Antioxidant Activity of Yichun Blue Honeysuckle (YBHS) Berry Counteracts CCl4-Induced Toxicity in Liver Injury Model of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. TBARS Assay
2.2.3. SOD Assay
2.2.4. CAT Assay
2.2.5. GPx Assay
2.2.6. Glutathione Assay
2.2.7. In Vitro Antioxidant Activity by DPPH Assay
2.3. Statistical Analysis
3. Results
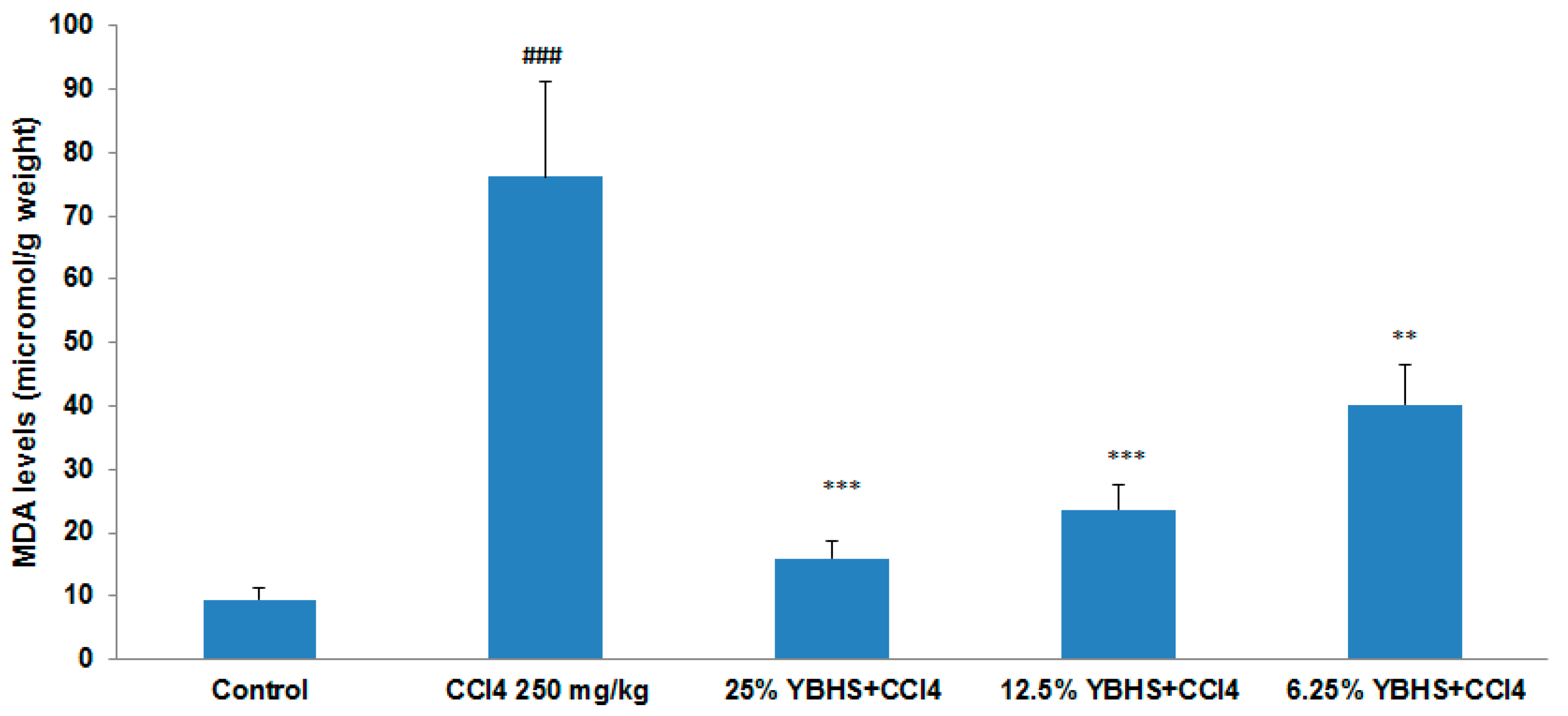
3.1. YBHS Decreases Lipid Peroxidation in CCl4-Induced Liver Injury Model
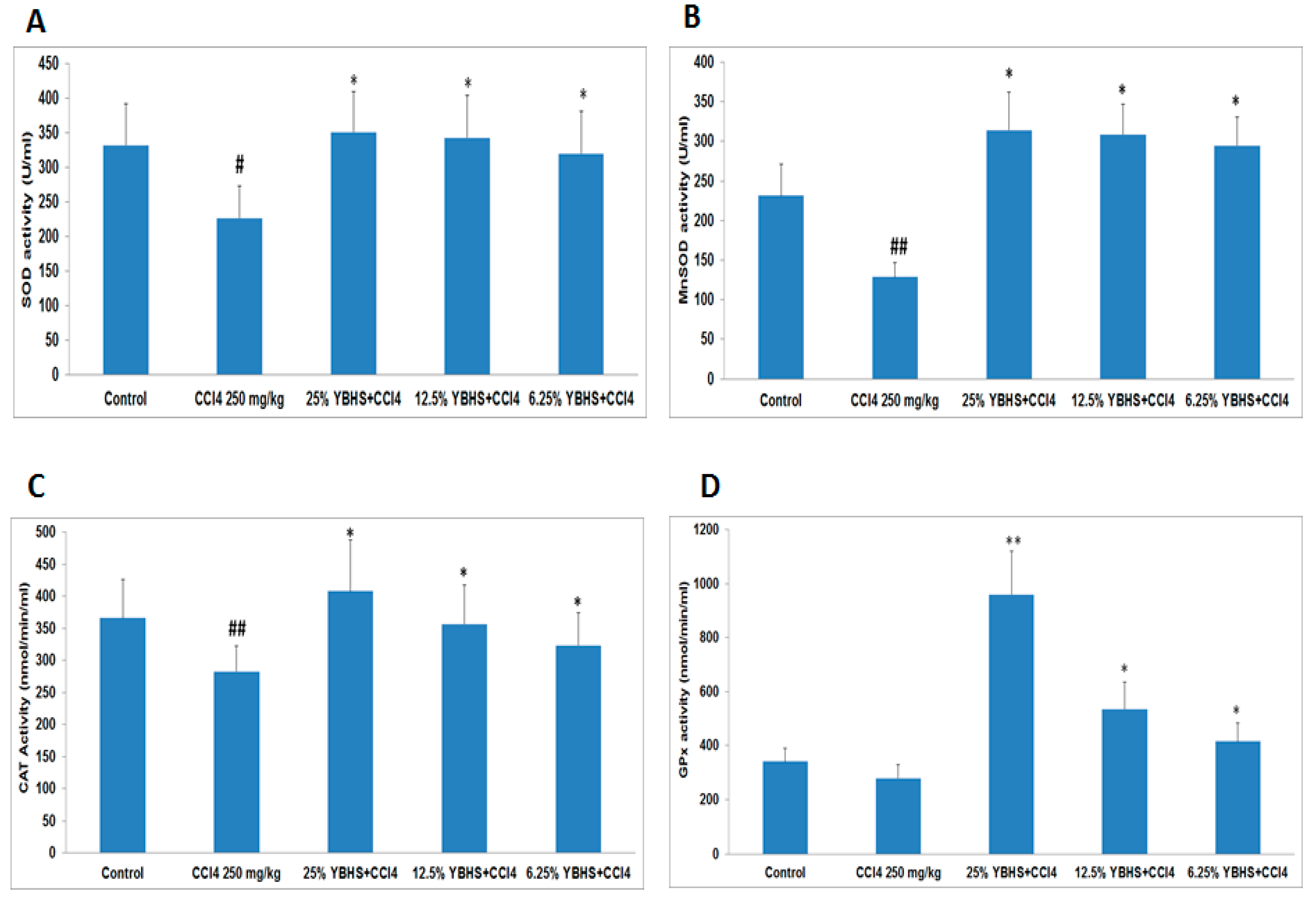
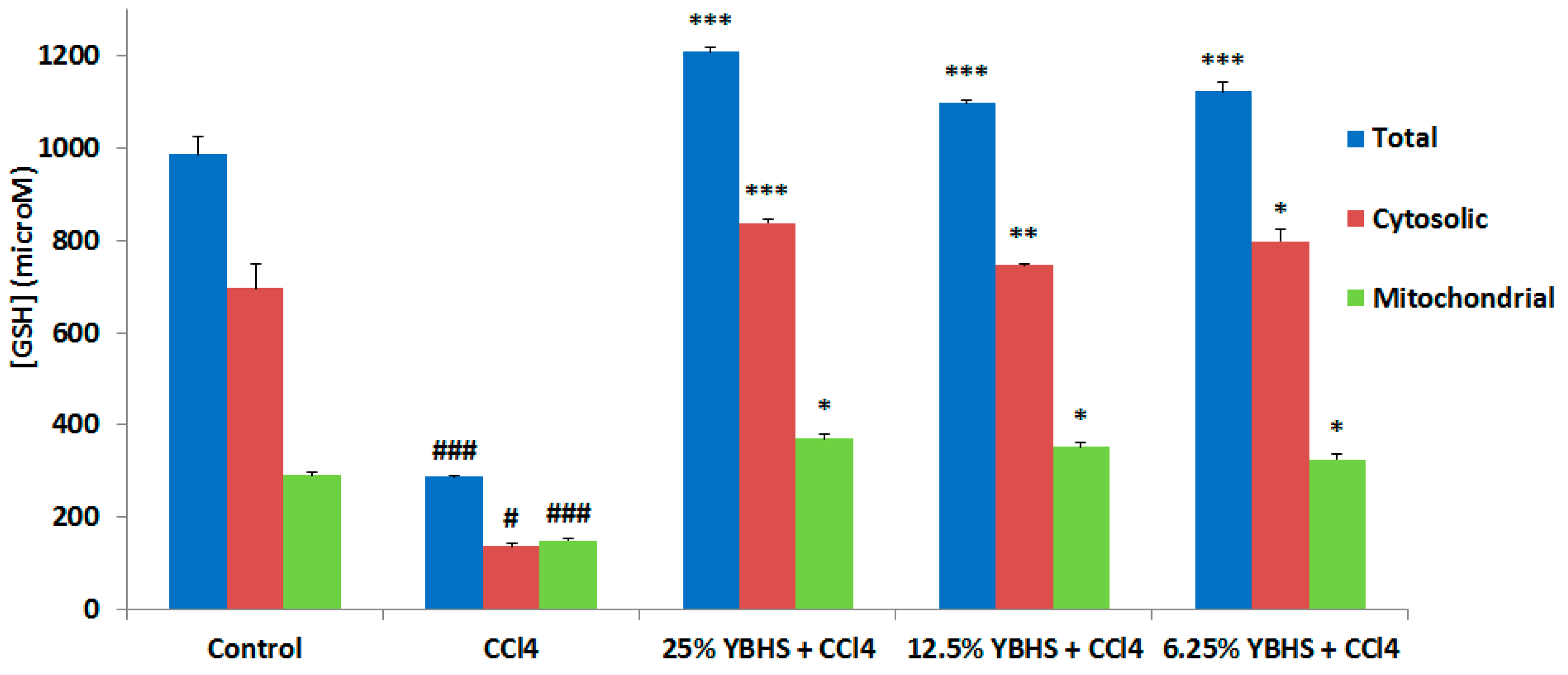
3.2. YBHS Activates the Endogenous Antioxidant System
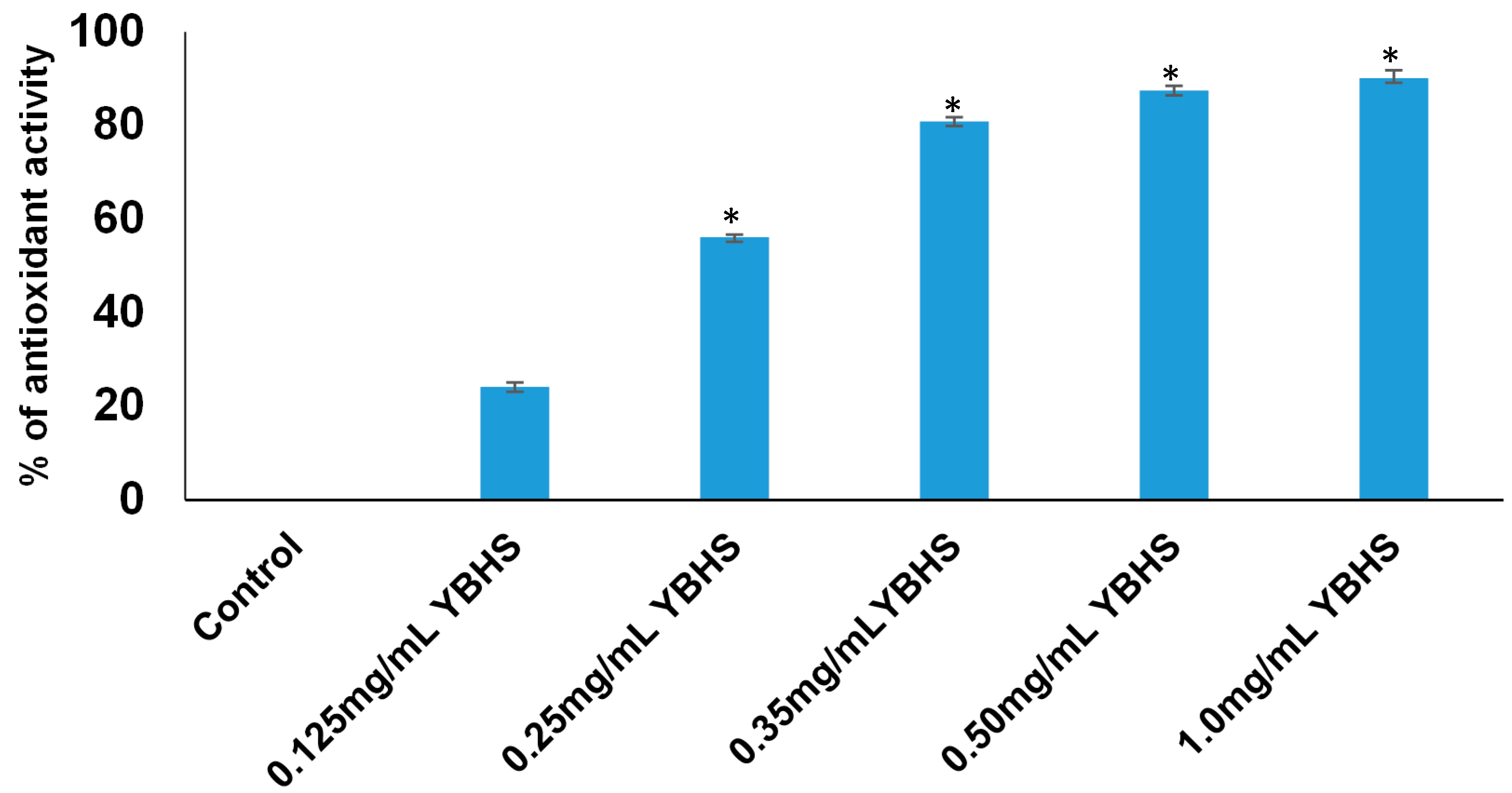
3.3. Free Radical Scavenging Activity of YBHS
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Alzheimer’s disease and oxidative stress: A review. Curr. Med. Chem. 2014, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Dean, O.M.; van den Buuse, M.; Berk, M.; Copolov, D.L.; Mavros, C.; Bush, A.I. N-acetyl cysteine restores brain glutathione loss in combined 2-cyclohexene-1-one and d-amphetamine-treated rats: Relevance to schizophrenia and bipolar disorder. Neurosci. Lett. 2011, 499, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.S.; Kim, H.J.; Kang, J.H.; Kudo, R.; Hosoya, T.; Kumazawa, S.; Jun, M.; Kim, O.Y.; Ahn, M.R. Anthocyanin profile and antioxidant activity of various berries cultivated in korea. Nat. Prod. Commun. 2015, 10, 963–968. [Google Scholar] [PubMed]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Namiesnik, J.; Vearasilp, K.; Nemirovski, A.; Leontowicz, H.; Leontowicz, M.; Pasko, P.; Martinez-Ayala, A.L.; Gonzalez-Aguilar, G.A.; Suhaj, M.; Gorinstein, S. In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin. Appl. Biochem. Biotechnol. 2014, 172, 2849–2865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, B.; Lin, Y.; Ma, Y.; Zhang, Q.; Meng, X.J. Effects of Lonicera caerulea berry extract on lipopolysaccharide-induced toxicity in rat liver cells: Antioxidant, anti-inflammatory, and anti-apoptotic activities. J. Funct. Foods 2017, 33, 217–226. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera) and their biological effects. Molecules 2011, 17, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Ningappa, M.B.; Dinesha, R.; Srinivas, L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem. 2008, 106, 720–728. [Google Scholar] [CrossRef]
- Recknagel, R.O. Carbon tetrachloride hepatotoxicity. Pharmacol. Rev. 1967, 19, 145–208. [Google Scholar] [PubMed]
- Liu, S.L.; Degli Esposti, S.; Yao, T.; Diehl, A.M.; Zern, M.A. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 1995, 22, 1474–1481. [Google Scholar] [PubMed]
- Wang, M.Y.; Nowicki, D.; Anderson, G.; Jensen, J.; West, B. Liver protective effects of Morinda citrifolia (Noni). Plant Foods Hum. Nutr. 2008, 63, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Laouar, A.; Klibet, F.; Bourogaa, E.; Benamara, A.; Boumendjel, A.; Chefrour, A.; Messarah, M. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac. J. Trop. Med. 2017, 10, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitao, S.G. Screening of brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Kovanen, P.T. The mast cell—A potential link between inflammation and cellular cholesterol deposition in atherogenesis. Eur. Heart J. 1993, 14 (Suppl. K), 105–117. [Google Scholar] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, R.; Nagashima, M.; Sakamoto, M.; Yamaguchi, N.; Hirohashi, S.; Yokota, J.; Kasai, H. Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res. 1994, 54, 3171–3172. [Google Scholar] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, A.; Albano, E.; Banni, S.; Botti, B.; Corongiu, F.; Dessi, M.A.; Iannone, A.; Vannini, V.; Dianzani, M.U. Free-radical metabolism of carbon tetrachloride in rat liver mitochondria. A study of the mechanism of activation. Biochem. J. 1987, 246, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Palikova, I.; Valentova, K.; Oborna, I.; Ulrichova, J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J. Agric. Food Chem. 2009, 57, 6584–6589. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Reznicek, V.; Mlcek, J.; Jurikova, T.; Balik, J.; Sochor, J.; Kramarova, D. Antioxidant and radical oxygen species scavenging activities of 12 cultivars of blue honeysuckle fruit. Hortic. Sci. 2011, 38, 63–70. [Google Scholar]
- Bresciani, G.; da Cruz, I.B.; Gonzalez-Gallego, J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar] [PubMed]
- Miyamoto, Y.; Koh, Y.H.; Park, Y.S.; Fujiwara, N.; Sakiyama, H.; Misonou, Y.; Ookawara, T.; Suzuki, K.; Honke, K.; Taniguchi, N. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol. Chem. 2003, 384, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Bielinski, D.F.; Carrihill-Knoll, K.L.; Rabin, B.M.; Shukitt-Hale, B. Protective effects of blueberry- and strawberry diets on neuronal stress following exposure to 56Fe particles. Brain Res. 2014, 1593, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jainu, M.; Devi, C.S. Antioxidant effect of methanolic extract of Solanum nigrum berries on aspirin induced gastric mucosal injury. Indian J. Clin. Biochem. 2004, 19, 57–61. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-Y.; Srinivasan, M.; Dasari, S.; Narvekar, P.; Samy, A.L.P.A.; Dontaraju, V.S.; Peng, L.; Anderson, G.L.; Munirathinam, G. Antioxidant Activity of Yichun Blue Honeysuckle (YBHS) Berry Counteracts CCl4-Induced Toxicity in Liver Injury Model of Mice. Antioxidants 2017, 6, 50. https://doi.org/10.3390/antiox6030050
Wang M-Y, Srinivasan M, Dasari S, Narvekar P, Samy ALPA, Dontaraju VS, Peng L, Anderson GL, Munirathinam G. Antioxidant Activity of Yichun Blue Honeysuckle (YBHS) Berry Counteracts CCl4-Induced Toxicity in Liver Injury Model of Mice. Antioxidants. 2017; 6(3):50. https://doi.org/10.3390/antiox6030050
Chicago/Turabian StyleWang, Mian-Ying, Madhuwanti Srinivasan, Subramanyam Dasari, Parnal Narvekar, Angela Lincy Prem Antony Samy, Venkata Satish Dontaraju, Lin Peng, Gary L. Anderson, and Gnanasekar Munirathinam. 2017. "Antioxidant Activity of Yichun Blue Honeysuckle (YBHS) Berry Counteracts CCl4-Induced Toxicity in Liver Injury Model of Mice" Antioxidants 6, no. 3: 50. https://doi.org/10.3390/antiox6030050
APA StyleWang, M.-Y., Srinivasan, M., Dasari, S., Narvekar, P., Samy, A. L. P. A., Dontaraju, V. S., Peng, L., Anderson, G. L., & Munirathinam, G. (2017). Antioxidant Activity of Yichun Blue Honeysuckle (YBHS) Berry Counteracts CCl4-Induced Toxicity in Liver Injury Model of Mice. Antioxidants, 6(3), 50. https://doi.org/10.3390/antiox6030050




