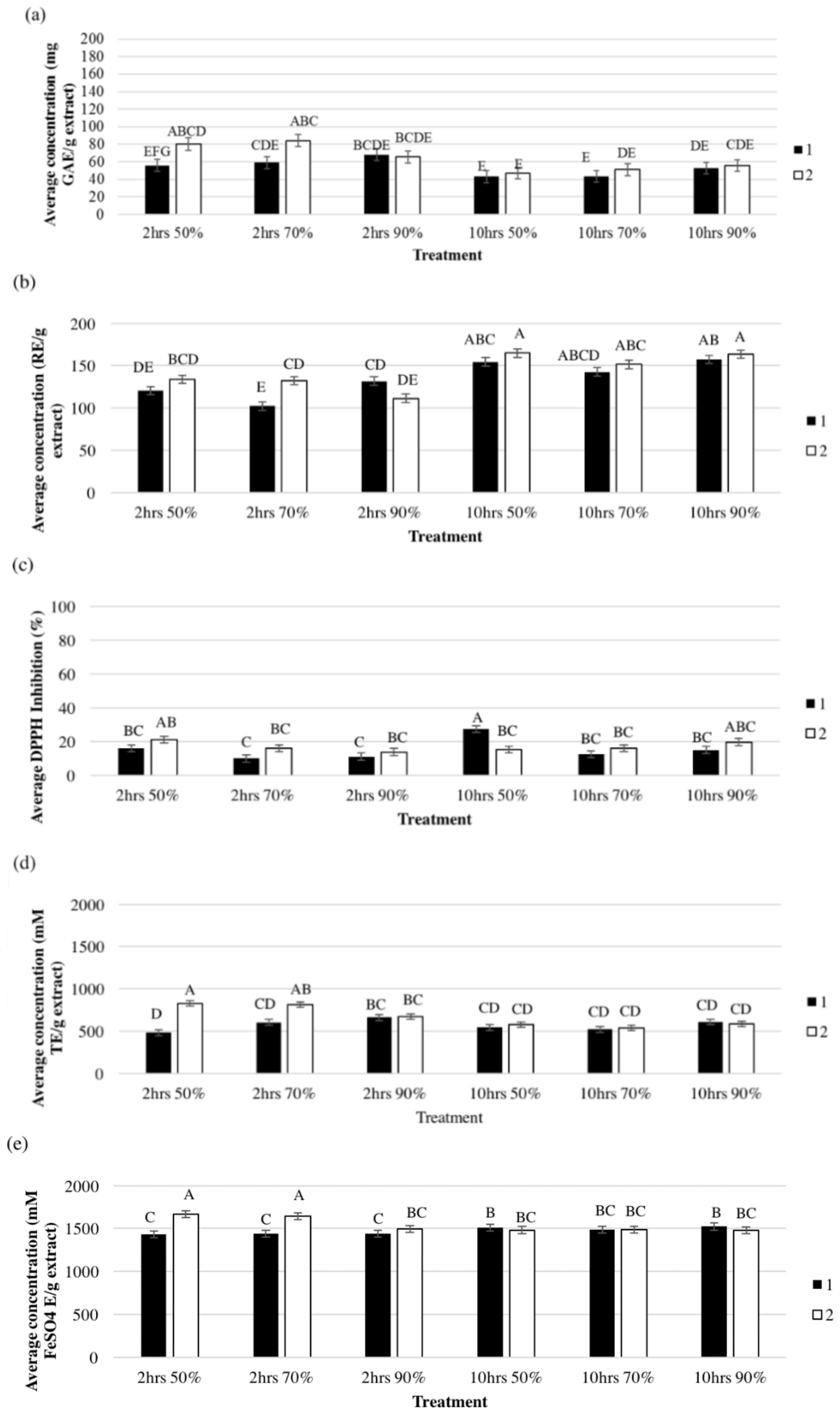
3.2. Effect of Extraction Solvent on TPC, TFC, and Antioxidant Activity
3.3. Effect of Extraction Time and Order on the Antioxidant Compounds
3.4. Effect of Extraction Order and Solvent Type on Antioxidant Activity
3.5. Effect of Solvent Concentration
3.6. Effect of Extraction Order and Solvent Concentration on the Antioxidant Activity
3.7. Pearson Correlations for the Measured Activities in AR Extract
3.8. Analysis of AR Extract Using HPLC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamat, J.P.; Boloor, K.K.; Devasagayam, T.P.; Venkatachalam, S. Antioxidant properties of Asparagus racemosus against damage induced by γ-radiation in rat liver mitochondria. J. Ethnopharmacol. 2000, 71, 425–435. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Espejo-Calvo, J.; Jiménez-Araujo, A. Effect of the extraction method on phytochemical composition and antioxidant activity of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 116, 484–490. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sharmin, F.A.; Akhter, S.; Bhuiyan, M.A.; Shahriar, M. Investigation of cytotoxicity and in-vitro antioxidant activity of Asparagus racemosus root extract. ICPJ 2012, 1, 250–257. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, M.; Rai, S.K.; Singh, B.; Tiwari, M.; Chandra, R. The effect of the aqueous extract of the roots of Asparagus racemosus on hepatocarcinogenesis initiated by diethylnitrosamine. Phytother. Res. 2008, 22, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Dahanukar, S.; Thatte, U.; Pai, N.; Mose, P.; Karandikar, S. Protective effect of Asparagus racemosus against induced abdominal sepsis. Research 1986, 24, 125–128. [Google Scholar]
- Elena, K. First report of Phomopsis asparagi causing stem blight of asparagus in Greece. Plant Pathol. 2006, 55, 300. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Preparation of bioactive extracts from asparagus by-product. FBP 2013, 91, 74–82. [Google Scholar] [CrossRef]
- Sahu, R.; Saxen, J. Screening of total phenolic and flavonoid content in conventional and non-conventional species of Curcuma. J. Pharmacogn. Phytochem. 2013, 2, 176–179. [Google Scholar]
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Huang, Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 2015, 52, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Khavari-Nejad, R.-A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran. J. Pharm. Res. 2014, 13, 163–170. [Google Scholar] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput microplate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- De-Ancos, B.; Sgroppo, S.; Plaza, L.; Cano, M.P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agric. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, E.J. The use of microwave and pulsed electric field as a pretreatment step in ultrasonic extraction of polyphenols from defatted hemp seed cake (Cannabis sativa) using response surface methodology. Food Bioprocess Technol. 2014, 7, 3064–3076. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar]
- Carter, W.P.; Pierce, J.A.; Luo, D.; Malkina, I.L. Environmental chamber study of maximum incremental reactivities of volatile organic compounds. Atmos. Environ. 1995, 29, 2499–2511. [Google Scholar] [CrossRef]
- McMartin, K.E.; Ambre, J.J.; Tephly, T.R. Methanol poisoning in human subjects: Role for formic acid accumulation in the metabolic acidosis. Am. J. Med. 1980, 68, 414–418. [Google Scholar] [CrossRef]
- Perini, M.; Camin, F. Δ18o of ethanol in wine and spirits for authentication purposes. J. Food Sci. 2013, 78, C839–C844. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Lesaint, O.; Vorobiev, E. A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innov. Food Sci. Emerg. Technol. 2013, 19, 124–132. [Google Scholar] [CrossRef]
- Guillén, R.; Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidants from asparagus spears: Phenolics. Acta Hortic. 2008, 776, 247–253. [Google Scholar] [CrossRef]
- Lee, E.J.; Yoo, K.S.; Patil, B.S. Development of a rapid HPLC-UV method for simultaneous quantification of protodioscin and rutin in white and green asparagus spears. J. Food Sci. 2010, 75, C703–C709. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.J.; Lee, C.W.; Son, K.H.; Son, J.K.; Lee, S.K.; Kim, H.P. Methyl protodioscin from the roots of Asparagus cochinchinensis attenuates airway inflammation by inhibiting cytokine production. Evid.-Based Complement. Altern. Med. 2015, 2015, 640846. [Google Scholar] [CrossRef] [PubMed]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42. [Google Scholar]
- Bekhit, A.E.-D.A.; Cheng, V.J.; Harrison, R.; Ye, Z.; Bekhit, A.A.; Ng, T.; Kong, L. Technological aspects of by-product utilization. In Valorization of Wine Making By-Products; Chapter 4; CRC Press: Boca Raton, FL, USA, 2016; p. 117. [Google Scholar]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.-F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Bazykina, N.; Nikolaevskii, A.; Filippenko, T.; Kaloerova, V. Optimization of conditions for the extraction of natural antioxidants from raw plant materials. Pharm. Chem. J. 2002, 36, 46–49. [Google Scholar] [CrossRef]
- Diankov, S.; Karsheva, M.; Hinkov, I. Extraction of natural antioxidants from lemon peels. Kinetics and antioxidant capacity. J. Chem. Technol. Met. 2011, 46, 315–319. [Google Scholar]
- Payne, A.C.; Mazzer, A.; Clarkson, G.J.J.; Taylor, G. Antioxidant assays—Consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Sci. Nutr. 2013, 1, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.G.; Ikawa, M.; Peirce, L.C. Caffeic acid identified as an inhibitory compound in asparagus root filtrate. HortScience 1991, 26, 1525–1527. [Google Scholar]
- Huang, X.F.; Luo, J.; Zhang, Y.; Kong, L.Y. Chemical constituents of Asparagus officinalis. Chin. J. Nat. Med. 2006, 4, 181–184. [Google Scholar]
- Lake, R.; Falloon, P.; Cook, D. Replant problem and chemical components of asparagus roots. N. Z. J. Crop Hortic. Sci. 1993, 21, 53–58. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-Activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, H.; Ren, X.; Yu, H.; Lei, Y.; Chen, Q. Neuroprotective effect of three caffeic acid derivatives via ameliorate oxidative stress and enhance pka/creb signaling pathway. Behav. Brain Res. 2017, 328, 81–86. [Google Scholar] [CrossRef] [PubMed]
| Extraction Time | Extraction Solvent | Extraction Order | Average Yield (%) |
|---|---|---|---|
| 2-h | 50% Methanol | 1 | 42.4 ± 1.8 a |
| 70% Methanol | 1 | 42.1 ± 1.8 a | |
| 90% Methanol | 1 | 41.8 ± 1.8 a | |
| 50% Ethanol | 1 | 44.0 ± 1.8 a | |
| 70% Ethanol | 1 | 44.3 ± 1.8 a | |
| 90% Ethanol | 1 | 36.4 ± 1.8 a | |
| Water | 1 | 38.4 ± 1.8 a | |
| 50% Methanol | 2 | 3.2 ± 1.8 b | |
| 70% Methanol | 2 | 3.4 ± 1.8 b | |
| 90% Methanol | 2 | 3.0 ± 1.8 b | |
| 50% Ethanol | 2 | 3.6 ± 1.8 b | |
| 70% Ethanol | 2 | 5.1 ± 1.8 b | |
| 90% Ethanol | 2 | 7.0 ± 1.8 b | |
| Water | 2 | 4.2 ± 1.8 b | |
| 10-h | 50% Ethanol | 1 | 53.6 ± 3.4 x |
| 70% Ethanol | 1 | 60.1 ± 3.4 x | |
| 90% Ethanol | 1 | 29.8 ± 3.4 y | |
| 50% Ethanol | 2 | 12.8 ± 3.4 z | |
| 70% Ethanol | 2 | 6.4 ± 3.4 z | |
| 90% Ethanol | 2 | 7.2 ± 3.4 z |
| Solvent | Total Polyphenols (mg GAE/g Extract) | Total Flavonoids (mg RE/g Extract) | DPPH Inhibition % | ORAC Antioxidant Activity (mM TE/g Extract) | FRAP Antioxidant Activity (mM FeSO4 E/g Extract) | Caffeic Acid (mg/g Extract) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Order | Order | Order | Order | Order | |||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Methanol 50% | 61.5 efg | 78.8 efg | 128.6 d | 126.6 d | 23.6 d | 40.8 abcd | 557.6 de | 596.9 ade | 1445.0 d | 1572.5 bc | 2.5 ab | 2.7 ab |
| Methanol 70% | 67.9 efg | 84.7 defg | 122.5 de | 127.7 d | 47.0 a | 42.3 abcd | 776.1 abc | 676.5 abcd | 1439.8 d | 1632.1 ab | 2.2 ab | 2.7 ab |
| Methanol 90% | 64.9 efg | 91.4 def | 118.0 de | 123.3 de | 39.9 abcd | 29.1 abcd | 591.1 de | 699.3 abcd | 1451.0 d | 1638.4 ab | 2.6 ab | 2.5 ab |
| Ethanol 50% | 56.1 h | 80.2 efg | 120.4 de | 134.0 d | 41.8 abcd | 38.3 abcd | 481.1 e | 829.5 a | 1435.1 d | 1668.6 a | 1.7 b | 2.8 a |
| Ethanol 70% | 59.1 efg | 84.2 defg | 102.1 e | 132.3 d | 27.1 cd | 41.2 abcd | 604.3 de | 811.8 ab | 1438.3 d | 1646.3 a | 1.7 b | 3.0 a |
| Ethanol 90% | 68.4 efg | 65.7 efg | 131.9 de | 119.4 d | 26.1 abcd | 32.1 abcd | 661.2 abcd | 670.7 abcd | 1439.8 d | 1494.0 d | 3.0 a | 2.3 ab |
| Water | 52.0 g | 63.7 defg | 109.3 de | 110.9 de | 41.5 abcd | 23.8 d | 593.2 de | 655.6 abcd | 1422.4 d | 1487.6 d | 2.1 ab | 2.1 ab |
| Time | Factor | Total Polyphenols | Total Flavonoids | DPPH Inhibition % | ORAC | FRAP |
|---|---|---|---|---|---|---|
| 2 h | Extraction Solvent | 0.000 | 0.000 | 0.270 | 0.127 | 0.000 |
| Concentration | 0.161 | 0.200 | 0.012 | 0.131 | 0.218 | |
| Extraction Order | 0.000 | 0.000 | 0.100 | 0.000 | 0.000 | |
| Extraction Solvent Concentration | 0.600 | 0.011 | 0.002 | 0.005 | 0.000 | |
| Extraction Solvent × Extraction Order | 0.641 | 0.095 | 0.001 | 0.000 | 0.000 | |
| Concentration × Extraction Order | 0.154 | 0.053 | 0.339 | 0.021 | 0.099 | |
| Extraction Solvent × Concentration × Extraction Order | 0.071 | 0.039 | 0.002 | 0.000 | 0.000 | |
| 10 h | Concentration | 0.000 | 0.000 | 0.000 | 0.040 | 0.662 |
| Extraction Order | 0.000 | 0.000 | 0.298 | 0.613 | 0.101 | |
| Concentration × Extraction Order | 0.001 | 0.163 | 0.000 | 0.526 | 0.413 | |
| Comparing 2 h and 10 h | Extraction Time | 0.000 | 0.000 | 0.015 | 0.000 | 0.002 |
| Concentration | 0.279 | 0.000 | 0.000 | 0.610 | 0.000 | |
| Extraction Order | 0.000 | 0.000 | 0.160 | 0.000 | 0.000 | |
| Extraction Time × Concentration | 0.060 | 0.499 | 0.451 | 0.059 | 0.000 | |
| Extraction Time × Extraction Order | 0.005 | 0.000 | 0.014 | 0.000 | 0.000 | |
| Concentration × Extraction Order | 0.336 | 0.004 | 0.010 | 0.000 | 0.000 | |
| Extraction Time × Concentration × Extraction Order | 0.489 | 0.073 | 0.004 | 0.013 | 0.000 |
| Extraction Time | Antioxidant Assay | Polyphenols | Flavonoids | DPPH | ORAC | |
|---|---|---|---|---|---|---|
| 2-h | Flavonoids | Pearson’s coefficient | 0.322 | |||
| p-value | 0.000 | |||||
| DPPH Inhibition % | Pearson’s coefficient | 0.110 | 0.269 | |||
| p-value | 0.228 | 0.003 | ||||
| ORAC | Pearson’s coefficient | 0.201 | 0.199 | 0.162 | ||
| p-value | 0.025 | 0.028 | 0.076 | |||
| FRAP | Pearson’s coefficient | 0.455 | 0.338 | 0.084 | 0.450 | |
| p-value | 0.000 | 0.000 | 0.353 | 0.000 | ||
| 10-h | Flavonoids | Pearson’s coefficient | 0.433 | |||
| p-value | 0.001 | |||||
| DPPH Inhibition % | Pearson’s coefficient | −0.165 | −0.110 | |||
| p-value | 0.232 | 0.428 | ||||
| ORAC | Pearson’s coefficient | 0.171 | 0.280 | −0.161 | ||
| p-value | 0.217 | 0.041 | 0.244 | |||
| FRAP | Pearson’s coefficient | 0.003 | 0.109 | −0.007 | −0.075 | |
| p-value | 0.982 | 0.432 | 0.962 | 0.587 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symes, A.; Shavandi, A.; Zhang, H.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Bekhit, A.E.-D.A. Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts. Antioxidants 2018, 7, 52. https://doi.org/10.3390/antiox7040052
Symes A, Shavandi A, Zhang H, Mohamed Ahmed IA, Al-Juhaimi FY, Bekhit AE-DA. Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts. Antioxidants. 2018; 7(4):52. https://doi.org/10.3390/antiox7040052
Chicago/Turabian StyleSymes, Abbey, Amin Shavandi, Hongxia Zhang, Isam A. Mohamed Ahmed, Fahad Y. Al-Juhaimi, and Alaa El-Din Ahmed Bekhit. 2018. "Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts" Antioxidants 7, no. 4: 52. https://doi.org/10.3390/antiox7040052
APA StyleSymes, A., Shavandi, A., Zhang, H., Mohamed Ahmed, I. A., Al-Juhaimi, F. Y., & Bekhit, A. E.-D. A. (2018). Antioxidant Activities and Caffeic Acid Content in New Zealand Asparagus (Asparagus officinalis) Roots Extracts. Antioxidants, 7(4), 52. https://doi.org/10.3390/antiox7040052






