Specialized Pro-Resolving Lipid Mediators and Dietary Omega-3/6 Fatty Acids in Selected Inflammatory Skin Diseases: A Systematic Review
Abstract
1. Introduction
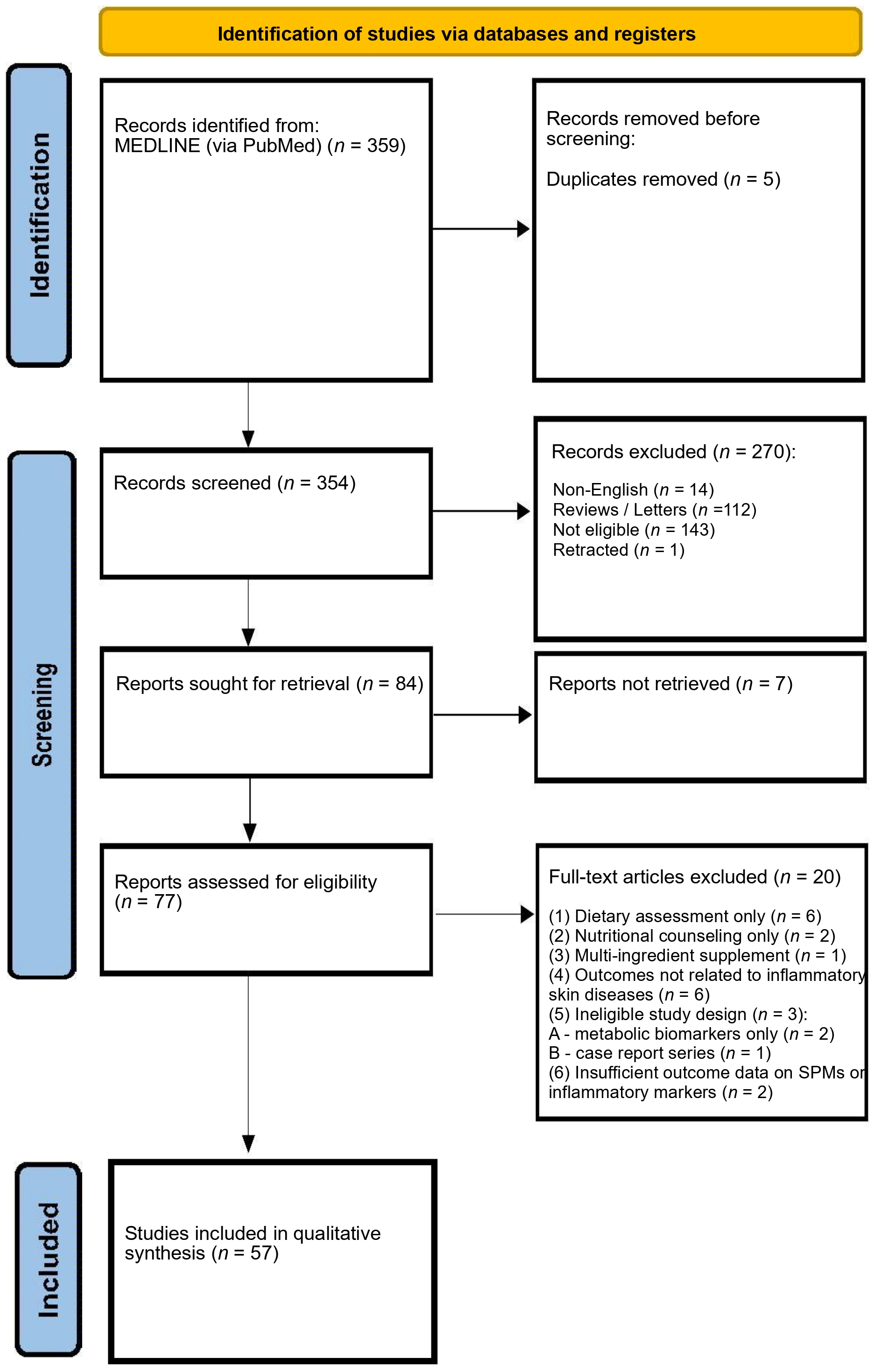
2. Materials and Methods
3. Results
| Author | Year | Population | Design/Intervention | Comparator | Key Observation |
|---|---|---|---|---|---|
| Experimental and Preclinical Studies | |||||
| Chen et al. [45] | 2011 | NHDF and NHEK | Treatment with lipoxin A4 | Untreated control cells | LXA4 suppressed the expression of IL-6 and IL-8 in keratinocytes and fibroblasts. |
| Liu et al. [46] | 2017 | IMQ-induced psoriasiform dermatitis mice model LPS-induced keratinocytes | Treatment with BML111 (LXA4 receptor agonist) | IMQ-induced mice without treatment (vehicle control) | BML111, a lipoxin A4 receptor agonist, treatment reduced IMQ-induced psoriasiform dermatitis. The effect of BML111 and lipoxin A4 is mediated by through HMGB and subsequent downregulation of inflammatory pathways. |
| Xu et al. [47] | 2018 | IMQ-induced psoriasiform dermatitis mice model | Pretreatment with RvD1 | IMQ-induced mice without treatment (vehicle control) | Pretreatment with RvD1 mitigates IMQ-induced psoriasiform dermatitis by targeting ALX/FPR2, and consequent inhibition of the IL-23/IL-17 axis. |
| Sawada et al. [48] | 2018 | IMQ-induced psoriasisiform dermatitis mice model | Treatment with RvE1 | Untreated or IMQ-induced control mice | RvE1 markedly reduced inflammatory infiltration, epidermal hyperplasia, and IL-23 expression in psoriatic skin. RvE1 inhibited IL-23 production and migration of dendritic cells and IL-17–producing γδ T cells through BLT1 antagonism. |
| Park et al. [49] | 2021 | IMQ-induced psoriasiform dermatitis mice model NHEK | Treatment with PD1 | Untreated or IMQ-induced control | PD1 mitigated psoriatic symptoms, epidermal thickening, erythema, and scaling. PD1 reduced pro-inflammatory cytokines and suppressed STAT1 and NF-κB activation in skin lesions. PD1 alleviated systemic inflammation and cytokine expression in keratinocytes. |
| Sorokin et al. [50] | 2023 | Mouse model of psoriasis-like inflammation | Dietary supplementation with DHA or EPA | Normal diet control | DHA supplementation increases skin levels of RvD5, protectin DX, and maresin 2.EPA supplementation reduces skin PGE2 and TXB2 levels. |
| Observational and Mechanistic Human Studies | |||||
| Sorokin et al. [51] | 2018 | Psoriatic patients | Psoriatic patients compared with healthy controls: blood samples, lesional and nonlesional skin punch biopsies. | Healthy controls (matched blood and skin samples) | 14-HDHA, 17-HDHA, precursors of D-series resolvins and protectins) are elevated in psoriatic skin lesions RvD2 is detected in ~50% of psoriasis and healthy individuals (no significant quantitative difference reported) 14,15-EpETE; 17,18-EpETE are mostly undetectable in both psoriasis and control groupsA shift toward ω-6 pro-inflammatory dominance and relative deficiency of ω-3-derived pro-resolving mediators in psoriasis |
| Sorokin et al. [52] | 2018 | Psoriatic patients (blood samples, lesional and nonlesional skin punch biopsies.) NHEK | NHEK cultivated in complete keratinocyte growth media-2 and subsequently treated with vehicle or TNFα or RvD1/RvD5 or TNFα, RvD1 and RvD5. | Vehicle-treated or TNFα-only treated NHEK | RvD5, protectin DX, and lipoxin are found only in lesional PSO skin, while protectin D1 is present in nonlesional PSO skin. RvD1 and RvD5 decrease IL-24 and S100A12 expression in human keratinocytes. |
| Author | Year | Population | Design/Intervention | Comparator | Key Observation |
|---|---|---|---|---|---|
| Experimental and Preclinical Studies | |||||
| Karrys et al. [53] | 2018 | Human keratinocytes (HEKn) homozygous for LCE3C_LCE3B deletion | In vitro DHA and curcumin treatment (VDR ligands) | Untreated/TNFα-stimulated cells | DHA and curcumin increased LCE3A/D/E expression, reduced MAPK activation, suggesting anti-inflammatory and skin barrier–repair effects relevant to psoriasis |
| Wannick et al. [54] | 2018 | Mouse model of Aldara-induced psoriasis-like dermatitis | Preclinical study; treatment with Compound A, a potent synthetic FFA4/GPR120 agonist | Vehicle-treated mice | Compound A did not improve psoriasis-like dermatitis, suggesting that sole FFA4 activation is insufficient to mediate the anti-inflammatory effects of ω3-PUFAs |
| Morin et al. [55] | 2021 | In vitro psoriatic and healthy skin substitutes | 10 μM DHA supplementation in culture medium | Unsupplemented control media | DHA reduced abnormal keratinocyte differentiation, decreased PGE2 and 12-HETE, rebalanced PPAR expression, and lowered TNF-α, attenuating psoriatic features. |
| Simard et al. [56] | 2021 | reconstructed 3D skin substitutes: psoriatic and healthy | In vitro 3D skin models derived from healthy and psoriatic donor cells, with keratinocytes cultured in 10 μM α-linolenic acid (ALA, ω-3 PUFA) medium | Skin substitutes cultured in standard medium (ALA) | ALA reduced keratinocyte proliferation (↓ Ki67), improved differentiation (↑ filaggrin, loricrin), incorporated into epidermal phospholipids, metabolized to EPA and ω-3 DPA, increased 15-HEPE and 18-HEPE, decreased ω-6 lipid mediators, and activated ERK1/2 signaling, normalizing psoriatic phenotype |
| Son et al. [57] | 2022 | FFA4 WT and KO BALB/c mice with imiquimod-induced psoriasis-like skin lesions | In vivo animal study; treatment with Compound A (30 mg/kg), a selective FFA4 agonist | Vehicle-treated mice (imiquimod only); FFA4 KO mice | Compound A reduced PASI, epidermal thickness, keratinocyte proliferation, TH17/TH1 cytokines, and CD4+IL-17A+ T cells in FFA4 WT mice; no effect in KO mice, indicating FFA4-mediated anti-psoriatic ω-3 PUFA effects. |
| Morin et al. [58] | 2023 | In vitro healthy and psoriatic skin substitutes with polarized T cells | Culture media supplemented with 10 μM EPA | Unsupplemented psoriatic skin model | EPA increased epidermal EPA, DPA, and DHA, elevated anti-inflammatory lipid mediators (PGE3, 12-HEPE, EPEA), and reduced ω-6–derived metabolites, restoring lipid homeostasis. |
| ω-3 Fatty Acids Intervention Studies on the Course of Psoriasis | |||||
| Gupta et al. [59] | 1989 | 18 patients with stable plaque psoriasis treated with UVB | 15-week RCT; diet enriched in fish oil (3.6 g of EPA 2.4 g of DCHA) | diet enriched in olive oil (76% olecic acid, 9% palmitic acid, 7% linoleic acid, 2%, stearic acid). | Fish oil supplementation led to greater improvement in BSA, redness, scaling, erythema, epidermal thickness, and overall global response compared to olive oil diet. |
| Dewsbury et al. [60] | 1989 | 11 psoriatic patients treated simultaneously with topical EPA and vehicle creams on different lesions | 7-week single-blind within-patient comparison; topical EPA | placebo (vehicle) cream | 8/11 patients showed subjective and objective improvement with EPA-treated plaques vs. placebo |
| Kojima et al. [61] | 1991 | 9 patients with chronic stable psoriasis | EPA supplementation 3.6 g/day for up to 12 months | Baseline/pre-treatment status | Modest but sustained clinical improvement; increased plasma EPA and LTB5, reduced LTB4 |
| Grimminger et al. [62] | 1993 | 21 patients with guttate psoriasis | Double-blind RCT;IV ω-3 emulsion (4.2 g EPA+DHA daily for 10 days) | IV ω-6 lipid emulsion | ω-3 group showed rapid, significant clinical improvement and increased EPA-derived mediators; ω-6 group showed only slight changes |
| Søyland et al. [63] | 1993 | 124 adults with stable plaque psoriasis | 4-month, double-blind RCT; six capsules daily of highly concentrated fish-oil ethyl esters (51% EPA, 32% DHA) | Six capsules daily of corn oil (oleic acid 18:1 n-9, linoleic acid 18:2 ω-6) | Fish-oil raised serum ω-3 PUFA but showed no PASI or symptom improvement vs. corn oil; minor reductions in scaling and infiltration seen in both groups. |
| Henneicke-von Zepelin et al. [64] | 1993 | 52 patients with moderate to severe psoriasis | Multicenter, double-blind RCT; topical ω-3 PUFA cream (1% or 10%) applied for 8 weeks | Placebo cream applied to matched control lesion on the same patient | Both treated and placebo lesions improved vs. baseline, but no significant difference between groups. Topical omega-3 PUFA was well tolerated; one case of mild perilesional eczema. |
| Mayser et al. [65] | 1998 | 83 patients with chronic plaque psoriasis | Double-blind RCT; IV ω-3 lipid emulsion (8.4 g EPA + DHA daily for 14 days) | IV ω-6 lipid emulsion | Greater PASI reduction in ω-3 group (p = 0.048); 37% achieved ≥50% PASI improvement vs. 23% controls; increased plasma EPA and anti-inflammatory eicosanoids |
| Danno et al. [66] | 1998 | 40 patients with moderate plaque psoriasis | 12-week randomized open study; combination therapy etretinate + 1800 mg/day EPA | etretinat | The combination therapy was more effective than monotherapy, especially with regard to improvement >75% |
| Guida et al. [67] | 2014 | 44 obese patients with mild-to-severe plaque psoriasis on stable immunosuppressive therapy | 6-month RCT; an energy-restricted diet enriched in ω-3 PUFAs (2.6 g/day) and low in ω-6 PUFAs | usual diet without nutritional intervention | ω-3 enriched diet significantly improved PASI, itch, and DLQI scores at 3 and 6 months (p < 0.05), and reduced body weight, waist circumference, triglycerides, total cholesterol, and ω-6/ω-3 ratio compared to controls. |
| Kristensen et al. [68] | 2017 | 145 patients with psoriatic arthritis | Randomized, double-blind trial; 3 g/day marine ω-3 PUFA for 24 weeks | 3 g/day olive oil | Improved disease activity measures, including PASI and reduced NSAID/paracetamol use; decreased leukotriene B4 and increased leukotriene B5 formation. |
| Petrovic et al. [69] | 2023 | 64 patients with mild psoriasis | Double blind RCT; Daily oral herring roe oil capsules providing 2.6 g ω-3 PUFA (DHA:EPA 3:1 ratio) | Matching placebo capsules with coconut oil (medium-chain triglycerides) | Significant PASI reduction after 26 weeks vs. placebo. ↓ CD38+ CD4+/CD8+ T cells and CD56^bright NK cells, maintained monocytes, ↓ CCL2, ↑ IFN-γR1 and CXCL10. Cytokine changes correlated with clinical improvement, indicating anti-inflammatory ω-3 PUFA effects. |
| Huang et al. [70] | 2024 | Genome-wide association study datasets | Two-sample Mendelian randomization assessing the causal relationship between fatty acid levels (ω-3, ω-6, and others) and psoriasis risk. | Genetic variants associated with lower or higher fatty acid levels. | Elevated genetically predicted ω-3 fatty acids were associated with lower psoriasis risk; no causal effect observed for other FA types. |
| Author | Year | Population | Design/Intervention | Comparator | Key Observation |
|---|---|---|---|---|---|
| Khayef et al. [71] | 2012 | 13 males with inflammatory acne | Supplementation of fish oil capsules daily for 12 weeks that contained a total of 930 mg EPA, 720 mg DHA, and 174 mg DPA per 3 capsules. | No control group. | 8 patients reduced acne symptoms, but no significant changes in total lesion count or redness was observed. |
| Jung et al. [72] | 2014 | 45 patients with mild to moderate acne | A 10-week double-blind RCT, parallel study including 45 patients divided in 2 groups: supplementing omega-3 (500 mg/day EPA and 500 mg/day DHA) or suplementing GLA 200 mg/day | A control group on regular diet. | Reduced mean-count inflammatory and non-inflammatory acne lesions in treatment groups compared to placebo group. |
| Aslan et al. [73] | 2016 | 31 female patients with moderate or severe acne | Cross-sectional study measuring serum FA and inflammatory mediators | 21 healthy controls | Significantly decreased EPA levels, increased AA/EPA and GLA/EPA ratios, increased serum LPL, increased sPLA2 in acne vulgaris patients |
| Zainab et al. [74] | 2021 | 60 patients treating acne with oral isotretinoin | Double-blind RCT including 34 patients on isotretinoin (0.5 mg/kg) plus omega-3 (1 mg/kg) | 26 patients on oral isotretinoin (0.5 mg/kg) and placebo | Omega-3 group had significantly fewer cases of cheilitis, lip dryness, and xerosis compared to placebo (p < 0.05). |
| Guertler et al. [75] | 2024 | 60 patients with acne | 16-week interventional study;Mediterranean diet + algal DHA/EPA (600–800 mg DHA, 300–400 mg EPA/day). | No control group; within-subject comparison across visits | Acne patients exhibited an EPA/DHA deficiency; supplementation significantly improved acne severity and reduced DLQI scores |
| Huang et al. [76] | 2024 | 46 acne vulgaris patients taking isotretinoin; three- and six-week-old male Sprague–Dawley rats | RCT: isotretinoin ± ω-3 fatty acids (2.4 g/day for 12 weeks); evaluation by clinical assessment, biochemical markers, gut microbiota. Parallel animal study using acne-induced rats and fecal microbiota transplantation (FMT). | Isotretinoin alone vs. isotretinoin + ω-3 fatty acids; 20 healthy controls. | Combined ω-3 + isotretinoin produced greater clinical improvement, reduced TG, increased HDL, and restored gut microbiota diversity. In rats, ω-3 supplementation reduced inflammation and comedones; FMT from ω-3-treated donors replicated the benefit, confirming a gut–skin axis mechanism. |
| Zhang et al. [77] | 2025 | Data from randomized controlled trials on acne patients and genetic datasets (European ancestry) for MR analysis. | Mendelian randomization evaluating causal links between serum UFA metabolites and acne. | genetic non-exposure variants | MR analysis identified EPA and AA as protective factors and DGLA as a risk factor (Enzymes FADS1/FADS2 implicated in acne regulation). |
| Author | Year | Population | Design/Intervention | Comparator | Key Observation |
|---|---|---|---|---|---|
| Experimental and Preclinical Studies | |||||
| Weise et al. [78] | 2011 | BALB/c mouse model of allergen-induced dermatitis | Dietary DHA + AA (24 mg/kg DHA + 48 mg/kg AA daily for 64 days) | Control diet, DHA alone, or AA alone | Oral DHA + AA reduced dermatitis severity and Ki67 expression, increased Foxp3+ T cells and IL-10, and suppressed keratinocyte TSLP; DHA or AA alone ineffective. |
| Kim et al. [79] | 2012 | DNFB-induced mouse AD model | Experimental study. Intraperitoneal RvE1 (100 or 200 ng/mouse/day) on days 7–13 after AD induction. | DNFB-treated (induction-only) and vehicle-treated controls | RvE1 in dose-dependent manner ameliorated DNFB-induced AD via downregulation of IgE and Th1/Th2 cytokine responses, and limiting immune cell infiltration (CD4+ T, CD8+ T, eosinophils, and mast cells) |
| Han et al. [80] | 2015 | DNCB-induced experimental ADmouse model | Experimental study. Oral DHA (100 mg/kg/day in drinking water) for 30 days; DHA-M2 macrophages transfused intravenously at days 12 and 19. | DNCB-induced AD without DHA or DHA-M2 transfusion; hydrocortisone group for comparison | DHA reduced IgE, histamine, ear thickness, epidermal thickness, and inflammatory cell infiltration. DHA increased CD4+Foxp3+ Tregs in LN and skin; suppressed Th1/Th2/Th17 cytokines; increased TGF-β, CTLA-4 DHA-M2 macrophages transferred protective effects, reduced inflammation, increased Tregs at inflammation sites. |
| Yoshida et al. [81] | 2016 | NC/Nga mice with AD-like dermatitis | DHA/EPA administered with FK506 | FK506 alone | Combined DHA/EPA + FK506 reduced dermatitis severity, LTB4 levels, immune cell infiltration, serum IgE, and IL-13/IL-17A secretion; effects reversed by LTB4 injection, indicating LTB4-dependent mechanism. |
| Fujii et al. [82] | 2018 | HR-AD mice with diet-induced AD–like symptoms | Oral administration of EPA ethyl ester (EPA-E) at 600 or 3000 mg/10 mL/kg once daily for 15 days | Vehicle (oleic acid) and HR-AD diet alone | High-dose EPA-E improved AD-like skin symptoms EPA-E lowered TSLP and IL-4 but not IL-5 expression EPA-E administration restored the depleted covalently bound ceramides, which resulted in improved barrier function |
| Lee at al. [83] | 2019 | mouse models of AD and psoriasis | Dietary supplementation with 5% EPA for 4 weeks prior to disease induction; AD induced with Dermatophagoides farinae body extract ointment, psoriasis induced with 5% imiquimod cream | Control diet | EPA altered skin lipid profiles by reducing AA-derived mediators (PGE2, TXB2, LTB4) and increasing EPA metabolites (e.g., RvE1), but failed to reduce scratching or dermatitis severity in AD and psoriasis models. |
| Sato et al. [84] | 2025 | Rat hind paw edema (PAF-induced) and mouse model of AD | Topical squid phospholipids (1% or 5% ointment) extracted from Todarodes pacificus, rich in DHA, EPA, and AA | Vehicle (Vaseline), soybean PC (1%), DHA (1.2%) | ω-3–rich squid phospholipids (DHA/EPA) reduced PAF-induced inflammation and AD-like skin lesions in mice DHA or soybean PC alone ineffective |
| Observational and Mechanistic Human Studies | |||||
| Montes et al. [85] | 2013 | 211 non-atopic mothers and their infants followed-up to 14 months | Prospective cohort assessing maternal and cord plasma FA composition during pregnancy and risk of infant atopic eczema | Non-eczema vs. eczema infants | Higher maternal and cord ω-3 LC-PUFAs (particularly DHA) associated with lower eczema risk |
| Mihály et al. [86] | 2014 | 20 adult AD patients and 20 healthy controls | Cross-sectional study assessing plasma and PBMC fatty acid composition, FADS2 and SCD1 expression | Healthy control group | AD patients showed elevated FADS2 expression in PBMCs and increased FADS2-derived ω-6 PUFAs, with reduced ω-3 PUFA levels; suggesting altered desaturase activity may drive ω-6 dominance and impaired ω-3-mediated resolution pathways. |
| Gardner et al. [87] | 2020 | 1131 mother–child pairs from a USA prenatal cohort (CANDLE study) | Observational prospective cohort. Maternal plasma phospholipid PUFAs measured during 2nd trimester; child AD assessed by ISAAC-based questionnaire at 4–6 years. | Comparison by maternal PUFA profiles (ω-3 PUFA, ω-6 PUFA, ω-6:ω-3 ratio, and combined EPA+DHA quartiles). | Maternal ω-6 PUFA exposure during pregnancy was positively associated with childhood AD, particularly among atopic mothers. ω-3 PUFAs showed no protective association overall, though moderate levels of EPA + DHA might reduce risk. No consistent link was observed for the ω-6:ω-3 ratio. |
| Mao et al. [88] | 2025 | 268,589 UK Biobank participants (after exclusion of prevalent AD cases | Prospective cohort study examining associations between FA profiles and risk of AD over a median 14.4-year follow-up | Comparison across FA quintiles and genetic subgroups | Higher plasma ω -3 and non-DHA ω-3 reduced AD risk, while elevated ω -6/ω -3 ratio increased risk; SFA, MUFA, ω -6, and LA showed no association. ω -3 attenuated genetic risk linked to rs1692120 and rs174448, with ~9% of AD cases potentially preventable through ω -3 supplementation. |
| Interventional Human Studies | |||||
| Bjørneboe et al. [89] | 1987 | 31 adults with moderate to severe AD and personal or family history of atopy | 12-week double-blind RCT. Intervention: capsules containing fish oil (~1.8 g EPA and 1.2 g DHA). | Placebo (olive oil) capsules. Mild topical steroids allowed. | Significant patient-reported improvement in itch, scaling, and total score; no significant physician-rated change or steroid use difference. |
| Berth-Jones et al. [90] | 1993 | 123 patients with AD | 16-week double-blindRCT Active arms: (1) Evening primrose oil, (2) Evening primrose + fish oil,; allowed topical steroids/emollients as required. | Placebo capsules topical steroids/emollients as required. | No significant improvement in disease severity, percentage of skin affected, topical steroid use, or patient symptom |
| Watanabe et al. [91] | 1999 | 64 patients with AD | Topical EPA/DHA ointment (1.2% DHA + 0.6% EPA, applied 2–3×/day for 4 weeks) | Placebo: hydrophilic ointment base applied to contralateral limb (n = 12 subset) | Significant improvement in erythema, papules, scaling, itching, and thickening; >80% improvement vs. 0–40% with placebo |
| Mayser et al. [92] | 2002 | 22 hospitalized patients with moderate-to-severe AD | Double-blind RCTl daily IV infusion of ω-3 fatty acid-based lipid emulsion for 10 days | ω-6 lipid emulsion | IV ω-3 lipid infusion improved AD severity and elevated plasma/membrane EPA and EPA/AA ratio, inducing EPA-derived mediators without affecting lymphocyte function. |
| Dunstan et al.l. [93] | 2003 | 98 atopic pregnant women; follow-up of their infants at high risk of allergic disease to 12 months | Double-blind RCT. Maternal supplementation with fish oil: 4 × 1 g/day capsules (total 3.7 g ω-3 PUFAs: 56% DHA, 27.7% EPA) from mid-pregnancy until delivery. | Olive oil capsules (placebo) | No significant difference in overall incidence of atopic dermatitis between groups. Infants in the fish oil group exhibited lower allergen sensitization rates and significantly reduced severity of AD. |
| Koch et al. [94] | 2008 | 53 adults with atopic eczema; 44 completed (DHA n = 21, Control n = 23) | 8-week double-blind RCT DHA 5.35 g + EPA 0.37 g/day | Isoenergetic control capsules containing MCT. | DHA supplementation significantly improved SCORAD score, reduced affected skin area, decreased plasma TG, increased HDL. Marked rise in ω-3 PUFA levels and reduced ω-6/ω-3 ratio. Ex vivo PBMC showed significant reduction in induced IgE production. |
| Furuhjelm et al. [95] | 2009 | 145 pregnant women with family history of allergic disease; follow-up of their infants to 24 months | Double-blind, RCT. Maternal supplementation ω-3 fatty acids (1.6 g/day EPA + 1.1 g/day DHA) from gestational week 25 until delivery | Placebo: soybean oil (2.5 g/day linoleic acid + 0.28 g/day α-linolenic acid) | ω-3 supplementation in pregnancy reduced IgE-associated eczema in infants during the first 2 years. Overall eczema frequency similar, but fewer IgE-mediated cases in the ω-3 group. Eczema severity (SCORAD) did not differ between groups. Trend toward lower IgE-associated allergic disease overall, especially in children of non-allergic mothers. Higher DHA and lower AA/EPA ratios in mothers and infants were linked to reduced allergic risk. |
| Noakes et al. [96] | 2012 | 123 pregnant women with low fish intake and atopic family history | RCT: 2 portions of salmon/week from 20 wks gestation to delivery (~3.45 g EPA + DHA; 28 µg vit D3) | Habitual low-fish diet | Increased cord plasma EPA + DHA and decreased AA; reduced CBMC IL-2, IL-4, IL-5, IL-10, TNF-α, and PGE2 responses; no difference in IgE, AD incidence, or severity at 6 months |
| Palmer et al. [97] | 2012 | Pregnant women with a personal or family history of allergic disease and their infants followed up to 1 year | Double-blind RCT. From 21 weeks’ gestation until delivery, mothers received fish oil capsules daily (800 mg DHA, 100 mg EPA). | Control group: vegetable oil capsules | Increased cord plasma DHA + EPA; decreased AA; reduced incidence of atopic eczema at 1 year; no difference in overall IgE-associated allergy or food allergy. |
| Wu et al. [98] | 2013 | 60 infants (aged 1–12 months, mean 4.2 months) with acute/subacute facial atopic eczema diagnosed by AAD criteria. | Double-blind RCT. Topical 0.1% 15(R/S)-methyl-lipoxin A4 (LXA4) cream applied twice daily for 10 days. | Placebo cream and 0.1% mometasone furoate (Eloson® Schering-Plough, Shanghai, China) cream. | LXA4 improved erythema and pruritus by day 3, papules/vesicles/scaling by day 5, and lichenification by day 10; effects persisted 1 week post-treatment. Efficacy comparable to mometasone (EASI, SSS), both improved IDQOL. No adverse events; relapse at day 40: LXA4 63%, mometasone 53% |
| Komulainen et al. [99] | 2023 | 439 pregnant women (BMI ≥ 25 kg/m2, <18 weeks gestation, no chronic disease) and 287 infants followed to 24 months | Double-blind RCT. Maternal supplementation: (1) Fish oil (2.4 g ω-3/day; DHA 1.9 g + EPA 0.22 g), (2) Probiotics, (3) Fish oil + probiotics, (4) Double placebo. | Placebo capsules (medium-chain triglycerides) and/or probiotics (microcrystalline cellulose). | No reduction in risk of physician-diagnosed atopic eczema at 12 or 24 months in any intervention group (all p > 0.05). |
| Figueroa-Garduño et al. [100] | 2023 | 193 Mexican mother–child pairs from the POSGRAD cohort; follow-up of infants to 5 years | Double-blind RCT. Prenatal DHA 400 mg/day from 18 to 22 weeks gestation to delivery. Assessment of maternal urinary arsenic at mid-pregnancy. | Soy/corn-oil placebo group without DHA supplementation. | DHA supplementation decreased early-childhood AD and counteracted the effect of prenatal arsenic exposure. |
| Niseteo et al. [101] | 2024 | 52 children (1–8 years) with moderate-to-severe AD | 4-month—triple-blind RCT. Fish oil syrup (600 mg EPA, 400 mg DHA, 10 mg GLA, 5 µg vit D3 daily) + standard AD care | Placebo syrup (MCT oil) | After 4 months, SCORAD significantly decreased in the intervention group vs. no change in placebo. PO-SCORAD, itch, sleep disturbance, and FDLQI improved significantly in the intervention group. No difference in TCS use. |
3.1. Psoriasis
3.2. Acne Vulgaris
3.3. Atopic Dermatitis
3.4. Factors Contributing to Outcome Differences
4. Discussion
4.1. Biosynthesis of Lipid Mediators
4.2. Overview of SPMs Classes
4.2.1. Lipoxins
4.2.2. Resolvins
4.2.3. Protectins
4.2.4. Maresins
4.3. Lipids as a Structural and Bioactive Regulators of Cutaneous Homeostasis
4.4. Psoriasis
4.4.1. Lipid Dysregulation in Psoriasis
4.4.2. Evidence for Omega-3 Fatty Acids Supplementation
4.4.3. Evidence for SPMs in Psoriasis
4.5. Hidradenitis Suppurativa
4.6. Acne Vulgaris
4.7. Atopic Dermatitis
4.8. Integrated Summary of Findings and Overall Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 14,15-EpETE/17,18-EpETE | 14,15-Epoxyeicosatetraenoic Acid/17,18-Epoxyeicosatetraenoic Acid |
| 14-HDHA/17-HDHA | 14-Hydroxydocosahexaenoic Acid/17-Hydroxydocosahexaenoic Acid |
| 5-HETE/12-HETE/12-HEPE/15-HEPE | 5-Hydroxyeicosatetraenoic Acid/12-Hydroxyeicosatetraenoic Acid/12-Hydroxyeicosapentaenoic Acid/15-Hydroxyeicosapentaenoic Acid |
| 5-oxo-ETE | 5-Oxo-Eicosatetraenoic Acid |
| AA | Arachidonic Acid |
| AC | Acne Comedonica |
| AD/AD-lika | Atopic dermatitis/Atopic dermatitis like |
| ALA | α-Linolenic Acid |
| ALX/FPR2 | Lipoxin A4 Receptor/Formyl Peptide Receptor 2 |
| AP | Acne Papulopustulosa |
| AT/ATL | Aspirin triggered/Aspirin triggered lipoxin |
| BALB/c | Bagg Albino Laboratory-bred Mouse Strain c |
| BLT1 | Leukotriene B4 Receptor 1 |
| BSA | Body Surface Area |
| CBMC | Cord blood mononuclear cell |
| CCL2 | C-C motif chemokine ligand 2 |
| CD80/86 | Co-stimulatory molecules expressed on antigen-presenting cells |
| CI | Confidence Interval |
| COX/COX-2/acetyl-COX-2 | Cyclooxygenase/Cyclooxygenase-2/aspirin-acetylated cyclooxygenase-2 |
| CRP | C-Reactive Protein |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CYP-450 | Cytochrome P450 Enzyme System |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DGLA | Dihomo-γ-Linolenic Acid |
| DHA | Docosahexaenoic Acid |
| DHA-M2 | Docosahexaenoic acid–induced M2 macrophages |
| DHET | Dihydroxyeicosatrienoic Acid |
| DLQI | Dermatology Life Quality Index |
| DNCB | 2,4-dinitrochlorobenzene |
| DNFB | 2,4-dinitrofluorobenzene |
| DPA | Docosapentaenoic Acid |
| EASI | Eczema Area and Severity Index |
| ECM | Extracellular Matrix |
| ELOVL | Elongation of Very Long Chain Fatty Acids Protein (family) |
| EPA/EPA-E | Eicosapentaenoic Acid/eicosapentaenoic acid ethyl ester |
| ERK1/2 | Extracellular Signal–Regulated Kinases 1/2 |
| FAs | Fatty acids |
| FABP | Fatty Acid–Binding Protein |
| FADS2 | Fatty acid desaturase 2 |
| FDLQI | Family Dermatology Life Quality Index |
| FFA4R/GPR120 | Free Fatty Acid Receptor 4 |
| FK506 | Tacrolimus |
| FMT | Fecal Microbiota Transplantation |
| GLA | γ-linolenic acid |
| GPCR | G Protein–Coupled Receptor |
| HDL | High-density lipoprotein |
| HETE | Hydroxyeicosatetraenoic Acid |
| HEPE | Hydroxy-eicosapentaenoic Acid |
| HMGB | High-Mobility Group Box protein |
| HpEPE | Hydroperoxy-eicosapentaenoic Acid |
| HpETE | Hydroxyperoxy-eicosatetraenoic Acid |
| HR-AD | Hairless atopic dermatitis |
| HRO | Herring Roe Oil |
| HS | Hidradenitis Suppurativa |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| IRI/I–R | Ischemia–Reperfusion Injury |
| ISAAC | International Study of Asthma and Allergies in Childhood |
| IV | Intravenous |
| IMQ | Imiquimod |
| KO | Knockout |
| LCE3B–C | Late Cornified Envelope Genes 3B and 3C |
| LC-PUFA | Long-chain polyunsaturated fatty acid |
| LN | Lymph node |
| LOX/5-LOX/12-LOX/15-LOX | Lipoxygenase/5- Lipoxygenase/12- Lipoxygenase/15- Lipoxygenase |
| LPL | Lipoprotein Lipase |
| LPS | Lipopolysaccharide |
| LT/LTA4/5/LTE4/5 | Leukotriene A4/5—E4/5 |
| LXA4/LXA5 | Lipoxin A4/A5 |
| M2 | Alternatively Activated Macrophage Phenotype |
| MAPK | Mitogen-Activated Protein Kinase |
| MaR1/MaR2 | Maresin-1/Maresin 2 |
| MCT | Medium-chain triglyceride |
| MCTR | Maresin Conjugates in Tissue Regeneration |
| MD | Mean difference |
| MR | Mendelian Randomization |
| MUFA | Monounsaturated fatty acid |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NHDF | Normal Human Dermal Fibroblasts |
| NHEK | Normal Human Epidermal Keratinocytes |
| NK | Natural Killer Cells |
| NRF2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| PASI | Psoriasis Area and Severity Index |
| PAF | Platelet-activating factor |
| PBMC | peripheral blood mononuclear cell |
| PG/PGE2/PGE3/PGH2/PGG2 | Prostaglandin/E2/E3/H2/G2 |
| PGI2 | Prostacyclin |
| PSO | Psoriasis |
| PUFA | Polyunsaturated Fatty Acid |
| PD1 | Protectin D1 |
| PDX | Protectin DX |
| PLA2/PLA2G4E/sPLA2 | Phospholipase A2/Phospholipase A2 Group IVE/Secretory Phospholipase A2 |
| PPAR | Peroxisome Proliferator–Activated Receptor |
| PRS | Polygenic risk score |
| RCT | Randomized Controlled Trial |
| QoL | Quality of life |
| RvD1-RvD6 | Resolvin D1-D6 |
| RvE/RvE1–RvE4 | Resolvin E-series/E1/E2/E3/E4 |
| S100A12 | S100 Calcium-Binding Protein A12 |
| SCORAD | SCORing Atopic Dermatitis |
| SFA | Saturated fatty acid |
| SNP | Single-nucleotide polymorphism |
| SPMs | Specialized Pro-Resolving Mediators |
| SSS | Symptom Severity Score |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TCM | Central Memory T cells |
| TCS | Topical corticosteroid |
| TEWL | Transepidermal Water Loss |
| TG | Trigliceride |
| TGF-β | Transforming growth factor-β |
| Th/Th2/Th17 | Helper T Cell/Type 2 Helper T Cell/Type 17 Helper T Cell |
| TN | Naïve T cells |
| TNF-α | Tumor Necrosis Factor α |
| Treg | Regulatory T cell |
| TSLP | Thymic stromal lymphopoietin |
| TXA2/TXB2 | Thromboxane A2/B2 |
| UFA | Unsaturated Fatty Acid |
| UV | Ultraviolet |
| VDR | Vitamin D Receptor |
| WT | Wild-Type |
| ω-3 | Omega-3 Fatty Acids |
| ω-6 | Omega-6 Fatty Acids |
References
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2-Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Maddox, J.F.; Petasis, N.A.; Akritopoulou-Zanze, I.; Papayianni, A.; Brady, H.R.; Colgan, S.P.; Madara, J.L. Design of Lipoxin A4 Stable Analogs That Block Transmigration and Adhesion of Human Neutrophils. Biochemistry 1995, 34, 14609–14615. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Serhan, C. Macrophage Proresolving Mediators-the When and Where. Microbiol. Spectr. 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. A Search for Endogenous Mechanisms of Anti-Inflammation Uncovers Novel Chemical Mediators: Missing Links to Resolution. Histochem. Cell Biol. 2004, 122, 305–321. [Google Scholar] [CrossRef]
- Fierro, I.M.; Colgan, S.P.; Bernasconi, G.; Petasis, N.A.; Clish, C.B.; Arita, M.; Serhan, C.N. Lipoxin A4 and Aspirin-Triggered 15-Epi-Lipoxin A4 Inhibit Human Neutrophil Migration: Comparisons Between Synthetic 15 Epimers in Chemotaxis and Transmigration with Microvessel Endothelial Cells and Epithelial Cells. J. Immunol. 2003, 170, 2688–2694. [Google Scholar] [CrossRef]
- Hosseini, Z.; Marinello, M.; Decker, C.; Sansbury, B.E.; Sadhu, S.; Gerlach, B.D.; Bossardi Ramos, R.; Adam, A.P.; Spite, M.; Fredman, G. Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. Arter. Thromb. Vasc. Biol. 2021, 41, 1062–1075. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; De La Rosa, X.; Serhan, C.N. Maresin 1 Activates LGR6 Receptor Promoting Phagocyte Immunoresolvent Functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Fredman, G.; Khan, S. Specialized Pro-resolving Mediators Enhance the Clearance of Dead Cells. Immunol. Rev. 2023, 319, 151–157. [Google Scholar] [CrossRef]
- Qiao, N.; Lin, Y.; Wang, Z.; Chen, J.-Y.; Ge, Y.-Y.; Yao, S.-L.; Gong, J. Maresin1 Promotes M2 Macrophage Polarization Through Peroxisome Proliferator–Activated Receptor-γ Activation to Expedite Resolution of Acute Lung Injury. J. Surg. Res. 2020, 256, 584–594. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Chiurchiù, V.; Perruche, S.; You, S. Regulation of T-Cell Immune Responses by Pro-Resolving Lipid Mediators. Front. Immunol. 2021, 12, 768133. [Google Scholar] [CrossRef] [PubMed]
- Ramon, S.; Bancos, S.; Serhan, C.N.; Phipps, R.P. Lipoxin A4 Modulates Adaptive Immunity by Decreasing Memory B-cell Responses via an ALX/FPR 2-dependent Mechanism. Eur. J. Immunol. 2014, 44, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Duffney, P.F.; Falsetta, M.L.; Rackow, A.R.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Key Roles for Lipid Mediators in the Adaptive Immune Response. J. Clin. Investig. 2018, 128, 2724–2731. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; He, L. A Review of Skin Immune Processes in Acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef]
- Schön, M.P. Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders. Front. Immunol. 2019, 10, 1764. [Google Scholar] [CrossRef]
- Burke, O.M.; Frerichs, V.R.; Garcia, D.F.; Stone, R.C.; Lev-Tov, H.; Czarnowicki, T.; Keane, R.W.; Ojeh, N.; Marjanovic, J.; Pastar, I.; et al. The Impact of Innate Immunity and Epigenetics in the Pathogenesis of Hidradenitis Suppurativa. Front. Immunol. 2025, 16, 1593253. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Xu, M.; Zhong, M.; Peng, X.; Zeng, K.; Huang, X. The Roles of Innate Immune Cells in Atopic Dermatitis. J. Innate Immun. 2024, 16, 385–396. [Google Scholar] [CrossRef]
- Orzan, O.A.; Tutunaru, C.V.; Ianoși, S.L. Understanding the Intricate Pathophysiology of Psoriasis and Related Skin Disorders. Int. J. Mol. Sci. 2025, 26, 749. [Google Scholar] [CrossRef]
- Spiteri, J.; Mintoff, D.; Grech, L.; Pace, N.P. Transcriptomic Signatures and Molecular Pathways in Hidradenitis Suppurativa—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7704. [Google Scholar] [CrossRef]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Schell, S.L.; Cong, Z.; Sennett, M.L.; Gettle, S.L.; Longenecker, A.L.; Goldberg, S.R.; Kirby, J.S.; Helm, M.F.; Nelson, A.M. Keratinocytes and Immune Cells in the Epidermis Are Key Drivers of Inflammation in Hidradenitis Suppurativa Providing a Rationale for Novel Topical Therapies. Br. J. Dermatol. 2023, 188, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Orsmond, A.; Bereza-Malcolm, L.; Lynch, T.; March, L.; Xue, M. Skin Barrier Dysregulation in Psoriasis. Int. J. Mol. Sci. 2021, 22, 10841. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Almegaard, A.; Schultz, A.N.; Zibert, J.R. Effect of Omega-3 Supplementation on Quality of Life in Patients with Psoriasis: A Digital Survey-Based Study. Acta Derm. Venereol. 2019, 99, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Jerzyńska, A.; Polańska, A.; Trafalska, E.; Jankowska, A.; Podlecka, D.; Brzozowska, A. Prenatal Polyunsaturated Fatty Acids and Atopic Dermatitis and Food Allergy in Children from Polish Mother and Child Cohort Study. Int. J. Occup. Med. Environ. Health 2023, 36, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Ohya, Y.; Matsunaga, I.; Yoshida, T.; Hirota, Y.; Oda, H. Relationship between Dietary Fat and Fish Intake and the Prevalence of Atopic Eczema in Pregnant Japanese Females: Baseline Data from the Osaka Maternal and Child Health Study. Asia Pac. J. Clin. Nutr. 2008, 17, 612–619. [Google Scholar]
- Nwaru, B.I.; Erkkola, M.; Lumia, M.; Kronberg-Kippilä, C.; Ahonen, S.; Kaila, M.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; et al. Maternal Intake of Fatty Acids during Pregnancy and Allergies in the Offspring. Br. J. Nutr. 2012, 108, 720–732. [Google Scholar] [CrossRef]
- Trak-Fellermeier, M.A.; Brasche, S.; Winkler, G.; Koletzko, B.; Heinrich, J. Food and Fatty Acid Intake and Atopic Disease in Adults. Eur. Respir. J. 2004, 23, 575–582. [Google Scholar] [CrossRef]
- Weiland, S.K.; von Mutius, E.; Hüsing, A.; Asher, M.I. Intake of Trans Fatty Acids and Prevalence of Childhood Asthma and Allergies in Europe. ISAAC Steering Committee. Lancet 1999, 353, 2040–2041. [Google Scholar] [CrossRef]
- Collier, P.M.; Ursell, A.; Zaremba, K.; Payne, C.M.; Staughton, R.C.; Sanders, T. Effect of Regular Consumption of Oily Fish Compared with White Fish on Chronic Plaque Psoriasis. Eur. J. Clin. Nutr. 1993, 47, 251–254. [Google Scholar] [PubMed]
- Dotterud, C.K.; Storrø, O.; Simpson, M.R.; Johnsen, R.; Øien, T. The Impact of Pre- and Postnatal Exposures on Allergy Related Diseases in Childhood: A Controlled Multicentre Intervention Study in Primary Health Care. BMC Public Health 2013, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Cameli, N.; Lorenzi, S.; De Padova, M.P.; Marasca, C.; Izzo, R.; Monfrecola, G. A Dietary Supplement to Reduce Side Effects of Oral Isotretinoin Therapy in Acne Patients. G. Ital. Dermatol. Venereol. 2014, 149, 441–445. [Google Scholar] [PubMed]
- Li, Y.; Li, X. PUFA, Psoriasis and Atherosclerotic CVD: Evidence from the National Health and Nutrition Examination Survey Database 2003–2006 and 2009–2014. Br. J. Nutr. 2025, 133, 1331–1341. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Peat, J.K.; Webb, K.; Tovey, E.R.; Marks, G.B.; Mellis, C.M.; Leeder, S.R. The Childhood Asthma Prevention Study (CAPS): Design and Research Protocol of a Randomized Trial for the Primary Prevention of Asthma. Control Clin. Trials 2001, 22, 333–354. [Google Scholar] [CrossRef]
- Ordnung, M.; Mank, M.; Stahl, B.; Kurz, D.; Marosvölgyi, T.; Decsi, T.; Rothenbacher, D.; Genuneit, J.; Siziba, L.P. Potential Sex Differences in Human Milk Fatty Acids and Their Association with Atopic Dermatitis: Results of the Ulm SPATZ Health Study. Pediatr. Allergy Immunol. 2023, 34, e13992. [Google Scholar] [CrossRef]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.E.P.; Huber, M.; van Ree, R.; Simões-Wüst, A.P.; Dagnelie, P.C.; van den Brandt, P.A. Fatty Acids in Breast Milk and Development of Atopic Eczema and Allergic Sensitisation in Infancy. Allergy 2011, 66, 58–67. [Google Scholar] [CrossRef]
- Wright, S.; Bolton, C. Breast Milk Fatty Acids in Mothers of Children with Atopic Eczema. Br. J. Nutr. 1989, 62, 693–697. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lawlor, K.C. Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium Acnes and Staphylococcus Aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef]
- Sarandi, E.; Krueger-Krasagakis, S.; Tsoukalas, D.; Evangelou, G.; Sifaki, M.; Kyriakakis, M.; Paramera, E.; Papakonstantinou, E.; Rudofsky, G.; Tsatsakis, A. Novel Fatty Acid Biomarkers in Psoriasis and the Role of Modifiable Factors: Results from the METHAP Clinical Study. Biomolecules 2024, 14, 1114. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Shi, R.; Qin, H.; Chen, W.; Yu, Z.; Ding, Y.; Peng, C.; Shi, Y. Sex Differences in the Association between Plasma Polyunsaturated Fatty Acids Levels and Moderate-to-Severe Plaque Psoriasis Severity: A Cross-Sectional and Longitudinal Study. J. Transl. Med. 2023, 21, 834. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.G.; Kim, K.; Logan, A.C. Acne Vulgaris, Mental Health and Omega-3 Fatty Acids: A Report of Cases. Lipids Health Dis. 2008, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.J.; Mahammad, N.; Midtun Flatekvål, H.; Jullumstrø Feuerherm, A.; Johansen, B. cPLA2α Enzyme Inhibition Attenuates Inflammation and Keratinocyte Proliferation. Biomolecules 2020, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.H.; Habicht, A.; Damsbo, P.; Wilms, J.; Johansen, B.; Gniadecki, R. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Man Study (Phase 0) to Assess the Safety and Efficacy of Topical Cytosolic Phospholipase A2 Inhibitor, AVX001, in Patients with Mild to Moderate Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Takahara, M.; Xie, L.; Takeuchi, S.; Tu, Y.; Nakahara, T.; Uchi, H.; Moroi, Y.; Furue, M. Lipoxin A(4), a Potential Anti-Inflammatory Drug Targeting the Skin. J. Dermatol. Sci. 2011, 62, 67–69. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Duan, X.; Poorun, D.; Xu, J.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; Ming, Z.; et al. Lipoxin A4 and Its Analog Suppress Inflammation by Modulating HMGB1 Translocation and Expression in Psoriasis. Sci. Rep. 2017, 7, 7100. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Hu, F.; Poorun, D.; Liu, X.; Wang, X.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; et al. Resolvin D1 Attenuates Imiquimod-Induced Mice Psoriasiform Dermatitis through MAPKs and NF-κB Pathways. J. Dermatol. Sci. 2018, 89, 127–135. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 Attenuates Murine Psoriatic Dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef]
- Park, K.-D.; Kim, N.; Kang, J.; Dhakal, H.; Kim, J.Y.; Jang, Y.H.; Lee, W.J.; Lee, S.-J.; Kim, S.-H. Protectin D1 Reduces Imiquimod-Induced Psoriasiform Skin Inflammation. Int. Immunopharmacol. 2021, 98, 107883. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Arnardottir, H.; Svirydava, M.; Ng, Q.; Baumer, Y.; Berg, A.; Pantoja, C.J.; Florida, E.M.; Teague, H.L.; Yang, Z.-H.; et al. Comparison of the Dietary Omega-3 Fatty Acids Impact on Murine Psoriasis-like Skin Inflammation and Associated Lipid Dysfunction. J. Nutr. Biochem. 2023, 117, 109348. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.-X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Investig. Dermatol. 2018, 138, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Norris, P.C.; English, J.T.; Dey, A.K.; Chaturvedi, A.; Baumer, Y.; Silverman, J.; Playford, M.P.; Serhan, C.N.; Mehta, N.N. Identification of Proresolving and Inflammatory Lipid Mediators in Human Psoriasis. J. Clin. Lipidol. 2018, 12, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Karrys, A.; Rady, I.; Chamcheu, R.-C.N.; Sabir, M.S.; Mallick, S.; Chamcheu, J.C.; Jurutka, P.W.; Haussler, M.R.; Whitfield, G.K. Bioactive Dietary VDR Ligands Regulate Genes Encoding Biomarkers of Skin Repair That Are Associated with Risk for Psoriasis. Nutrients 2018, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Wannick, M.; Bezdek, S.; Guillen, N.; Thieme, M.; Meshrkey, F.; Mousavi, S.; Seeling, M.; Nimmerjahn, F.; Mócsai, A.; Zillikens, D.; et al. Oral Administration of the Selective GPR120/FFA4 Agonist Compound A Is Not Effective in Alleviating Tissue Inflammation in Mouse Models of Prototypical Autoimmune Diseases. Pharmacol. Res. Perspect. 2018, 6, e00438. [Google Scholar] [CrossRef]
- Morin, S.; Simard, M.; Flamand, N.; Pouliot, R. Biological Action of Docosahexaenoic Acid in a 3D Tissue-Engineered Psoriatic Skin Model: Focus on the PPAR Signaling Pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159032. [Google Scholar] [CrossRef]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guérin, S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401.e13. [Google Scholar] [CrossRef]
- Son, S.-E.; Koh, J.-M.; Im, D.-S. Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. Int. J. Mol. Sci. 2022, 23, 4482. [Google Scholar] [CrossRef]
- Morin, S.; Tremblay, A.; Dumais, E.; Julien, P.; Flamand, N.; Pouliot, R. Eicosapentaenoic Acid Influences the Lipid Profile of an In Vitro Psoriatic Skin Model Produced with T Cells. Biomolecules 2023, 13, 1413. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ellis, C.N.; Tellner, D.C.; Anderson, T.F.; Voorhees, J.J. Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy of Fish Oil and Low-Dose UVB in the Treatment of Psoriasis. Br. J. Dermatol. 1989, 120, 801–807. [Google Scholar] [CrossRef]
- Dewsbury, C.E.; Graham, P.; Darley, C.R. Topical Eicosapentaenoic Acid (EPA) in the Treatment of Psoriasis. Br. J. Dermatol. 1989, 120, 581. [Google Scholar] [CrossRef]
- Kojima, T.; Terano, T.; Tanabe, E.; Okamoto, S.; Tamura, Y.; Yoshida, S. Long-Term Administration of Highly Purified Eicosapentaenoic Acid Provides Improvement of Psoriasis. Dermatologica 1991, 182, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Grimminger, F.; Mayser, P.; Papavassilis, C.; Thomas, M.; Schlotzer, E.; Heuer, K.U.; Führer, D.; Hinsch, K.D.; Walmrath, D.; Schill, W.B. A Double-Blind, Randomized, Placebo-Controlled Trial of n-3 Fatty Acid Based Lipid Infusion in Acute, Extended Guttate Psoriasis. Rapid Improvement of Clinical Manifestations and Changes in Neutrophil Leukotriene Profile. Clin. Investig. 1993, 71, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Søyland, E.; Funk, J.; Rajka, G.; Sandberg, M.; Thune, P.; Rustad, L.; Helland, S.; Middelfart, K.; Odu, S.; Falk, E.S. Effect of Dietary Supplementation with Very-Long-Chain n-3 Fatty Acids in Patients with Psoriasis. N. Engl. J. Med. 1993, 328, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Henneicke-von Zepelin, H.H.; Mrowietz, U.; Färber, L.; Bruck-Borchers, K.; Schober, C.; Huber, J.; Lutz, G.; Kohnen, R.; Christophers, E.; Welzel, D. Highly Purified Omega-3-Polyunsaturated Fatty Acids for Topical Treatment of Psoriasis. Results of a Double-Blind, Placebo-Controlled Multicentre Study. Br. J. Dermatol. 1993, 129, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Mayser, P.; Mrowietz, U.; Arenberger, P.; Bartak, P.; Buchvald, J.; Christophers, E.; Jablonska, S.; Salmhofer, W.; Schill, W.B.; Krämer, H.J.; et al. Omega-3 Fatty Acid-Based Lipid Infusion in Patients with Chronic Plaque Psoriasis: Results of a Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. J. Am. Acad. Dermatol. 1998, 38, 539–547. [Google Scholar] [CrossRef]
- Danno, K.; Sugie, N. Combination Therapy with Low-Dose Etretinate and Eicosapentaenoic Acid for Psoriasis Vulgaris. J. Dermatol. 1998, 25, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Napoleone, A.; Trio, R.; Nastasi, A.; Balato, N.; Laccetti, R.; Cataldi, M. Energy-Restricted, n-3 Polyunsaturated Fatty Acids-Rich Diet Improves the Clinical Response to Immuno-Modulating Drugs in Obese Patients with Plaque-Type Psoriasis: A Randomized Control Clinical Trial. Clin. Nutr. 2014, 33, 399–405. [Google Scholar] [CrossRef]
- Kristensen, S.; Schmidt, E.B.; Schlemmer, A.; Rasmussen, C.; Johansen, M.B.; Christensen, J.H. Beneficial Effect of N-3 Polyunsaturated Fatty Acids on Inflammation and Analgesic Use in Psoriatic Arthritis: A Randomized, Double Blind, Placebo-Controlled Trial. Scand. J. Rheumatol. 2018, 47, 27–36. [Google Scholar] [CrossRef]
- Petrovic, A.; Bueide, I.; Tveit, K.S.; Hallaråker, H.; Bjørndal, B.; Holmes, T.D.; Davies, R.; Brokstad, K.A.; Bergum, B.; Appel, S. Herring Roe Oil in Treatment of Psoriasis—Influence on Immune Cells and Cytokine Network. Front. Immunol. 2023, 14, 1128986. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Wu, X.; Chen, C.; Cai, L. The Effects of Fatty Acids on Psoriasis: A Two-Sample Mendelian Randomization Study. J. Cosmet. Dermatol. 2024, 23, 2716–2725. [Google Scholar] [CrossRef]
- Khayef, G.; Young, J.; Burns-Whitmore, B.; Spalding, T. Effects of Fish Oil Supplementation on Inflammatory Acne. Lipids Health Dis. 2012, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of Dietary Supplementation with Omega-3 Fatty Acid and Gamma-Linolenic Acid on Acne Vulgaris: A Randomised, Double-Blind, Controlled Trial. Acta Derm. Venereol. 2014, 94, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Aslan, İ.; Özcan, F.; Karaarslan, T.; Kıraç, E.; Aslan, M. Decreased Eicosapentaenoic Acid Levels in Acne Vulgaris Reveals the Presence of a Proinflammatory State. Prostaglandins Other Lipid Mediat. 2017, 128–129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zainab, Z.; Malik, N.A.; Obaid, S.; Malik, S.; Aftab, K.; Mumtaz, M.; Pervez, A.; Syed, Z. Effectiveness of Oral Omega 3 In Reducing Mucocutaneous Side Effects of Oral Isotretinoin in Patients with Acne Vulgaris. J. Ayub Med. Coll. Abbottabad 2021, 33, 60–63. [Google Scholar]
- Guertler, A.; Neu, K.; Lill, D.; Clanner-Engelshofen, B.; French, L.E.; Reinholz, M. Exploring the Potential of Omega-3 Fatty Acids in Acne Patients: A Prospective Intervention Study. J. Cosmet. Dermatol. 2024, 23, 3295–3304. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, F.; Lai, J.; Jiang, S.; Tan, X.; Chen, L.; Xu, Y.; Xiong, X.; Deng, Y. The Adjuvant Treatment Role of ω-3 Fatty Acids by Regulating Gut Microbiota Positively in the Acne Vulgaris. J. Dermatol. Treat. 2024, 35, 2299107. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Pu, Y.; Dang, T.; Shi, Q.; Wu, W. Exploring Clinical and Genetic Evidence in Association between Unsaturated Fatty Acids and Acne. Eur. J. Nutr. 2025, 64, 130. [Google Scholar] [CrossRef]
- Weise, C.; Heunemann, C.; Loddenkemper, C.; Herz, U.; van Tol, E.A.F.; Worm, M. Dietary Docosahexaenoic Acid in Combination with Arachidonic Acid Ameliorates Allergen-Induced Dermatitis in Mice. Pediatr. Allergy Immunol. 2011, 22, 497–504. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, G.-D.; Jin, Y.-H.; Park, Y.S.; Park, C.-S. Omega-3 Fatty Acid-Derived Mediator, Resolvin E1, Ameliorates 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis in NC/Nga Mice. Int. Immunopharmacol. 2012, 14, 384–391. [Google Scholar] [CrossRef]
- Han, S.-C.; Koo, D.-H.; Kang, N.-J.; Yoon, W.-J.; Kang, G.-J.; Kang, H.-K.; Yoo, E.-S. Docosahexaenoic Acid Alleviates Atopic Dermatitis by Generating Tregs and IL-10/TGF-β-Modified Macrophages via a TGF-β-Dependent Mechanism. J. Investig. Dermatol. 2015, 135, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yasutomo, K.; Watanabe, T. Treatment with DHA/EPA Ameliorates Atopic Dermatitis-like Skin Disease by Blocking LTB4 Production. J. Med. Investig. 2016, 63, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Ohyanagi, C.; Kawaguchi, N.; Matsuda, H.; Miyamoto, Y.; Ohya, S.; Nabe, T. Eicosapentaenoic Acid Ethyl Ester Ameliorates Atopic Dermatitis-like Symptoms in Special Diet-Fed Hairless Mice, Partly by Restoring Covalently Bound Ceramides in the Stratum Corneum. Exp. Dermatol. 2018, 27, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Tominaga, M.; Yasukawa, K.; Ohba, M.; Takahashi, N.; Honda, K.; Okuno, T.; Takamori, K.; Yokomizo, T. Dietary Supplementation of Omega-3 Fatty Acid Eicosapentaenoic Acid Does Not Ameliorate Pruritus in Murine Models of Atopic Dermatitis and Psoriasis. J. Dermatol. Sci. 2019, 95, 130–133. [Google Scholar] [CrossRef]
- Sato, A.; Sato, G.; Yabuki, A.; Ogawa, Y.; Nemoto, H.; Ohira, M. Ameliorative Effects of Squid Phospholipids from Todarodes Pacificus on Atopic Dermatitis-like Lesions in NC/Nga Mice. Arch. Dermatol. Res. 2025, 317, 239. [Google Scholar] [CrossRef]
- Montes, R.; Chisaguano, A.M.; Castellote, A.I.; Morales, E.; Sunyer, J.; López-Sabater, M.C. Fatty-Acid Composition of Maternal and Umbilical Cord Plasma and Early Childhood Atopic Eczema in a Spanish Cohort. Eur. J. Clin. Nutr. 2013, 67, 658–663. [Google Scholar] [CrossRef]
- Mihály, J.; Marosvölgyi, T.; Szegedi, A.; Köröskényi, K.; Lucas, R.; Törőcsik, D.; Garcia, A.L.; Decsi, T.; Rühl, R. Increased FADS2-Derived n-6 PUFAs and Reduced n-3 PUFAs in Plasma of Atopic Dermatitis Patients. Ski. Pharmacol. Physiol. 2014, 27, 242–248. [Google Scholar] [CrossRef]
- Gardner, K.G.; Gebretsadik, T.; Hartman, T.J.; Rosa, M.J.; Tylavsky, F.A.; Adgent, M.A.; Moore, P.E.; Kocak, M.; Bush, N.R.; Davis, R.L.; et al. Prenatal Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Childhood Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 937–944. [Google Scholar] [CrossRef]
- Mao, R.; Meng, X.; Zhang, T.; Wang, F.; Zhong, Y.; Li, J. Evaluating the Impact of Omega-3 Fatty Acids and Genetic Susceptibility on Atopic Dermatitis in Adults. Mol. Nutr. Food Res. 2025, 69, e70002. [Google Scholar] [CrossRef]
- Bjørneboe, A.; Søyland, E.; Bjørneboe, G.E.; Rajka, G.; Drevon, C.A. Effect of Dietary Supplementation with Eicosapentaenoic Acid in the Treatment of Atopic Dermatitis. Br. J. Dermatol. 1987, 117, 463–469. [Google Scholar] [CrossRef]
- Berth-Jones, J.; Graham-Brown, R.A. Placebo-Controlled Trial of Essential Fatty Acid Supplementation in Atopic Dermatitis. Lancet 1993, 341, 1557–1560. [Google Scholar] [CrossRef]
- Watanabe, T.; Kuroda, Y. The Effect of a Newly Developed Ointment Containing Eicosapentaenoic Acid and Docosahexaenoic Acid in the Treatment of Atopic Dermatitis. J. Med. Investig. 1999, 46, 173–177. [Google Scholar]
- Mayser, P.; Mayer, K.; Mahloudjian, M.; Benzing, S.; Krämer, H.-J.; Schill, W.-B.; Seeger, W.; Grimminger, F. A Double-Blind, Randomized, Placebo-Controlled Trial of n-3 versus n-6 Fatty Acid-Based Lipid Infusion in Atopic Dermatitis. J. Parenter. Enter. Nutr. 2002, 26, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish Oil Supplementation in Pregnancy Modifies Neonatal Allergen-Specific Immune Responses and Clinical Outcomes in Infants at High Risk of Atopy: A Randomized, Controlled Trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Dölle, S.; Metzger, M.; Rasche, C.; Jungclas, H.; Rühl, R.; Renz, H.; Worm, M. Docosahexaenoic Acid (DHA) Supplementation in Atopic Eczema: A Randomized, Double-Blind, Controlled Trial. Br. J. Dermatol. 2008, 158, 786–792. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Fagerås, M.; Fälth-Magnusson, K.; Larsson, J.; Fredriksson, M.; Duchén, K. Allergic Disease in Infants up to 2 Years of Age in Relation to Plasma Omega-3 Fatty Acids and Maternal Fish Oil Supplementation in Pregnancy and Lactation. Pediatr. Allergy Immunol. 2011, 22, 505–514. [Google Scholar] [CrossRef]
- Noakes, P.S.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Miles, E.A.; Erlewyn-Lajeunesse, M.; Williams, A.P.; Godfrey, K.M.; Calder, P.C. Increased Intake of Oily Fish in Pregnancy: Effects on Neonatal Immune Responses and on Clinical Outcomes in Infants at 6 Mo. Am. J. Clin. Nutr. 2012, 95, 395–404. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Effect of N-3 Long Chain Polyunsaturated Fatty Acid Supplementation in Pregnancy on Infants’ Allergies in First Year of Life: Randomised Controlled Trial. BMJ 2012, 344, e184. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, X.-Q.; Liu, B.; Wu, H.-J.; Dong, L. Efficacy and Safety of 15(R/S)-Methyl-Lipoxin A(4) in Topical Treatment of Infantile Eczema. Br. J. Dermatol. 2013, 168, 172–178. [Google Scholar] [CrossRef]
- Komulainen, M.; Saros, L.; Vahlberg, T.; Nermes, M.; Jartti, T.; Laitinen, K. Maternal Fish Oil and/or Probiotics Intervention: Allergic Diseases in Children up to Two Years Old. Pediatr. Allergy Immunol. 2023, 34, e14004. [Google Scholar] [CrossRef]
- Figueroa-Garduño, I.; Escamilla-Núñez, C.; Barraza-Villarreal, A.; Hernández-Cadena, L.; Onofre-Pardo, E.N.; Romieu, I. Docosahexaenoic Acid Effect on Prenatal Exposure to Arsenic and Atopic Dermatitis in Mexican Preschoolers. Biol. Trace Elem. Res. 2023, 201, 3152–3161. [Google Scholar] [CrossRef]
- Niseteo, T.; Hojsak, I.; Ožanić Bulić, S.; Pustišek, N. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients 2024, 16, 2829. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Polyunsaturated Fatty Acids: Conversion to Lipid Mediators, Roles in Inflammatory Diseases and Dietary Sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel Series of Biologically Active Compounds Formed from Arachidonic Acid in Human Leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Jerab, D.; Blangero, F.; Da Costa, P.C.T.; De Brito Alves, J.L.; Kefi, R.; Jamoussi, H.; Morio, B.; Eljaafari, A. Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients 2025, 17, 1253. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of Eicosapentaenoic Acid on Major Coronary Events in Hypercholesterolaemic Patients (JELIS): A Randomised Open-Label, Blinded Endpoint Analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Liang, J.; Chen, L.; Yang, S.; Zhang, H.; Li, L.; Chen, Z.; Zhang, S.; Xian, H.; Tang, Y.; Deng, Y.; et al. A 12-Hour Rapid Titration Method for Cancer Pain: A Randomized, Controlled, Open-Label Study. Ann. Palliat. Med. 2021, 10, 88–96. [Google Scholar] [CrossRef]
- The ASCEND Study Collaborative Group. Effects of N−3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Da Silva, B.G.C.; Da Silva, T.G.; Mintem, G.C.; Bielemann, R.M.; Gigante, D.P. Omega-3 Supplementation and Diabetes: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4435–4448. [Google Scholar] [CrossRef]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and Signature Profiles for Pro-Resolving and Inflammatory Lipid Mediators in Human Tissue. Am. J. Physiol. Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Serhan, C.N. Specialized Pro-Resolving Lipid Mediators in the Inflammatory Response: An Update. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Ariel, A.; Chiang, N.; Arita, M.; Petasis, N.A.; Serhan, C.N. Aspirin-Triggered Lipoxin A4 and B4 Analogs Block Extracellular Signal-Regulated Kinase-Dependent TNF-Alpha Secretion from Human T Cells. J. Immunol. 2003, 170, 6266–6272. [Google Scholar] [CrossRef] [PubMed]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting Edge: Lipoxins Rapidly Stimulate Nonphlogistic Phagocytosis of Apoptotic Neutrophils by Monocyte-Derived Macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Roach, K.M.; Feghali-Bostwick, C.A.; Amrani, Y.; Bradding, P. Lipoxin A4 Attenuates Constitutive and TGF-Β1–Dependent Profibrotic Activity in Human Lung Myofibroblasts. J. Immunol. 2015, 195, 2852–2860. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.-H.; Wu, Q.; Miao, S.; Liu, Z.-J.; Wu, P.; Ye, D.-Y. Lipoxin A4 Activates Nrf2 Pathway and Ameliorates Cell Damage in Cultured Cortical Astrocytes Exposed to Oxygen-Glucose Deprivation/Reperfusion Insults. J. Mol. Neurosci. 2015, 56, 848–857. [Google Scholar] [CrossRef]
- Levy, B.D. Myocardial 15-Epi-Lipoxin A4 Generation Provides a New Mechanism for the Immunomodulatory Effects of Statins and Thiazolidinediones. Circulation 2006, 114, 873–875. [Google Scholar] [CrossRef]
- Clària, J.; Serhan, C.N. Aspirin Triggers Previously Undescribed Bioactive Eicosanoids by Human Endothelial Cell-Leukocyte Interactions. Proc. Natl. Acad. Sci. USA 1995, 92, 9475–9479. [Google Scholar] [CrossRef]
- József, L.; Zouki, C.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. Lipoxin A4 and Aspirin-Triggered 15-Epi-Lipoxin A4 Inhibit Peroxynitrite Formation, NF-κB and AP-1 Activation, and IL-8 Gene Expression in Human Leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The Resolution Code of Acute Inflammation: Novel pro-Resolving Lipid Mediators in Resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an Endogenous Lipid Mediator Derived from Omega-3 Eicosapentaenoic Acid, Protects against 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2021, 11, 631319. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.-Y.C.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Potent Immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 Receptor Stereoselectivity and Regulation of Inflammation and Proresolving MicroRNAs. Am. J. Pathol. 2012, 180, 2018–2027. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A Docosahexaenoic Acid-Derived Docosatriene Protects Human Retinal Pigment Epithelial Cells from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef]
- Liu, W.-C.; Yang, Y.-H.; Wang, Y.-C.; Chang, W.-M.; Wang, C.-W. Maresin: Macrophage Mediator for Resolving Inflammation and Bridging Tissue Regeneration—A System-Based Preclinical Systematic Review. Int. J. Mol. Sci. 2023, 24, 11012. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel Macrophage Mediators with Potent Antiinflammatory and Proresolving Actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.; Xu, Z.; Ji, R.; Zhu, M.; Petasis, N.A. Macrophage Proresolving Mediator Maresin 1 Stimulates Tissue Regeneration and Controls Pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E. Skin Lipid Barrier: Structure, Function and Metabolism. Allergy Asthma Immunol. Res. 2024, 16, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Higuchi, K.; Okuda, M.; Ohashi, Y.; Kawamata, A. Pseudo-Acylceramide with Linoleic Acid Produces Selective Recovery of Diminished Cutaneous Barrier Function in Essential Fatty Acid-Deficient Rats and Has an Inhibitory Effect on Epidermal Hyperplasia. J. Clin. Investig. 1994, 94, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Enomoto, Y.; Endo, S.; Hariya, H.; Moriguchi, T. Omega-3 Fatty Acids Mitigate Skin Damage Caused by Ultraviolet-B Radiation. Prostaglandins Leukot. Essent. Fat. Acids 2024, 203, 102641. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef]
- Töröcsik, D.; Weise, C.; Gericke, J.; Szegedi, A.; Lucas, R.; Mihaly, J.; Worm, M.; Rühl, R. Transcriptomic and Lipidomic Profiling of Eicosanoid/Docosanoid Signalling in Affected and Non-Affected Skin of Human Atopic Dermatitis Patients. Exp. Dermatol. 2019, 28, 177–189. [Google Scholar] [CrossRef]
- Liu, M.; Diaz-Torres, S.; Mitchell, B.L.; Toledo-Flores, D.; Gharhakhani, P.; Simpson, M.A.; Zhang, H.; Ong, J.-S.; Li, J.; Rentería, M.E. The Role of Lipid Metabolism in Acne Risk: Integrating Blood Metabolite and Genetic Insights. Ski. Health Dis. 2025, 5, 124–129. [Google Scholar] [CrossRef]
- McCusker, M.M.; Grant-Kels, J.M. Healing Fats of the Skin: The Structural and Immunologic Roles of the ω-6 and ω-3 Fatty Acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef]
- De Spirt, S.; Stahl, W.; Tronnier, H.; Sies, H.; Bejot, M.; Maurette, J.-M.; Heinrich, U. Intervention with Flaxseed and Borage Oil Supplements Modulates Skin Condition in Women. Br. J. Nutr. 2008, 101, 440–445. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Aberrations in Lipid Expression and Metabolism in Psoriasis. Int. J. Mol. Sci. 2021, 22, 6561. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in Parenteral Nutrition: Biological Aspects. J. Parenter. Enter. Nutr. 2020, 44, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosapentaenoic and Docosahexaenoic Acid Derived Specialised Pro-Resolving Mediators: Concentrations in Humans and the Effects of Age, Sex, Disease and Increased Omega-3 Fatty Acid Intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Tawada, C.; Kanoh, H.; Nakamura, M.; Mizutani, Y.; Fujisawa, T.; Banno, Y.; Seishima, M. Interferon-γ Decreases Ceramides with Long-Chain Fatty Acids: Possible Involvement in Atopic Dermatitis and Psoriasis. J. Investig. Dermatol. 2014, 134, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Chen, J.; Swindell, W.R.; Tsoi, L.C.; Xing, X.; Ma, F.; Uppala, R.; Sarkar, M.K.; Plazyo, O.; Billi, A.C.; et al. Phospholipase A2 Enzymes Represent a Shared Pathogenic Pathway in Psoriasis and Pityriasis Rubra Pilaris. J. Clin. Investig. 2021, 6, e151911. [Google Scholar] [CrossRef]
- Myśliwiec, H.; Baran, A.; Harasim-Symbor, E.; Myśliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum Fatty Acid Profile in Psoriasis and Its Comorbidity. Arch. Dermatol. Res. 2017, 309, 371–380. [Google Scholar] [CrossRef]
- Marchlewicz, M.; Polakowska, Z.; Maciejewska-Markiewicz, D.; Stachowska, E.; Jakubiak, N.; Kiedrowicz, M.; Rak-Załuska, A.; Duchnik, M.; Wajs-Syrenicz, A.; Duchnik, E. Fatty Acid Profile of Erythrocyte Membranes in Patients with Psoriasis. Nutrients 2024, 16, 1799. [Google Scholar] [CrossRef]
- Kozłowska, D.; Harasim-Symbor, E.; Myśliwiec, H.; Milewska, A.; Chabowski, A.; Flisiak, I. Lipid Profile Disturbances May Predispose Psoriatic Patients to Liver Dysfunction. Adv. Dermatol. Allergol. Postępy Dermatol. I Alergol. 2021, 38, 310–318. [Google Scholar] [CrossRef]
- Serhan, C.N.; Bäck, M.; Chiurchiù, V.; Hersberger, M.; Mittendorfer, B.; Calder, P.C.; Waitzberg, D.L.; Stoppe, C.; Klek, S.; Martindale, R.G.; et al. Expert Consensus Report on Lipid Mediators: Role in Resolution of Inflammation and Muscle Preservation. FASEB J. 2024, 38, e23699. [Google Scholar] [CrossRef]
- Yang, S.-J.; Chi, C.-C. Effects of Fish Oil Supplement on Psoriasis: A Meta-Analysis of Randomized Controlled Trials. BMC Complement. Altern. Med. 2019, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Riveira-Munoz, E.; He, S.-M.; Escaramís, G.; Stuart, P.E.; Hüffmeier, U.; Lee, C.; Kirby, B.; Oka, A.; Giardina, E.; Liao, W.; et al. Meta-Analysis Confirms the LCE3C_LCE3B Deletion as a Risk Factor for Psoriasis in Several Ethnic Groups and Finds Interaction with HLA-Cw6. J. Investig. Dermatol. 2011, 131, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dou, Y.; Lin, Y.; Chu, G.; Wang, J.; Ma, L. HMGB1 Regulates Th17 Cell Differentiation and Function in Patients with Psoriasis. Immun. Inflamm. Dis. 2024, 12, e1205. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.S.L.E.; Bloise, G.; Moltrasio, C.; Coelho, A.; Agrelli, A.; Moura, R.; Tricarico, P.M.; Jamain, S.; Marzano, A.V.; Crovella, S.; et al. Transcriptome Meta-Analysis Confirms the Hidradenitis Suppurativa Pathogenic Triad: Upregulated Inflammation, Altered Epithelial Organization, and Dysregulated Metabolic Signaling. Biomolecules 2022, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Alavi, A.; Wolk, K.; Wortsman, X.; McGrath, B.; Garg, A.; Szepietowski, J.C. Hidradenitis Suppurativa. Lancet 2025, 405, 420–438. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Matusiak, Ł.; Von Stebut, E.; Schultheis, M.; Kirschner, U.; Nikolakis, G.; Szepietowski, J.C. Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life 2021, 11, 34. [Google Scholar] [CrossRef]
- Gold, D.A.; Reeder, V.J.; Mahan, M.G.; Hamzavi, I.H. The Prevalence of Metabolic Syndrome in Patients with Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2014, 70, 699–703. [Google Scholar] [CrossRef]
- Dany, M.; Elston, D. Gene Expression of Sphingolipid Metabolism Pathways Is Altered in Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2017, 77, 268–273.e6. [Google Scholar] [CrossRef]
- Choi, E.; Mir, S.A.; Ji, S.; Ooi, X.T.; Chua, E.W.L.; Wei, Y.Y.; Wenk, M.R.; Bendt, A.K.; Chandran, N.S. Understanding the Systemic Burden of Disease in Hidradenitis Suppurativa from Plasma Lipidomic Analysis. J. Dermatol. Sci. 2022, 107, 133–141. [Google Scholar] [CrossRef]
- Penno, C.A.; Jäger, P.; Laguerre, C.; Hasler, F.; Hofmann, A.; Gass, S.K.; Wettstein-Ling, B.; Schaefer, D.J.; Avrameas, A.; Raulf, F.; et al. Lipidomics Profiling of Hidradenitis Suppurativa Skin Lesions Reveals Lipoxygenase Pathway Dysregulation and Accumulation of Proinflammatory Leukotriene B4. J. Investig. Dermatol. 2020, 140, 2421–2432.e10. [Google Scholar] [CrossRef]
- Geng, R.; Sibbald, R.G. Acne Vulgaris: Clinical Aspects and Treatments. Adv. Ski. Wound Care 2024, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, A.H.; Masood, S.; Saleh, H.M.; Schlessinger, J. Acne Vulgaris. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Smith, R.N.; Mann, N.J.; Braue, A.; Mäkeläinen, H.; Varigos, G.A. A Low-Glycemic-Load Diet Improves Symptoms in Acne Vulgaris Patients: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2007, 86, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne Vulgaris: A Review of the Pathophysiology, Treatment, and Recent Nanotechnology Based Advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthet. Dermatol. 2013, 6, 27–35. [Google Scholar] [PubMed]
- Jeremy, A.H.T.; Holland, D.B.; Roberts, S.G.; Thomson, K.F.; Cunliffe, W.J. Inflammatory Events Are Involved in Acne Lesion Initiation. J. Investig. Dermatol. 2003, 121, 20–27. [Google Scholar] [CrossRef]
- Leyden, J.J.; McGinley, K.J.; Mills, O.H.; Kligman, A.M. Propionibacterium Levels In Patients With And Without Acne Vulgaris. J. Investig. Dermatol. 1975, 65, 382–384. [Google Scholar] [CrossRef]
- Sobhan, M.; Seif Rabiei, M.A.; Amerifar, M. Correlation Between Lipid Profile and Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2020, 13, 67–71. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.Y.; Zhou, L.; Lu, B.; Lin, Y.; Huang, X.; Wei, B.; Wang, Q.; Wang, L.; Lu, J. Acne Patients Frequently Associated with Abnormal Plasma Lipid Profile. J. Dermatol. 2015, 42, 296–299. [Google Scholar] [CrossRef]
- Gokdemir, G.S.; Alp, S.; Nas, C.; Ecevit, H. Evaluation of the Relationship between Lipid Profile and Inflammatory Parameters in Patients with Acne Vulgaris. J. Med. Dent. Investig. 2024, 5, e240384. [Google Scholar] [CrossRef]
- Kelhälä, H.-L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 Pathway Is Activated in Acne Lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium Acnes Promotes Th17 and Th17/Th1 Responses in Acne Patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef]
- Fasseeh, A.N.; Elezbawy, B.; Korra, N.; Tannira, M.; Dalle, H.; Aderian, S.; Abaza, S.; Kaló, Z. Burden of Atopic Dermatitis in Adults and Adolescents: A Systematic Literature Review. Dermatol. Ther. 2022, 12, 2653–2668. [Google Scholar] [CrossRef]
- Mirrahimi, B.; Moazemi, M.; Eslami, N.; Jamshidi, E.; Mir, M.; Mohebbi, R.; Esmaily, H. Evaluating the Effect of Eicosapentaenoic Acid in Children With Atopic Dermatitis: A Randomized Triple-Blind Clinical Trial. J. Pediatr. Pharmacol. Ther. 2023, 28, 29–35. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar] [PubMed]
- Fania, L.; Moretta, G.; Antonelli, F.; Scala, E.; Abeni, D.; Albanesi, C.; Madonna, S. Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Resolution of Chronic Inflammation, Restoration of Epigenetic Disturbances and Correction of Dysbiosis as an Adjunctive Approach to the Treatment of Atopic Dermatitis. Cells 2024, 13, 1899. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, Y.; Wang, H.; Jiang, H. Effect of Prenatal Omega-3 Polyunsaturated Fatty Acid Supplementation on Childhood Eczema: A Systematic Review and Meta-Analysis. Int. Arch. Allergy Immunol. 2023, 184, 21–32. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Biełach-Bazyluk, A.; Jakubowicz-Zalewska, O.; Myśliwiec, H.; Flisiak, I. Specialized Pro-Resolving Lipid Mediators and Dietary Omega-3/6 Fatty Acids in Selected Inflammatory Skin Diseases: A Systematic Review. Antioxidants 2026, 15, 9. https://doi.org/10.3390/antiox15010009
Biełach-Bazyluk A, Jakubowicz-Zalewska O, Myśliwiec H, Flisiak I. Specialized Pro-Resolving Lipid Mediators and Dietary Omega-3/6 Fatty Acids in Selected Inflammatory Skin Diseases: A Systematic Review. Antioxidants. 2026; 15(1):9. https://doi.org/10.3390/antiox15010009
Chicago/Turabian StyleBiełach-Bazyluk, Angelika, Olivia Jakubowicz-Zalewska, Hanna Myśliwiec, and Iwona Flisiak. 2026. "Specialized Pro-Resolving Lipid Mediators and Dietary Omega-3/6 Fatty Acids in Selected Inflammatory Skin Diseases: A Systematic Review" Antioxidants 15, no. 1: 9. https://doi.org/10.3390/antiox15010009
APA StyleBiełach-Bazyluk, A., Jakubowicz-Zalewska, O., Myśliwiec, H., & Flisiak, I. (2026). Specialized Pro-Resolving Lipid Mediators and Dietary Omega-3/6 Fatty Acids in Selected Inflammatory Skin Diseases: A Systematic Review. Antioxidants, 15(1), 9. https://doi.org/10.3390/antiox15010009







