Screening of Antioxidant Activity of Gentian Lutea Root and Its Application in Oil-in-Water Emulsions
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Extraction of G. Lutea
2.3. Determination of the Total Phenolic Content (TPC)
2.4. Determination of Free Radical Scavenging Activity Assays
2.4.1. TEAC Assay
2.4.2. DPPH Assay
2.4.3. Superoxide Activity Xanthine/Xanthine Oxidase (X/XO)
2.5. Determination of Antioxidant Activity in o/w Emulsion
2.5.1. Removal of Tocopherols from Sunflower Oil
2.5.2. Preparation of Emulsion
2.5.3. Determination of Peroxide Value (PV)
2.5.4. Determination of Secondary Oxidation by Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Statistical Analysis
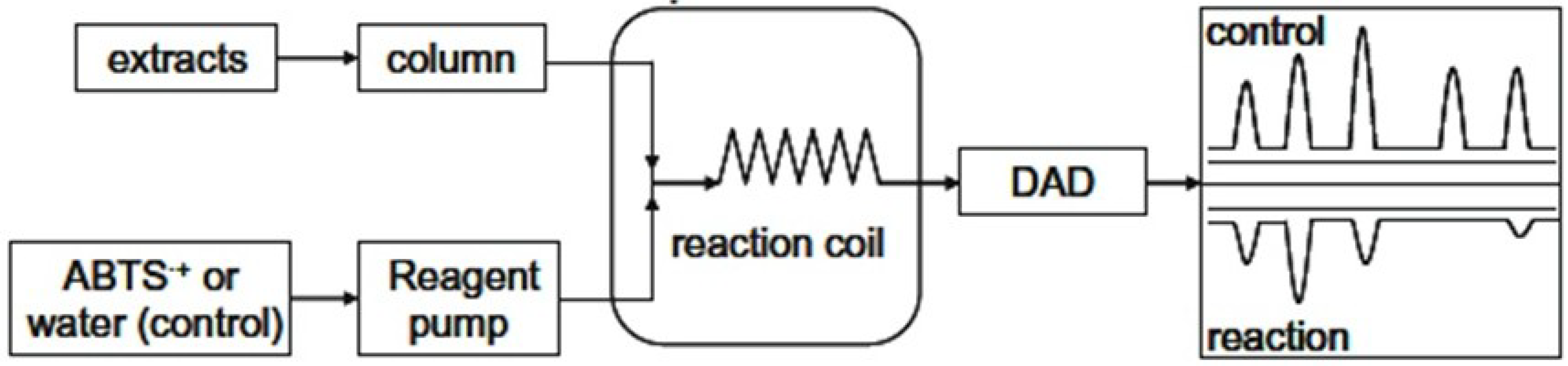
2.7. HPLC and Post-Column HPLC-ABTS•+ Radical Scavenging Method
3. Results and Discussion
3.1. Analysis of Total Polyphenols and Free Radical Activity Assays
| Activity G. Lutea | Extraction Solvent | |
|---|---|---|
| H2O | 50:50 MeOH:H2O | |
| Extraction yield (%) | 20.00 ± 0.9 | 29.10 ± 0.3 |
| Total phenolic content (g GAE/g DW) | 3.79 ± 1.7 | 12.03 ± 1.8 |
| DPPH (μmol of TE/g DW) | 12.34 ± 1.5 | 15.89 ± 0.5 |
| TEAC (μmol of TE/g DW) | 33.28 ± 1.5 | 48.90 ± 1.8 |
| Superoxide activity (mg/mL) | 30.00 ± 2.8 | 23.21 ± 2.8 |
3.2. Antioxidant Effect in Stored o/w Emulsion
3.2.1. Evolution of Peroxide Value (PV)
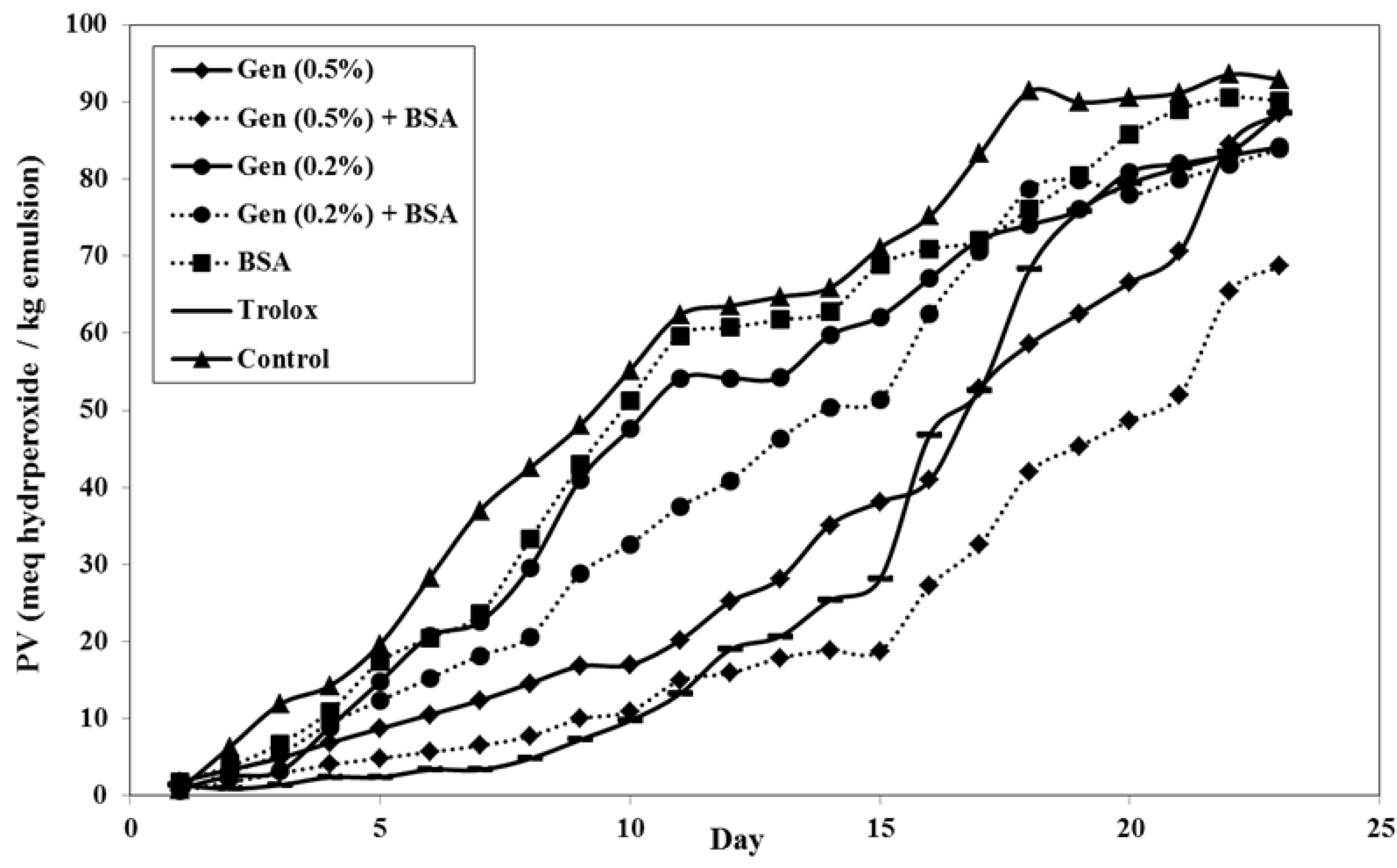
3.2.2. Evolution of pH over Time
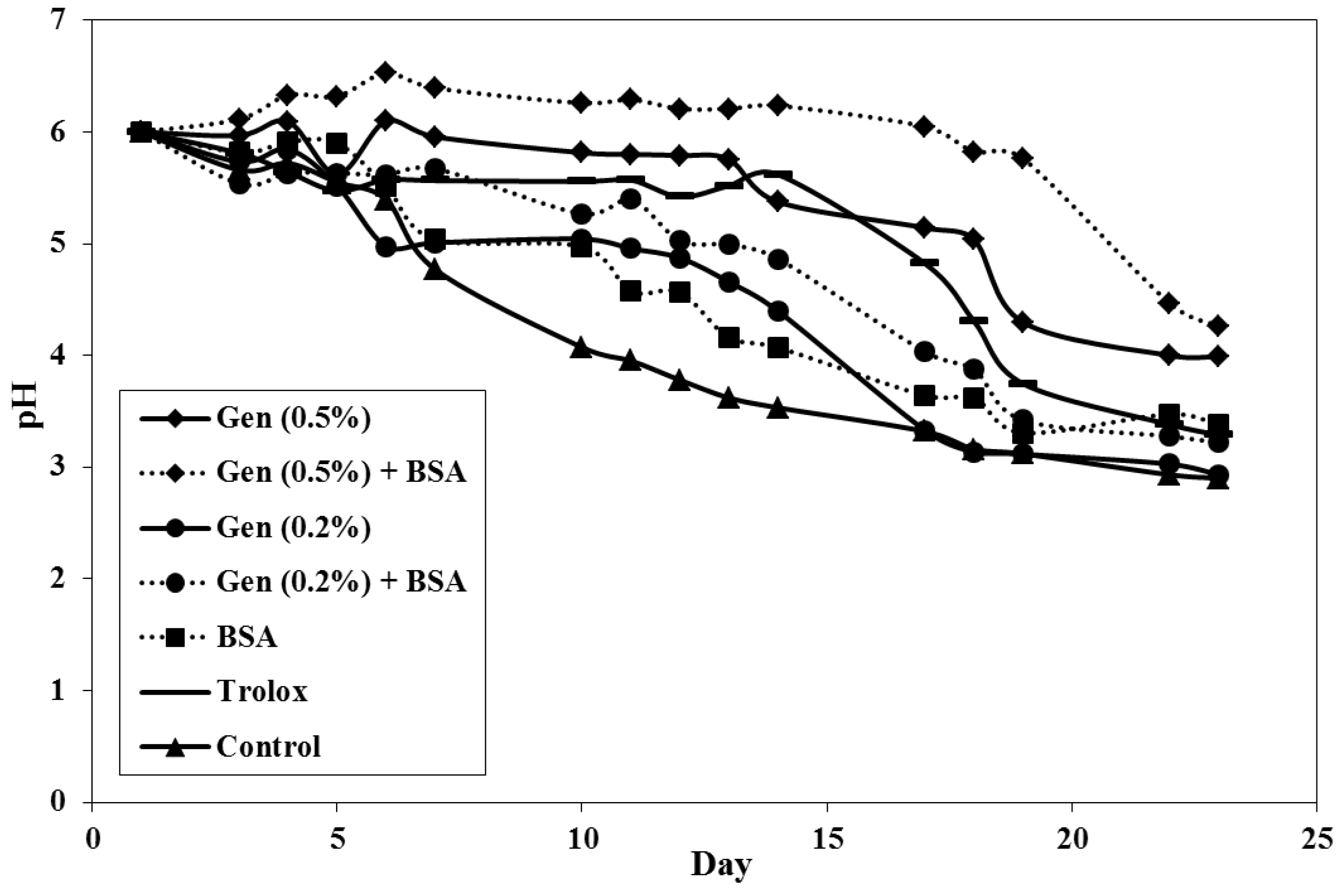
3.2.3. Evolution of Thiobarbituric Acid Reactive Substances (TBARS)
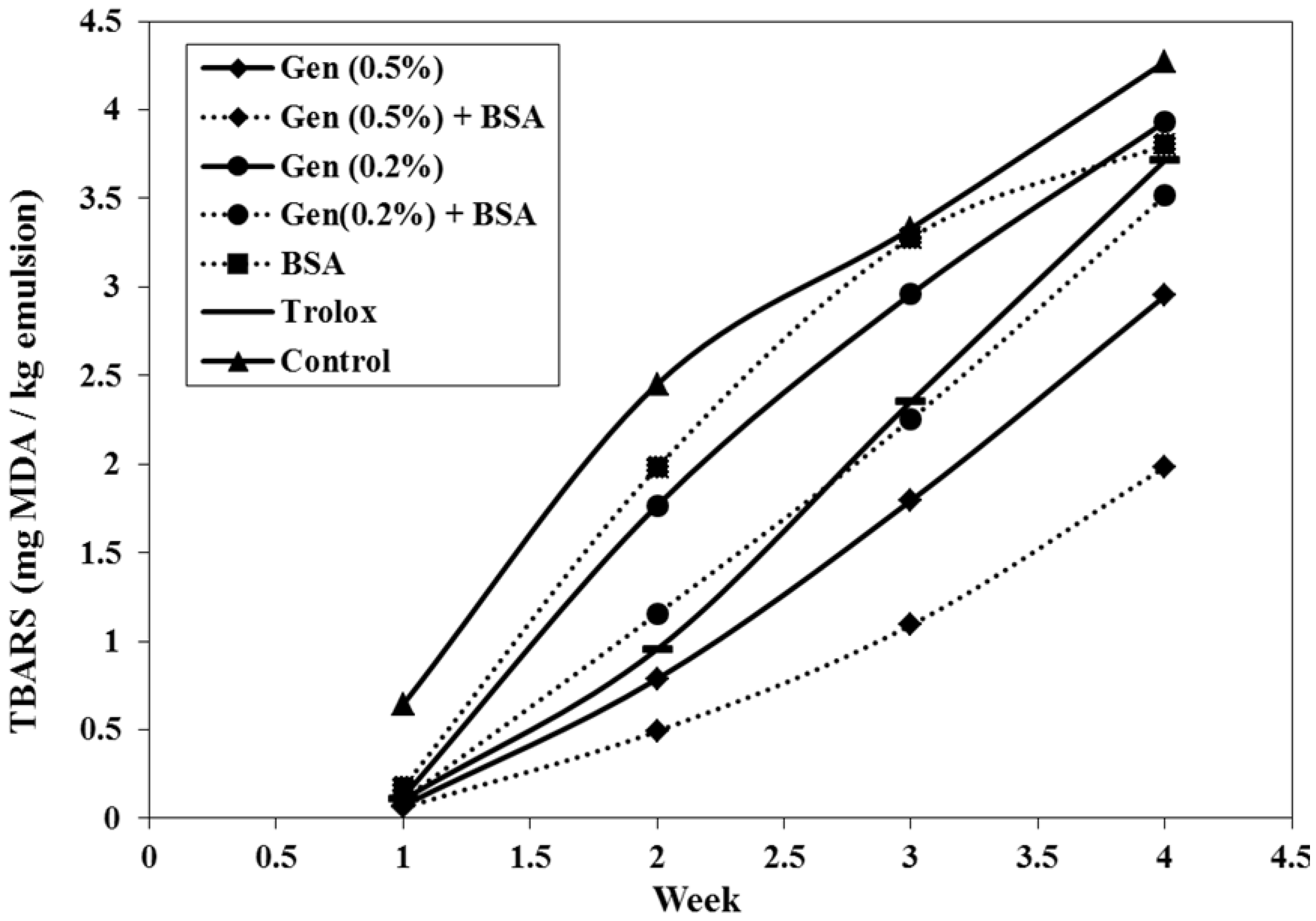
3.3. HPLC Analysis of G. Lutea and the Total Antioxidant Activity Based on Post-Column On-Line Coupling ABTS•+
| Sample | Concentration (mg/L) |
|---|---|
| Gentiopocroside | 1805 ± 62 |
| Sweroside | 72 ± 4 |
| Amarogentin | n.d |
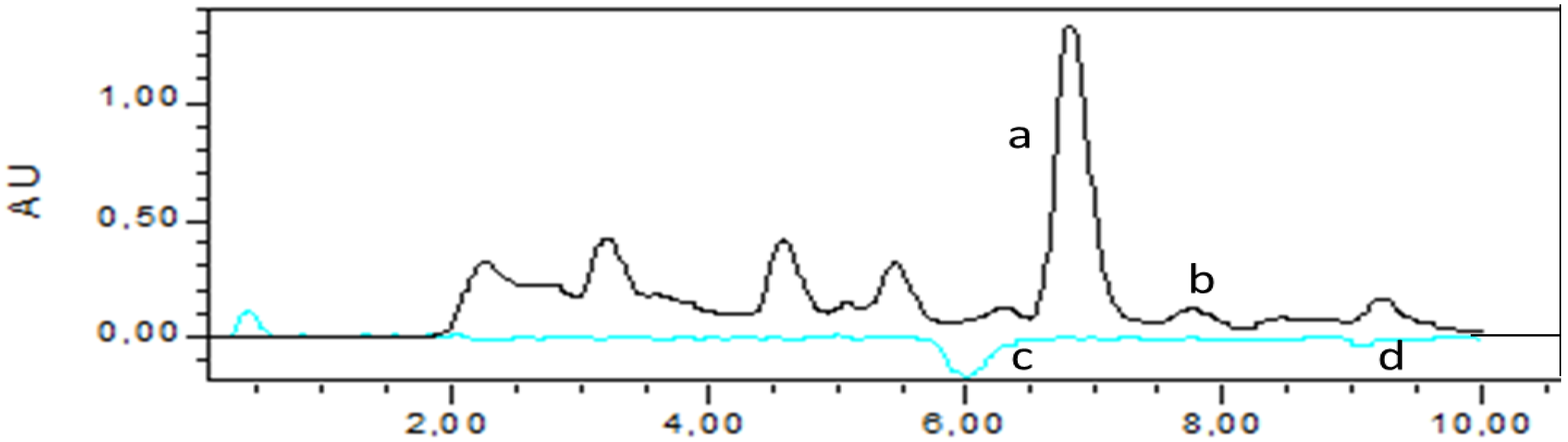
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balijagić, J.; Janković, T.; Zdunić, G.; Bosković, J.; Savikin, K.; Godevac, D.; Stanojković, T.; Jovancević, M.; Menković, N. Chemical profile, radical scavenging and cytotoxic activity of yellow gentian leaves (Genitaneae luteaefolium) grown in northern regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1487–1490. [Google Scholar] [PubMed]
- Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC-MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- Aberham, A.; Schwaiger, S.; Stuppner, H.; Ganzera, M. Quantitative analysis of iridoids, secoiridoids, xanthones and xanthone glycosides in Gentiana lutea L. roots by RP-HPLC and LC-MS. J. Pharm. Biomed. Anal. 2007, 45, 437–442. [Google Scholar] [CrossRef]
- Lian, L.H.; Wu, Y.L.; Wan, Y.; Li, X.; Xie, W.X.; Nan, J.X. Anti-apoptotic activity of gentiopicroside in d-galactosamine/Lipopolysaccharide-induced murine fulminant hepatic failure. Chem. Biol. Interact. 2010, 188, 127–133. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Mandal, S.; Das, A.; Roy, A.; Das, S.; Panda, C.K. Prevention of liver carcinogenesis by amarogentin through modulation of G 1/S cell cycle check point and induction of apoptosis. Carcinogenesis 2012, 33, 2424–2431. [Google Scholar] [CrossRef]
- Tan, R.X.; Kong, L.D.; Wei, H.X. Secoiridoid glycosides and an antifungal derivative from gentian 4 tibetica. Phytochemistry 1998, 47, 1223–1226. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Nahar, L.; Sarker, S. Bioactivity of gentiopicroside from the aerial parts of Centaurium erythraea. Fitoterapia 2003, 74, 151–154. [Google Scholar] [CrossRef]
- Jia, N.; Li, Y.; Wu, Y.; Xi, M.; Hur, G.; Zhang, X.; Cui, J.; Sun, W.; Wen, A. Comparison of the anti-inflammatory and analgesic effects of Gentiana macrophylla Pall. and Gentiana straminea Maxim., and identification of their active constituents. J. Ethnopharmacol. 2012, 144, 638–645. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Arambašić, J.; Mišić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef]
- Nastasijević, B.; Lazarević-Pašti, T.; Dimitrijević-Branković, S.; Pašti, I.; Vujačić, A.; Joksić, G.; Vasić, V. Inhibition of myeloperoxidase and antioxidative activity of Gentiana lutea extracts. J. Pharm. Biomed. Anal. 2012, 66, 191–196. [Google Scholar] [CrossRef]
- Jiang, D.J.; Dai, Z.; Li, Y.J. Pharmacological effects of xanthones as cardiovascular protective agents. Cardiovasc. Drug Rev. 2004, 22, 91–102. [Google Scholar] [CrossRef]
- Rana, V.S.; Rawat, M.S.M. A new xanthone glycoside and antioxidant constituents from the rhizomes of Swertia speciosa. Chem. Biodivers. 2005, 2, 1310–1315. [Google Scholar] [CrossRef]
- Wei, S.; Chen, G.; He, W.; Chi, H.; Abe, H.; Yamashita, K.; Yokoyama, M.; Kodama, H. Inhibitory effects of secoiridoids from the roots of Gentiana straminea on stimulus-induced superoxide generation, phosphorylation and translocation of cytosolic compounds to plasma membrane in human neutrophils. Phytother. Res. 2012, 26, 168–173. [Google Scholar] [CrossRef]
- Kintzios, S.; Papageorgiou, K.; Yiakoumettis, I.; Baricevic, D.; Kusar, A. Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. J. Pharm. Biomed. Anal. 2010, 53, 773–776. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Food chemistry and toxicology protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on color and texture deterioration. J. Food. Sci. 2005, 70, 427–432. [Google Scholar]
- Frankel, E.N.; Huang, S.; Aeschbach, R. Antioxidant activity of green teas in different lipid systems. J. Am. Oil Chem. Soc. 1997, 74, 1309–1315. [Google Scholar] [CrossRef]
- Kiokias, S.; Dimakou, C.; Oreopoulou, V. Activity of natural carotenoid preparations against the autoxidative deterioration of sunflower oil-in-water emulsions. Food Chem. 2009, 114, 1278–1284. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, H.; Liu, C. Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep. Purif. Technol. 2007, 57, 277–282. [Google Scholar] [CrossRef]
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J. Agric. Food Chem. 2006, 54, 8048–8057. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- López-Cruz, R.I.; Zenteno-Savín, T.; Galván-Magaña, F. Superoxide production, oxidative damage and enzymatic antioxidant defenses in shark skeletal muscle. Comp. Biochem. Physiol. 2010, 156, 50–56. [Google Scholar] [CrossRef]
- Yoshida, H.; Kajimoto, G.; Emura, S. Antioxidant effects of d-tocopherols at different concentrations in oils during microwave heating. J. Am. Oil Chem. Soc. 1993, 70, 989–995. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. AOCS Official Method Cd 8-53; Firestone, D., Ed.; Official Methods and Recommended Practices of the American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.; van Beek, T. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Anal. Chem. 2001, 73, 3373–3381. [Google Scholar] [CrossRef]
- Carnat, A.; Fraisse, D.; Carnat, A.-P.; Felgines, C.; Chaud, D.; Lamaison, J.-L. Influence of drying mode on iridoid bitter constituent levels in gentian root. J. Sci. Food Agric. 2005, 85, 598–602. [Google Scholar] [CrossRef]
- Kusar, A.; Zupancic, A.; Sentjurc, M.; Baricevic, D. Free radical scavenging activities of yellow gentian (Gentiana lutea L.) measured by electron spin resonance. Hum. Exp. Toxicol. 2006, 25, 599–604. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbó, R.; Jiménez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Deighton, N.; Brennan, R.; Finn, C.; Davies, H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000, 80, 1307–1313. [Google Scholar] [CrossRef]
- Gazzani, G.; Papetti, A.; Massolini, G.; Daglia, M. Anti- and prooxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J. Agric. Food Chem. 1998, 46, 4118–4122. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldra, F. Handbook of Analysis of Edible Animal By-Products; CRC Press: Gent, Belgium, 2011; p. 471. [Google Scholar]
- Almajano, M.P.; Gordon, M.H. Synergistic effect of BSA on antioxidant activities in model food emulsions. Am. Oil Chem. Soc. 2004, 81, 275–280. [Google Scholar] [CrossRef]
- Rampon, V.; Lethuaut, L.; Mouhous-Riou, N.; Genot, C. Interface characterization and aging of bovine serum albumin stabilized oil-in-water emulsions as revealed by front-surface fluorescence. J. Agric. Food Chem. 2001, 49, 4046–4051. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Aeschbach, R.; Prior, E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Gallego, G.; Peiró, S.; Almajano, M.P. Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 2013, 94, 911–918. [Google Scholar] [PubMed]
- Sørensen, A.-D.M.; Haahr, A.-M.; Becker, E.M.; Skibsted, L.H.; Bergenståhl, B.; Nilsson, L.; Jacobsen, C. Interactions between iron, phenolic compounds, emulsifiers, and pH in omega-3-enriched oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1740–1750. [Google Scholar] [CrossRef]
- Donnelly, J.L.; Decker, E.A.; McClements, D.J. Iron-catalyzed oxidation of menhaden oil as affected by emulsifiers. J. Food Sci. 1998, 63, 997–1000. [Google Scholar]
- Mancuso, J.R.; McClements, D.J.; Decker, E.A. Ability of iron to promote surfactant peroxide decomposition and oxidize alpha-tocopherol. J. Agric. Food Chem. 1999, 47, 4146–4149. [Google Scholar] [CrossRef]
- Mancuso, J.R.; McClements, D.J.; Decker, E.A. The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem. 1999, 47, 4112–4116. [Google Scholar] [CrossRef]
- Pangloli, P.; Melton, S.L.; Collins, J.L.; Penfield, M.P.; Saxton, A.M. Flavor and storage stability of potato chips fried in cottonseed and sunflower oils and palm olein/sunflower oil blends. J. Food Sci. 2002, 67, 97–103. [Google Scholar] [CrossRef]
- Wu, Q.-X.; Li, Y.; Shi, Y.-P. Antioxidant phenolic glucosides from Gentiana piasezkii. J. Asian Nat. Prod. Res. 2006, 8, 391–396. [Google Scholar] [CrossRef]
- Citová, I.; Ganzera, M.; Stuppner, H.; Solich, P. Determination of gentisin, isogentisin, and amarogentin in Gentiana lutea L. by capillary electrophoresis. J. Sep. Sci. 2008, 31, 195–200. [Google Scholar] [CrossRef]
- Skrzypczak, L.; Wesolowska, M.; Bajaj, Y.P.S. Medicinal and Aromatic Plants IV; Springer Verlag: Berlin, Germany, 1993; Volume 21, pp. 172–186. [Google Scholar]
- Ando, H.; Hirai, Y.; Fujii, M.; Hori, Y.; Fukumura, M.; Niiho, Y.; Nakajima, Y.; Shibata, T.; Toriizuka, K.; Ida, Y. The chemical constituents of fresh Gentian root. J. Nat. Med. 2007, 61, 269–279. [Google Scholar] [CrossRef]
- Hayashi, T.; Minamiyama, Y.; Miura, T.; Yamagishi, T.; Kaneshina, H. Cultivation of Gentiana lutea and chemical evaluation evaluation of gentiana radix. Hokkaidoritsu eisei Kenkyushoho 1990, 40, 103–106. [Google Scholar]
- Phoboo, S.; Pinto, M.D.S.; Barbosa, A.C.L.; Sarkar, D.; Bhowmik, P.C.; Jha, P.K.; Shetty, K. Phenolic-linked biochemical rationale for the anti-diabetic properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytother. Res. 2013, 27, 227–235. [Google Scholar] [CrossRef]
- Oku, H.; Ogawa, Y.; Iwaoka, E.; Ishiguro, K. Allergy-preventive effects of chlorogenic acid and iridoid derivatives from flower buds of Lonicera japonica. Biol. Pharm. Bull. 2011, 34, 1330–1333. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azman, N.A.M.; Segovia, F.; Martínez-Farré, X.; Gil, E.; Almajano, M.P. Screening of Antioxidant Activity of Gentian Lutea Root and Its Application in Oil-in-Water Emulsions. Antioxidants 2014, 3, 455-471. https://doi.org/10.3390/antiox3020455
Azman NAM, Segovia F, Martínez-Farré X, Gil E, Almajano MP. Screening of Antioxidant Activity of Gentian Lutea Root and Its Application in Oil-in-Water Emulsions. Antioxidants. 2014; 3(2):455-471. https://doi.org/10.3390/antiox3020455
Chicago/Turabian StyleAzman, Nurul Aini Mohd, Francisco Segovia, Xavier Martínez-Farré, Emilio Gil, and María Pilar Almajano. 2014. "Screening of Antioxidant Activity of Gentian Lutea Root and Its Application in Oil-in-Water Emulsions" Antioxidants 3, no. 2: 455-471. https://doi.org/10.3390/antiox3020455
APA StyleAzman, N. A. M., Segovia, F., Martínez-Farré, X., Gil, E., & Almajano, M. P. (2014). Screening of Antioxidant Activity of Gentian Lutea Root and Its Application in Oil-in-Water Emulsions. Antioxidants, 3(2), 455-471. https://doi.org/10.3390/antiox3020455







