Antioxidant and Photoprotective Activities of Viola philippica Polyol Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Polyol Extracts from Viola philippica
2.3. Total Phenolic and Flavonoid Content Determination
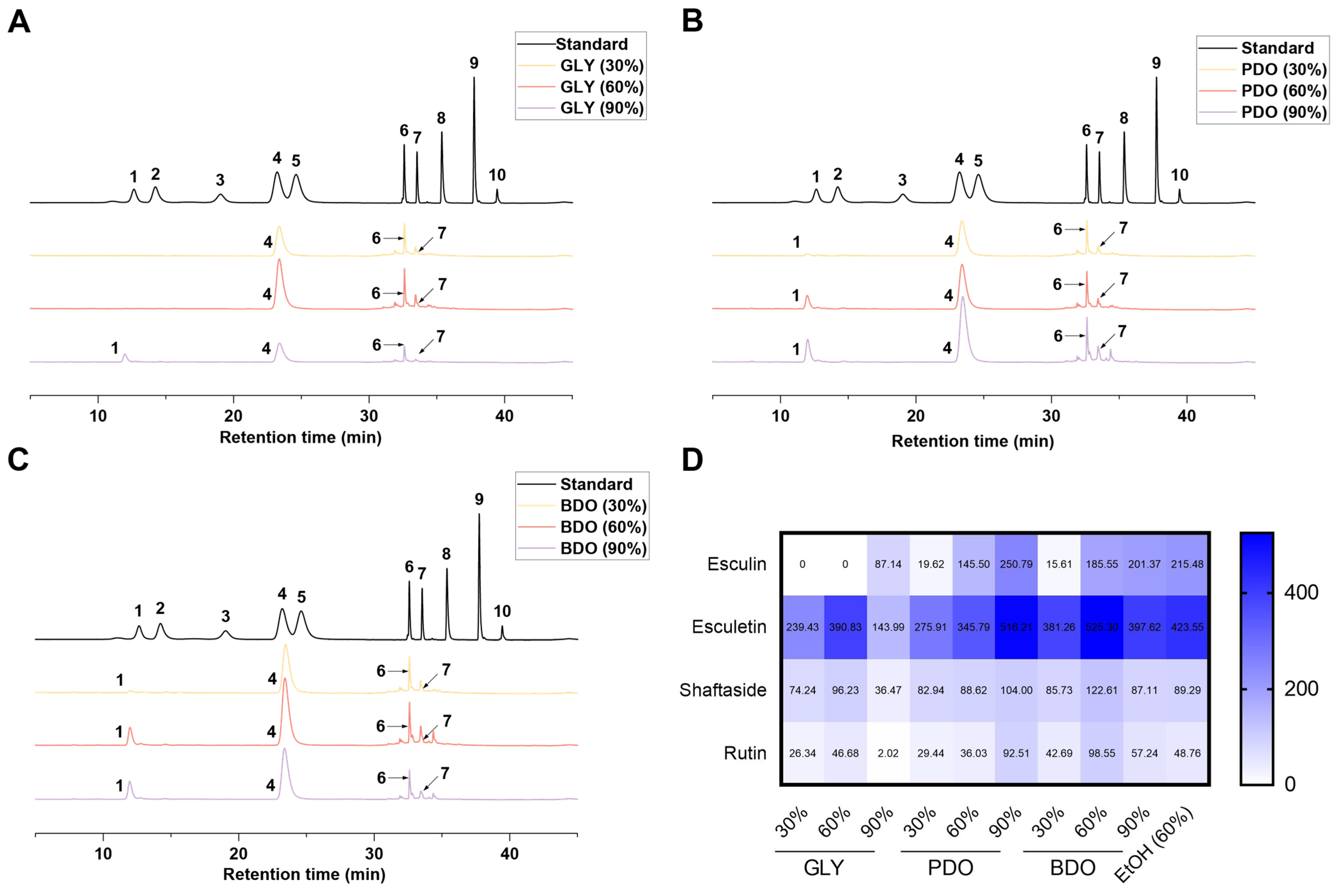
2.4. Analysis of Active Components in VP Extracts by HPLC and LC-MS
2.5. Determination of Antioxidant Potential
2.5.1. DPPH Free Radical Scavenging
2.5.2. ABTS Free Radical Scavenging
2.5.3. OH Scavenging
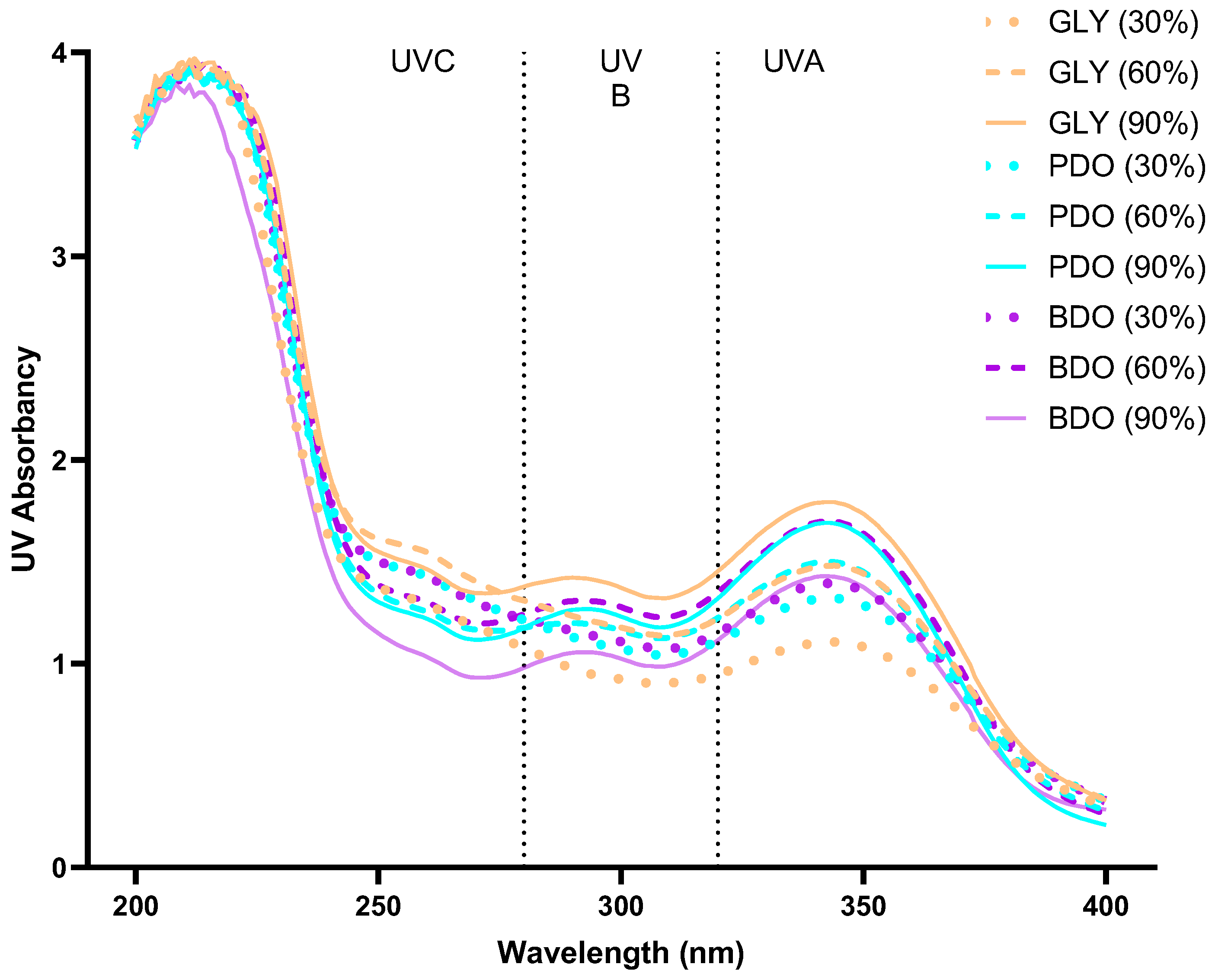
2.6. Ultraviolet Wavelength Scanning of VP Extracts
2.7. Cellular Analysis of VP Extracts
2.7.1. Cell Culture and Treatment
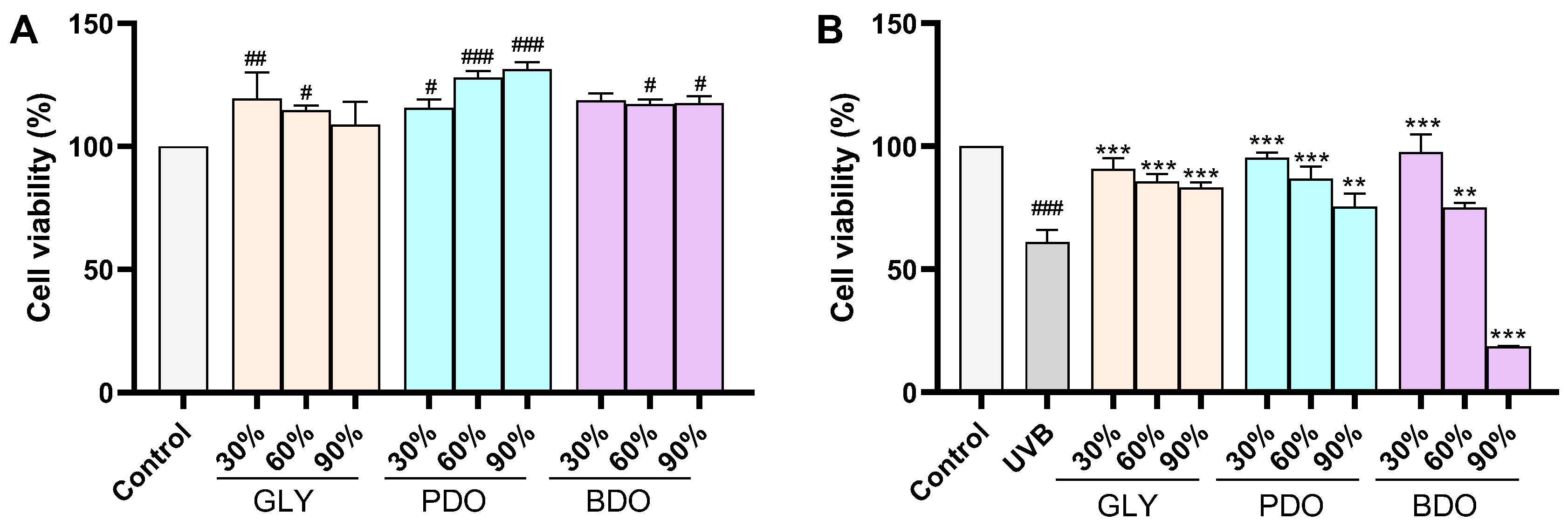
2.7.2. Cell Viability
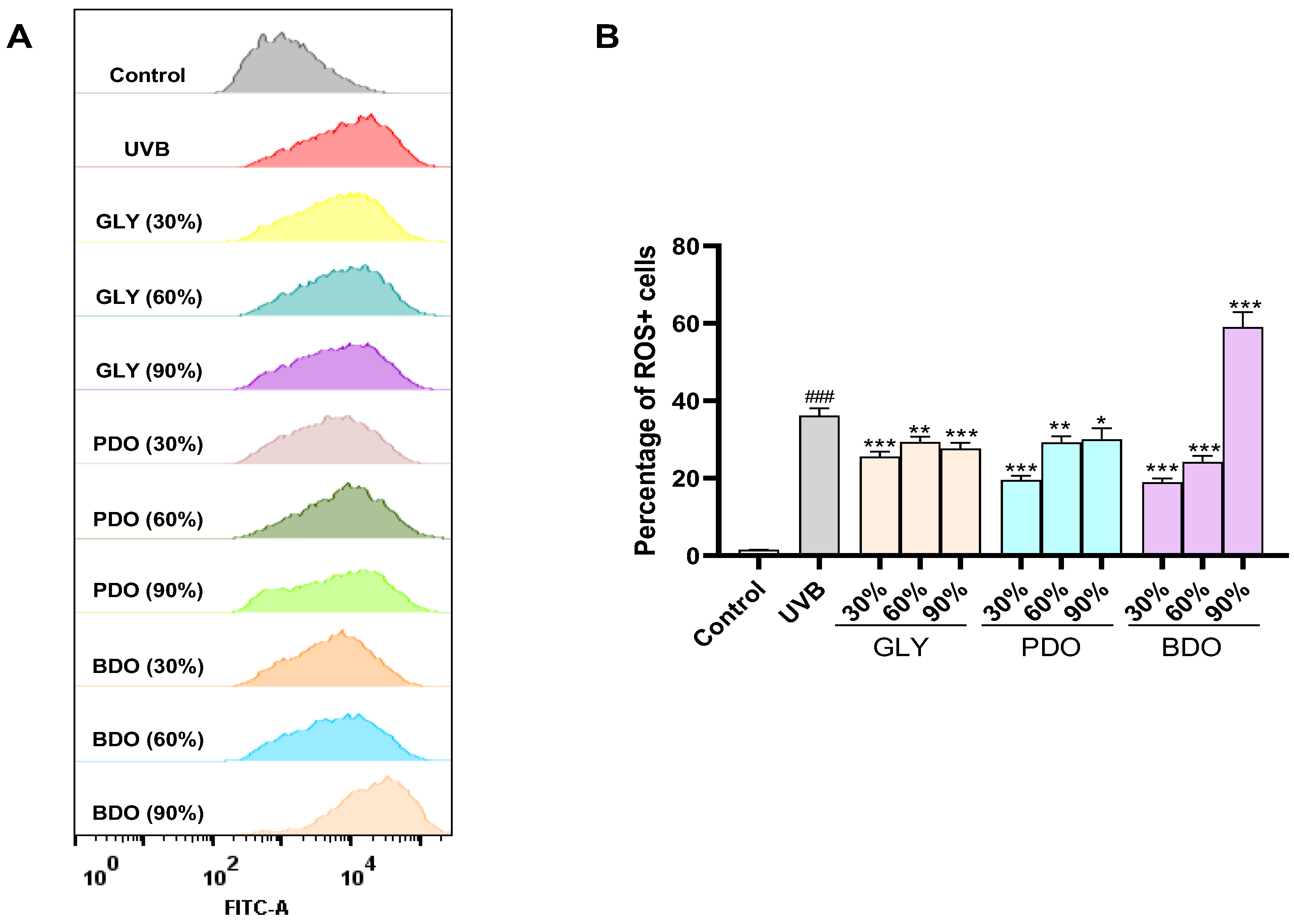
2.7.3. VP ROS Assay
2.7.4. Comet Assay
2.8. RNA-Seq
2.9. Data Analysis
3. Results
3.1. Analysis of Total Phenolic and Total Flavonoid Content of VP Polyol Extracts
3.2. Quantitative Analysis of Active Compounds in VP Extracts via HPLC
3.3. Free Radical Scavenging Ability of VP Extracts
3.4. Effect of Ultraviolet Wavelength Absorption by Extracts of VP
3.5. Protection Against UVB-Induced HaCaT Cell Damage by VP Extracts
3.5.1. Effect of VP Polyol Extracts on the Activity of UVB-Induced HaCaT Cells
3.5.2. Effect of VP Polyol Extracts on UVB-Induced Intracellular ROS in HaCaT Cells
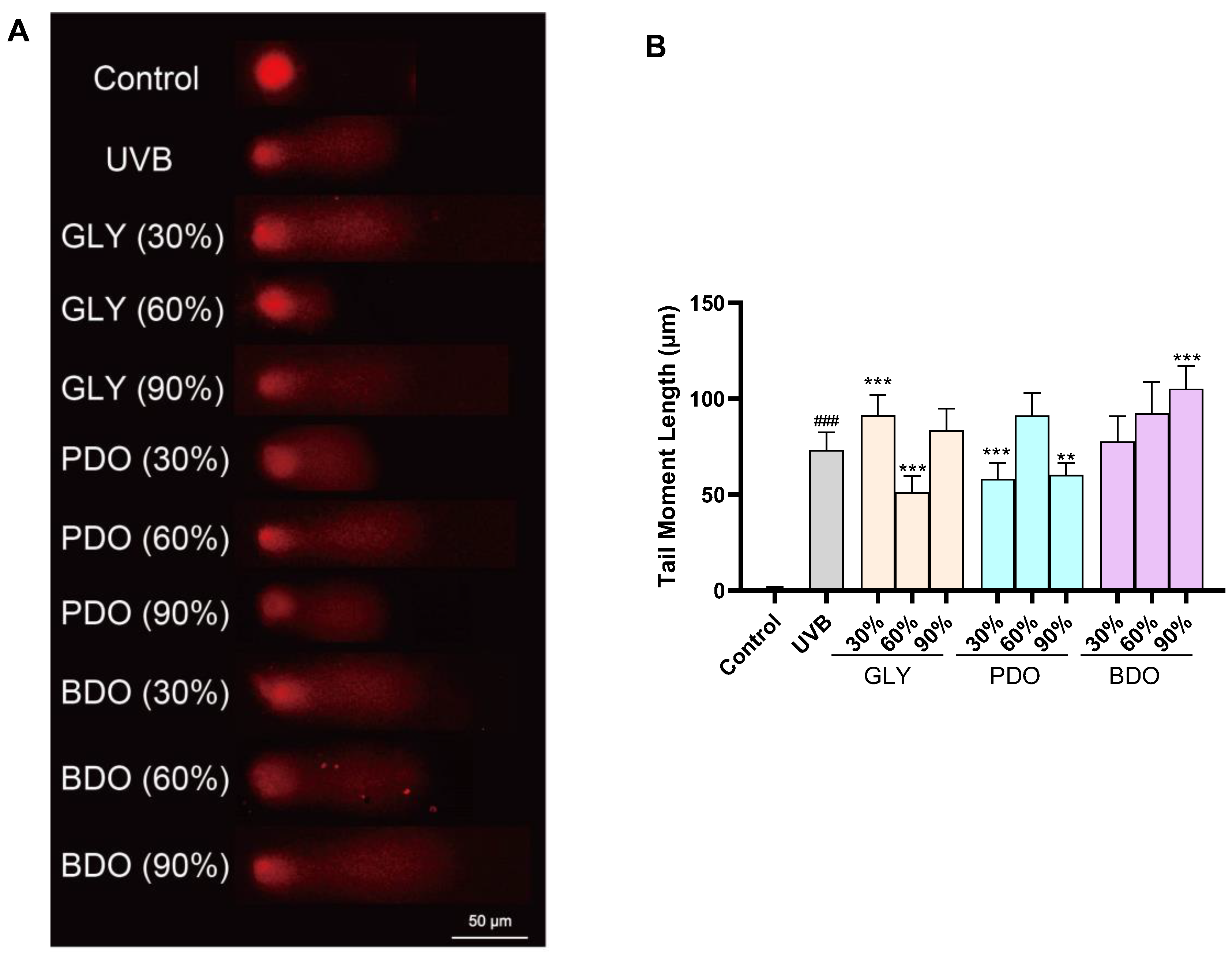
3.5.3. Effect of VP Polyol Extracts on UVB-Induced DNA Damage in HaCaT Cells
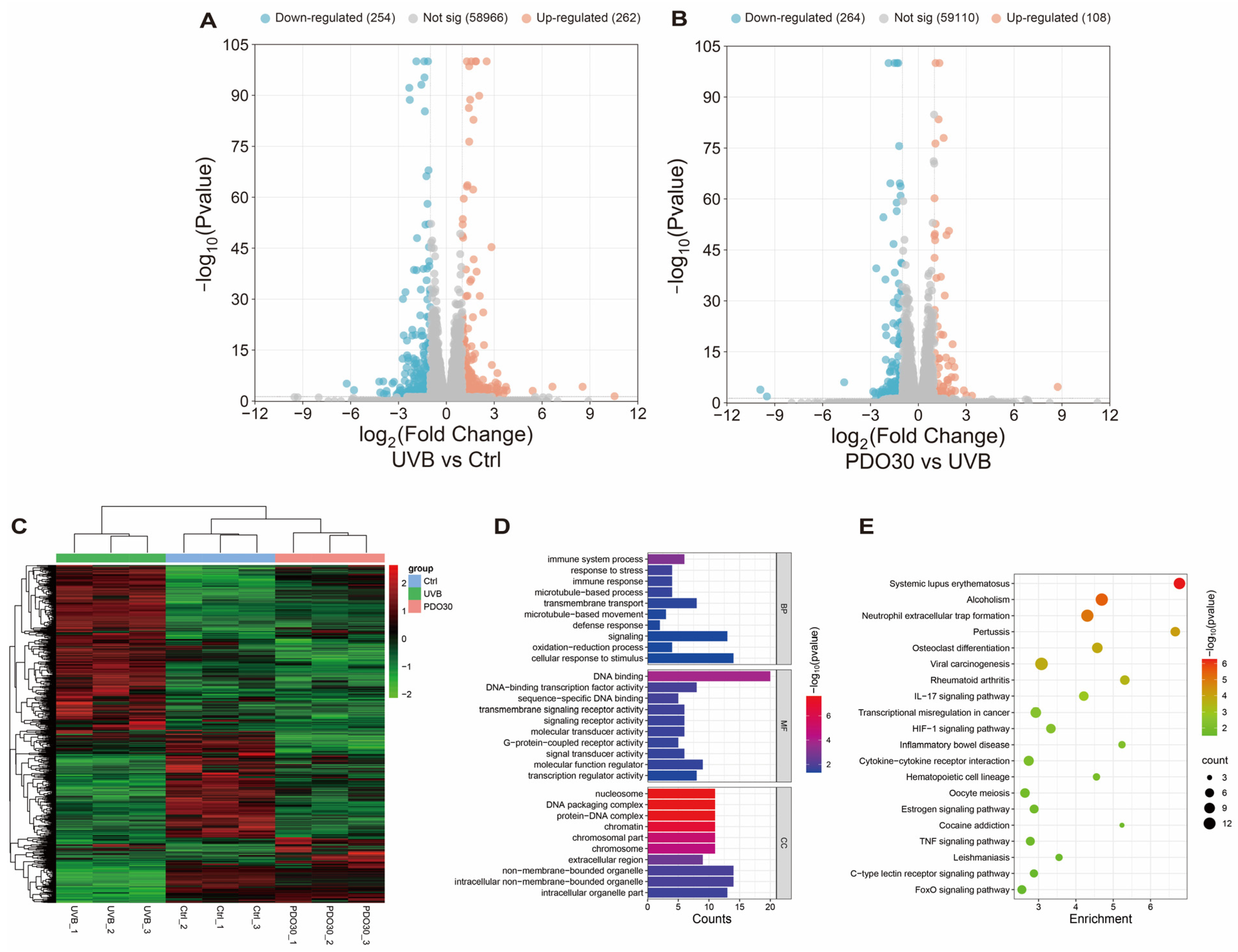
3.6. RNA-Seq Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Wang, Y.; Wu, C.; Ye, T.; Xia, H.; Huang, R.; Deng, J.; Li, Z.; Huang, Y.; et al. Recombinant filaggrin-2 improves skin barrier function and attenuates ultraviolet B (UVB) irradiation-induced epidermal barrier disruption. Int. J. Biol. Macromol. 2024, 281, 136064. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.A.H.; Abdik, E.A.; Abdik, H.; Aydın, S.; Şahin, F.; Taşlı, P.N. Zooming in across the Skin: A Macro-to-Molecular Panorama. Adv. Exp. Med. Biol. 2020, 1247, 157–200. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Molecular insights of human skin epidermal and dermal aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Skin. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Brüggen, M.C.; Stingl, G. Subcutaneous white adipose tissue: The deepest layer of the cutaneous immune barrier. J. Dtsch. Dermatol. Ges. 2020, 18, 1225–1227. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, R.; Xiong, Z.; Feng, Y.; Chen, L. Dysregulation of autophagy during photoaging reduce oxidative stress and inflammatory damage caused by UV. Front. Pharmacol. 2025, 16, 1562845. [Google Scholar] [CrossRef]
- Al-Sadek, T.; Yusuf, N. Ultraviolet Radiation Biological and Medical Implications. Curr. Issues Mol. Biol. 2024, 46, 1924–1942. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postep. Dermatol. Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Karekezi, J.; Nugroho, T.A.; Ndahigwa, E.N.; Choi, Y.J.; Kim, H.; Kim, H.J.; Park, S.W. Oyster hydrolysate ameliorates UVB-induced skin dehydration and barrier dysfunction. Life Sci. 2024, 358, 123149. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Q.; Lu, Y. Magnolia biondii flower extract attenuates UVB-induced skin damage through high-mobility group box protein B1. Int. J. Cosmet. Sci. 2024, 46, 775–785. [Google Scholar] [CrossRef]
- Campiche, R.; Sandau, P.; Kurth, E.; Massironi, M.; Imfeld, D.; Schuetz, R. Protective effects of an extract of the freshwater microalga Scenedesmus rubescens on UV-irradiated skin cells. Int. J. Cosmet. Sci. 2018, 40, 187–192. [Google Scholar] [CrossRef]
- Mansoor, K.; Aburjai, T.; Al-Mamoori, F.; Schmidt, M. Plants with cosmetic uses. Phytother. Res. 2023, 37, 5755–5768. [Google Scholar] [CrossRef]
- Mao, Y.; Kong, X.; Liang, Z.; Yang, C.; Wang, S.; Fan, H.; Ning, C.; Xiao, W.; Wu, Y.; Wu, J.; et al. Viola yedoensis Makino alleviates heat stress-induced inflammation, oxidative stress, and cell apoptosis in the spleen and thymus of broilers. J. Ethnopharmacol. 2024, 319, 117350. [Google Scholar] [CrossRef]
- Zeng, G.; Li, X.; Zhao, C.; Pang, Y.; Luo, X.; Tang, Z. Proximate Composition and In Vitro Bioactive Properties of Leaf Extracts from Seven Viola Species. Foods 2025, 14, 302. [Google Scholar] [CrossRef]
- Fan, P.; Yang, Y.; Liu, T.; Lu, X.; Huang, H.; Chen, L.; Kuang, Y. Anti-atopic effect of Viola yedoensis ethanol extract against 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin dysfunction. J. Ethnopharmacol. 2021, 280, 114474. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, J.; Yuan, Z.; Wang, A.; Liu, X.; Chen, Y.; Zhang, M.; Gao, Y.; Zhang, H.; Liu, Y. Bioactive compound schaftoside from Clinacanthus nutans attenuates acute liver injury by inhibiting ferroptosis through activation the Nrf2/GPX4 pathway. J. Ethnopharmacol. 2024, 328, 118135. [Google Scholar] [CrossRef]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Clementi, M.E. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Núñez, S.; López, V.; Moliner, C.; Valero, M.S.; Gómez-Rincón, C. Lipid lowering and anti-ageing effects of edible flowers of Viola x wittrockiana Gams in a Caenorhabditis elegans obese model. Food Funct. 2023, 14, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.; Bhatt, S.; Jain, N. Extraction, Phytochemical Screening and Antioxidant Potential of Hydroalcoholic Extract of Viola odorata Linn. J. Pharm. Res. Int. 2021, 33, 673–680. [Google Scholar] [CrossRef]
- Dhiman, S.; Singla, S.; Kumar, I.; Palia, P.; Kumar, P.; Goyal, S. Protection of Viola odorata L. against Neurodegenerative Diseases: Potential of the Extract and Major Phytoconstituents. Clin. Complement. Med. Pharmacol. 2023, 3, 100105. [Google Scholar] [CrossRef]
- Thonthula, S.; Sousa, S.D.; Dubuis, A.; Boudah, S.; Mehta, R.; Singh, A.; Eilstein, J.; Tabet, J.-C.; John, S.; Roy, D.; et al. Improved Skin Barrier Function Along with Hydration Benefits of Viola yedoensis Extract, Aesculin, and Schaftoside and LC-HRMS/MS Dereplication of Its Bio-Active Components. Int. J. Mol. Sci. 2024, 25, 12770. [Google Scholar] [CrossRef]
- Sikandar, T.; Anum Bhatti, A.; Mushtaq, Z.; Shahid, M.; Nighat, F.; Anwar, S. Evaluation of the bioactive role of indegenous Viola odorata leaf extracts. Pak. J. Bot. 2021, 53, 541–549. [Google Scholar] [CrossRef]
- Nekoukar, Z.; Zakariaei, Z.; Taghizadeh, F.; Musavi, F.; Banimostafavi, E.S.; Sharifpour, A.; Ebrahim Ghuchi, N.; Fakhar, M.; Tabaripour, R.; Safanavaei, S. Methanol poisoning as a new world challenge: A review. Ann. Med. Surg. 2021, 66, 102445. [Google Scholar] [CrossRef]
- Gina, M.; Ofenloch, R.; Schwebke, I.; Hübner, N.-O.; Brüning, T.; Fartasch, M. The Effect of Alcohol-Based Virucidal Hand Sanitizers on Skin Barrier Function—A Randomised Experimental Study. Contact Dermat. 2025, 93, 119–130. [Google Scholar] [CrossRef]
- Lim, M.W.; Quan Tang, Y.; Aroua, M.K.; Gew, L.T. Glycerol Extraction of Bioactive Compounds from Thanaka (Hesperethusa crenulata) Bark through LCMS Profiling and Their Antioxidant Properties. ACS Omega 2024, 9, 14388–14405. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Jabłonowska, M.; Ciganović, P.; Jablan, J.; Marguí, E.; Tomczyk, M.; Zovko Končić, M. Silybum marianum glycerol extraction for the preparation of high-value anti-ageing extracts. Ind. Crops Prod. 2021, 168, 113613. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Lin, L.-H. The liquid polyol extracts of camellia seed dregs used in sunscreen cosmetics. Chem. Pap. 2019, 73, 501–508. [Google Scholar] [CrossRef]
- Myo, H.; Buathong, N.; Saelee, M.; Saewan, N.; Khat-Udomkiri, N. Enhancement of bioactive compound extraction from Rhus chinensis leaves using the polyol-based ultrasound-assisted method: Antioxidant, anti-melanogenesis, and pollution defense properties. Ind. Crops Prod. 2025, 233, 121414. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of Total Polysaccharides and Total Flavonoids in Chrysanthemum morifolium Using Near-Infrared Hyperspectral Imaging and Multivariate Analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Q.; Gao, Z.; Bianca Xu, G.; Chen, H.; Chitrakar, B.; Sun, Y.; Zhao, W.; Lin, X.; Zhou, K.; et al. Characterization of procyanidin extracts from hawthorn (Crataegus pinnatifida) in human colorectal adenocarcinoma cell line Caco-2, simulated Digestion, and fermentation identified unique and novel prebiotic properties. Food Res. Int. 2023, 165, 112393. [Google Scholar] [CrossRef]
- Myat Win, S.; Saelee, M.; Myo, H.; Khat-Udomkiri, N. Microwave-Assisted Extraction of Phenolic Compounds and Antioxidants for Cosmetic Applications Using Polyol-Based Technology. J. Vis. Exp. 2024, 210, e67033. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Lin, L.-H. DPPH scavenging capacity of extracts from Camellia seed dregs using polyol compounds as solvents. Heliyon 2019, 5, e02315. [Google Scholar] [CrossRef] [PubMed]
- Myo, H.; Khat-udomkiri, N. Optimizing ultrasound-assisted extraction of bioactive compounds from Canthium horridum blume leaves utilizing polyols: A study on skin-related activities. Heliyon 2024, 10, e31150. [Google Scholar] [CrossRef]
- Opriş, O.; Soran, M.-L.; Lung, I.; Stegarescu, A.; Guţoiu, S.; Podea, R.; Podea, P. Optimization of extraction conditions of polyphenols, antioxidant capacity and sun protection factor from Prunus spinosa fruits. Application in sunscreen formulation. J. Iran. Chem. Soc. 2021, 18, 2625–2636. [Google Scholar] [CrossRef]
- Pattanakitjaroenchai, S.; Pitsawong, P.; Khat-Udomkiri, N. Exploration of cosmetic bioactive compounds from cocoa bean shell using polyol-based microwave-assisted extraction: Cytotoxicity, anti-tyrosinase, and anti-melanogenesis properties. Curr. Res. Green Sustain. Chem. 2025, 10, 100454. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takahashi, T.; Katsube, T.; Kudo, A.; Kuramitsu, O.; Ishiwata, M.; Matsumoto, S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 2013, 141, 552–556. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. Int. Sch. Res. Not. Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Borja-Martínez, M.; Pedreño, M.A.; Sabater-Jara, A.B. Broccoli Byproduct Extracts Attenuate the Expression of UVB-Induced Proinflammatory Cytokines in HaCaT Keratinocytes. Antioxidants 2024, 13, 1479. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Mo, Q.; He, Y.; Wang, C.; Wang, D.; Li, M. Forsythiaside A repairs UVB-induced skin inflammatory damage by IL-17 signaling pathway. Food Biosci. 2025, 64, 105845. [Google Scholar] [CrossRef]
- Boutin, A.T.; Weidemann, A.; Fu, Z.; Mesropian, L.; Gradin, K.; Jamora, C.; Wiesener, M.; Eckardt, K.U.; Koch, C.J.; Ellies, L.G.; et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 2008, 133, 223–234. [Google Scholar] [CrossRef]
- Nys, K.; Maes, H.; Andrei, G.; Snoeck, R.; Garmyn, M.; Agostinis, P. Skin mild hypoxia enhances killing of UVB-damaged keratinocytes through reactive oxygen species-mediated apoptosis requiring Noxa and Bim. Free Radic. Biol. Med. 2012, 52, 1111–1120. [Google Scholar] [CrossRef]
- Faßbender, S.; Sondenheimer, K.; Majora, M.; Schindler, J.; Opitz, F.V.; Pollet, M.; Haarmann-Stemmann, T.; Krutmann, J.; Weighardt, H. Keratinocytes Counteract UVB-Induced Immunosuppression in Mice through HIF-1a Signaling. J. Investig. Dermatol. 2022, 142, 1183–1193. [Google Scholar] [CrossRef]
| VP Extracts | TPC (mg GAEs/g) | TFC (mg REs/g) |
|---|---|---|
| EtOH (60%) | 63.58 ± 0.05 | 43.33 ± 0.07 |
| GLY (30%) | 57.25 ± 0.19 *** | 31.77 ± 0.07 *** |
| GLY (60%) | 64.75 ± 0.45 ** | 46.68 ± 0.27 *** |
| GLY (90%) | 34.73 ± 0.05 *** | 26.68 ± 0.32 *** |
| PDO (30%) | 61.09 ± 0.09 *** | 35.12 ± 0.07 *** |
| PDO (60%) | 66.53 ± 0.69 *** | 39.71 ± 0.34 *** |
| PDO (90%) | 71.45 ± 0.17 *** | 40.62 ± 0.22 *** |
| BDO (30%) | 64.57 ± 0.02 * | 34.72 ± 0.29 *** |
| BDO (60%) | 71.28 ± 0.59 *** | 40.65 ± 0.19 *** |
| BDO (90%) | 46.23 ± 0.05 *** | 32.72 ± 0.14 *** |
| VP Extracts | DPPH Radical IC50 (mg/mL) | ABTS Radical IC50 (mg/mL) | OH· Radical IC50 (mg/mL) |
|---|---|---|---|
| Vc (μM) | 20.34 ± 0.1 | 18.12 ± 0.6 | 359.15 ± 0.36 |
| EtOH (60%) | 0.4 ± 0.06 | 0.11 ± 0.01 | 181.6 ± 5.23 |
| GLY (30%) | 0.64 ± 0.03 | 0.11 ± 0.01 | 1.71 ± 0.17 |
| GLY (60%) | 0.49 ± 0.05 | 0.1 ± 0.01 | 3.65 ± 0.23 |
| GLY (90%) | 1.26 ± 0.07 | 0.11 ± 0.01 | 3.62 ± 0.42 |
| PDO (30%) | 0.6 ± 0.01 | 0.12 ± 0.01 | 1.58 ± 0.01 |
| PDO (60%) | 0.7 ± 0.01 | 0.12 ± 0.01 | 4.19 ± 1.21 |
| PDO (90%) | 0.5 ± 0.02 | 0.12 ± 0.01 | 339.45 ± 0.92 |
| BDO (30%) | 0.89 ± 0.04 | 0.12 ± 0.01 | 1.94 ± 0.16 |
| BDO (60%) | 0.56 ± 0.02 | 0.1 ± 0.01 | 5.95 ± 0.53 |
| BDO (90%) | 0.61 ± 0.03 | 0.19 ± 0.03 | 460.95 ± 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, J.; Li, Y.; Luo, L.; Zhang, W.; Tian, Y.; Tian, Y.; Li, Y.; Wang, Z.; Wu, M. Antioxidant and Photoprotective Activities of Viola philippica Polyol Extracts. Antioxidants 2025, 14, 884. https://doi.org/10.3390/antiox14070884
Li J, Ma J, Li Y, Luo L, Zhang W, Tian Y, Tian Y, Li Y, Wang Z, Wu M. Antioxidant and Photoprotective Activities of Viola philippica Polyol Extracts. Antioxidants. 2025; 14(7):884. https://doi.org/10.3390/antiox14070884
Chicago/Turabian StyleLi, Jiang, Jiancheng Ma, Ya Li, Lan Luo, Wenhuan Zhang, Yong Tian, Yuncai Tian, Yi Li, Zhongjuan Wang, and Mingyi Wu. 2025. "Antioxidant and Photoprotective Activities of Viola philippica Polyol Extracts" Antioxidants 14, no. 7: 884. https://doi.org/10.3390/antiox14070884
APA StyleLi, J., Ma, J., Li, Y., Luo, L., Zhang, W., Tian, Y., Tian, Y., Li, Y., Wang, Z., & Wu, M. (2025). Antioxidant and Photoprotective Activities of Viola philippica Polyol Extracts. Antioxidants, 14(7), 884. https://doi.org/10.3390/antiox14070884





