Depolymerization and Nanoliposomal Encapsulation of Grape Seed Condensed Tannins: Physicochemical Characterization, Stability, In Vitro Release and Bioaccessibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction and Characterization of Depolymerized Grape Seed Condensed Tannins
2.2.1. Chemical Depolymerization of Grape Seed Condensed Tannins
2.2.2. Chromatographic Analysis Using Ultra-High-Performance Liquid Chromatography (UHPLC)
2.2.3. Structural Free Radical Scavenging Activity
2.2.4. Total Polyphenol Content (TPC)
2.3. Obtaining and Characterizing Nanoliposomal Suspensions That Encapsulate Depolymerized Grape Seed Tannins (LTD)
2.3.1. Preparation of Nanoliposomal Suspensions
2.3.2. Encapsulation Efficiency (%EE) and Loaded Capacity (%LC)
2.3.3. Hydrodynamic Particle Diameter, Polydispersity Index, and Zeta Potential
2.3.4. Morphological Properties and Microstructure
2.3.5. Rheological Properties
2.3.6. LTD Suspension Stability
2.4. In Vitro Release of Nanoliposomal Suspensions Encapsulating Depolymerized Seed Tannins (LTD) in a Food Simulant
2.5. Bioaccessibility of Liposomal Suspensions Encapsulating Depolymerized Grape Seed Condensed Tannins (LTD)
2.6. Statistical Analysis
3. Results
3.1. Results of Obtaining and Characterizing Depolymerized Grape Seed Condensed Tannins
3.2. Results of Obtaining and Characterizing Nanoliposomal Suspensions Encapsulated Depolymerized Condensed Tannins
3.2.1. Results of Physicochemical Parameters of Liposomal Suspensions
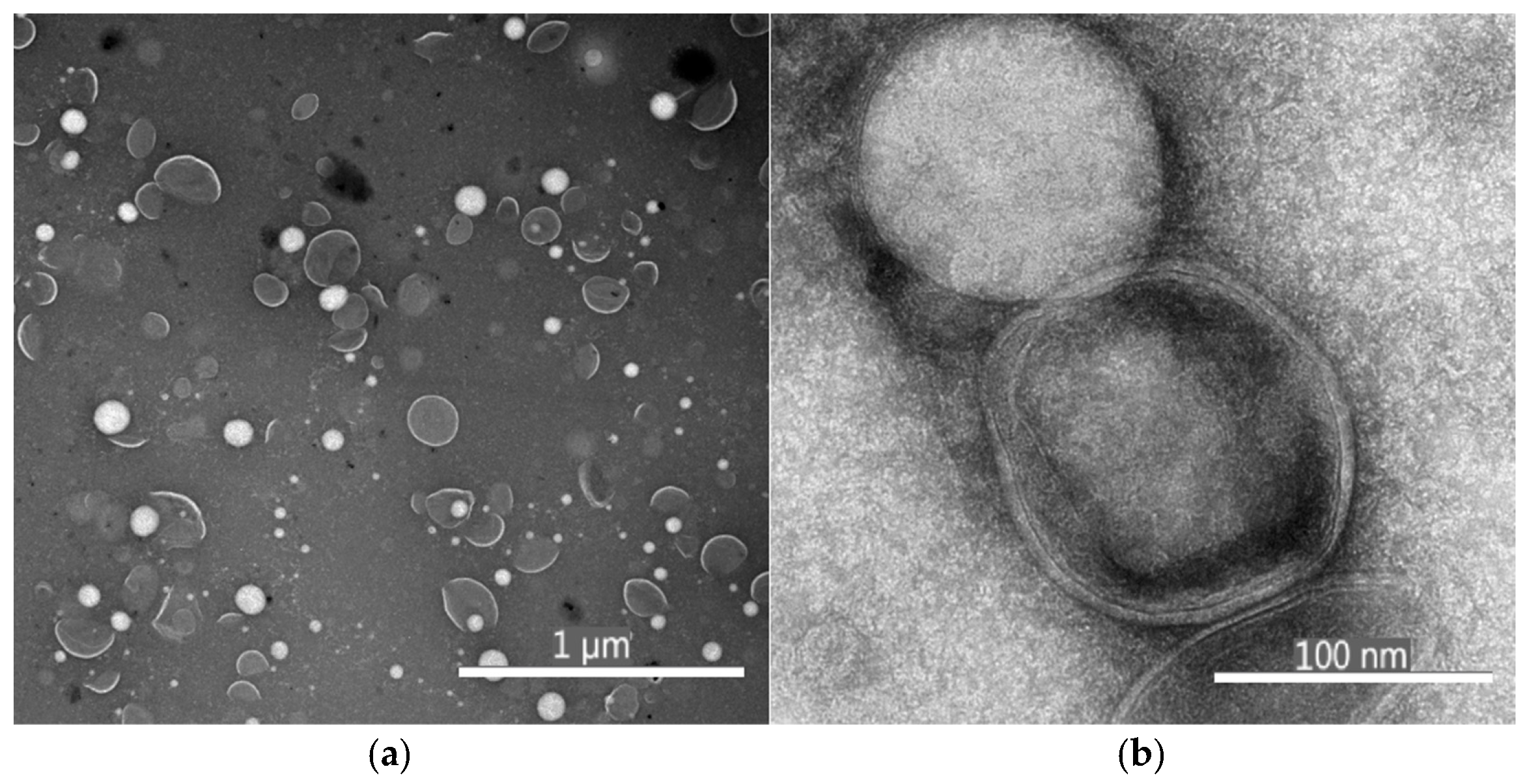
3.2.2. Results of Morphological Properties and Microstructure
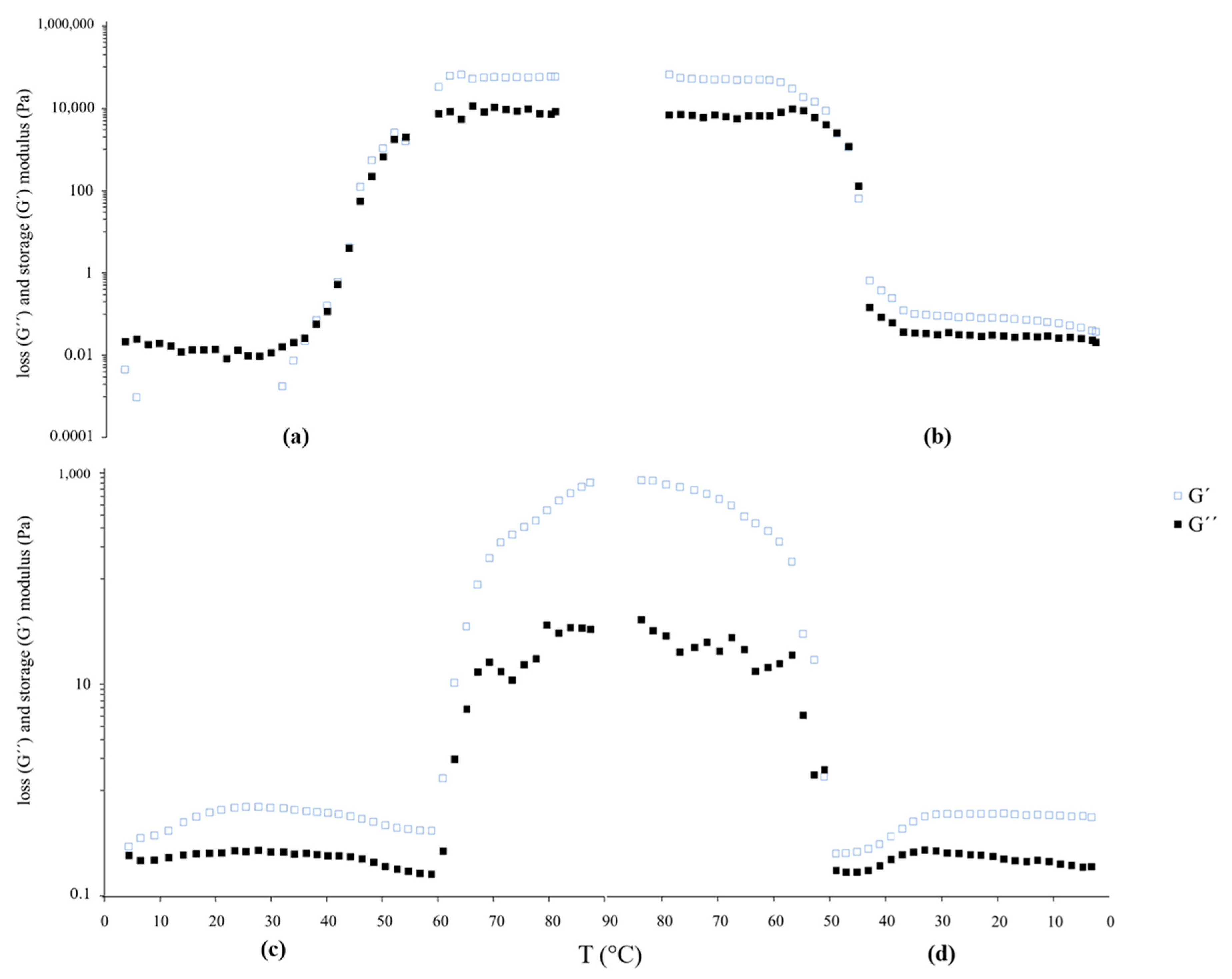
3.2.3. Results of the Rheological Behavior of Liposomal Suspensions
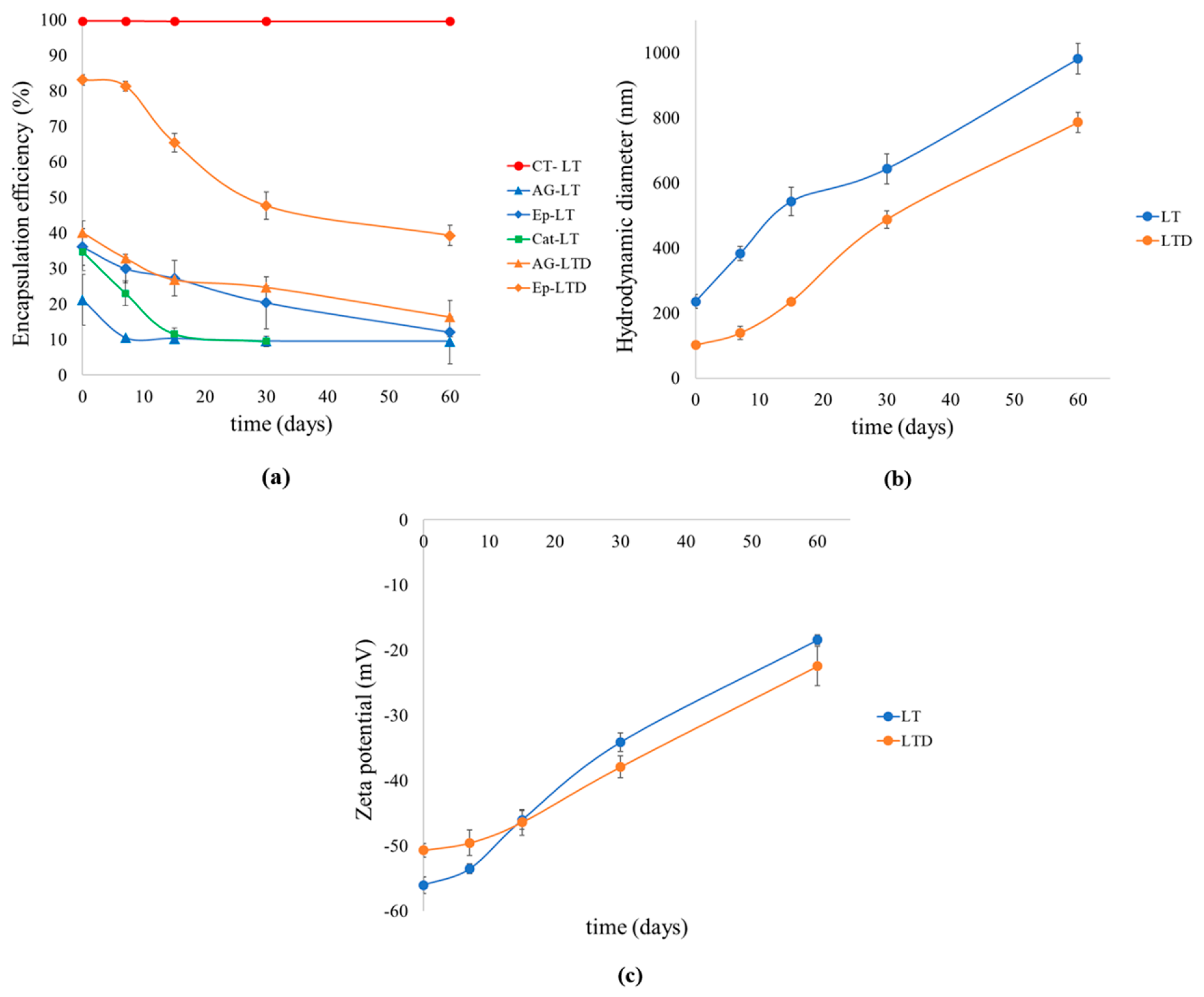
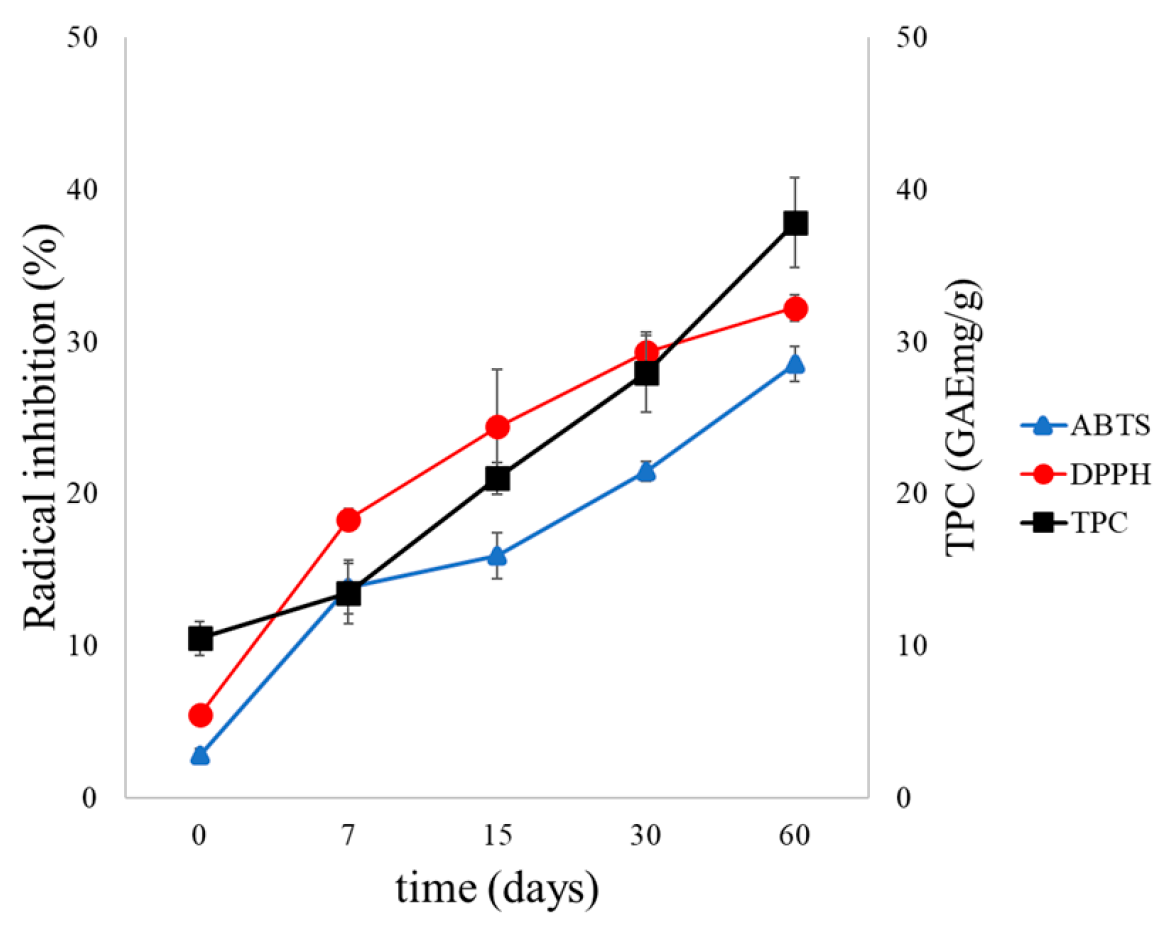
3.2.4. Results of Long-Term Stability of Liposomal Suspensions and Antioxidant Activity
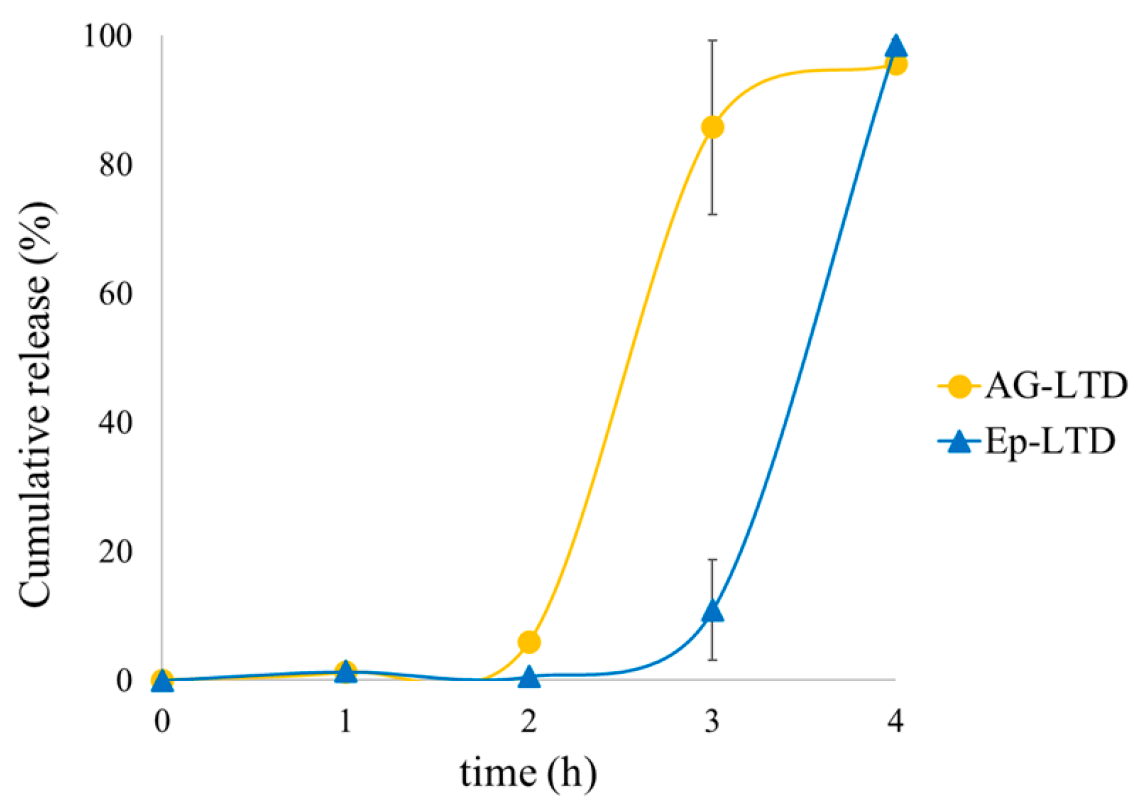
3.3. Results of in Vitro Release Testing of Liposomal Suspensions in a Food Simulant
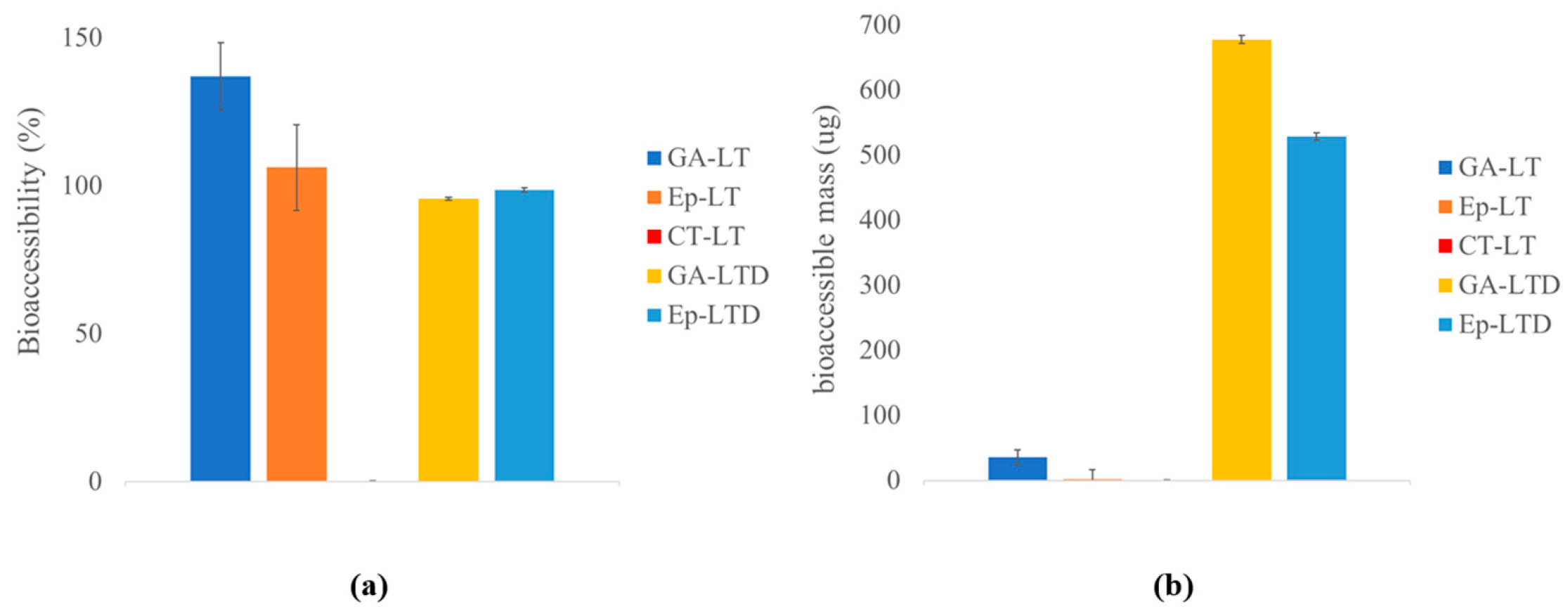
3.4. Results of Bioaccessibility and Digestion Release of Liposomal Suspensions
4. Discussion
4.1. Discussion of Obtaining and Characterizing Depolymerized Grape Seed Condensed Tannins
4.2. Discussion of Obtaining and Characterizing Nanoliposomal Suspensions Encapsulating Depolymerized Condensed Tannins
4.2.1. Physicochemical Parameters of LT and LTD
4.2.2. Morphological Properties and Microstructure of LTD
4.2.3. Rheological Behavior of LT and LTD
4.3. Discussion of Liposomal Suspensions’ Long-Term Stability and Antioxidant Activity
4.4. Discussion of in Vitro Release of Liposomal Suspensions in a Food Simulant
4.5. Discussion of Bioaccessibility and Digestion Release of Liposomal Suspensions
4.6. Applications and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | L-α-Lecithin Soybean with phosphatidylcholine purity ≥ 97% |
| ST | Condensed tannin suspensions |
| STD | Depolymerized condensed tannin suspensions |
| LT | Condensed tannins in liposome suspensions |
| LTD | Depolymerized condensed tannins nanoliposome suspensions |
| GA | Gallic acid |
| Ep | Epicatechin |
| Cat | Catechin |
| CT | Condensed tannins, polymeric fraction of ST |
| GA-LT | Gallic acid fraction encapsulated in LT |
| Cat-LT | Catechin fraction encapsulated in LT |
| Ep-LT | Epicatechin fraction encapsulated in LT |
| CT-LT | Condensed tannins fraction encapsulated in LT |
| GA-LTD | Gallic acid fraction encapsulated in LTD |
| Ep-LTD | Epicatechin fraction encapsulated in LTD |
References
- Mir-Cerdà, A.; Nunez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- González, C.M.; Llorca, E.; Quiles, A.; Hernando, I.; Moraga, G. An in vitro digestion study of tannins and antioxidant activity affected by drying “Rojo Brillante” persimmon. LWT 2022, 155, 112961. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Condensed tannins, a viable solution to meet the need for sustainable and effective multifunctionality in food packaging: Structure, sources, and properties. J. Agric. Food Chem. 2022, 70, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Servicio Agricola y Ganadero. Informe Final–Producción de Vinos 2024; Servicio Agricola y Ganadero: Santiago, Chile, 2024; Available online: https://www.sag.gob.cl/sites/default/files/Informe%20Final%20Cosecha%202024.pdf (accessed on 4 August 2025).
- Jia, B.; Wei, Z.; Kong, X.; Xia, S.; Gan, L.; Han, S. Antioxidant properties of larch tannins with different mean polymerization degrees: Controlled degradation based on hydroxyl radical degradation. J. Agric. Food Chem. 2022, 70, 9367–9376. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Simal-Gandara, J. Traditional applications of tannin rich extracts supported by scientific data: Chemical composition, bioavailability and bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Cui, C.; Shi, A.; Bai, S.; Yan, P.; Li, Q.; Bi, K. Novel antihypertensive prodrug from grape seed proanthocyanidin extract via acid-mediated depolymerization in the presence of captopril: Synthesis, process optimization, and metabolism in rats. J. Agric. Food Chem. 2018, 66, 3700–3707. [Google Scholar] [CrossRef]
- Wen, K.S.; Ruan, X.; Wang, J.; Yang, L.; Wei, F.; Zhao, Y.X.; Wang, Q. Optimizing nucleophilic depolymerization of proanthocyanidins in grape seeds to dimeric proanthocyanidin B1 or B2. J. Agric. Food Chem. 2019, 67, 5978–5988. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Awika, J.M.; Wang, L.; Qian, H.; Gu, L. Depolymerization of sorghum procyanidin polymers into oligomers using HCl and epicatechin: Reaction kinetics and optimization. J. Cereal Sci. 2016, 70, 170–176. [Google Scholar] [CrossRef]
- Liu, H.; Zou, T.; Gao, J.M.; Gu, L. Depolymerization of cranberry procyanidins using (+)-catechin,(−)-epicatechin, and (−)-epigallocatechin gallate as chain breakers. Food Chem. 2013, 141, 488–494. [Google Scholar] [CrossRef]
- Bai, R.; Cui, Y.; Luo, L.; Yuan, D.; Wei, Z.; Yu, W.; Sun, B. A semisynthetic approach for the simultaneous reaction of grape seed polymeric procyanidins with catechin and epicatechin to obtain oligomeric procyanidins in large scale. Food Chem. 2019, 278, 609–616. [Google Scholar] [CrossRef]
- Suo, H.; Tian, R.; Xu, W.; Li, L.; Cui, Y.; Zhang, S.; Sun, B. Novel Catechin–Tiopronin Conjugates Derived from Grape Seed Proanthocyanidin Degradation: Process Optimization, High-Speed Counter-Current Chromatography Preparation, as Well as Antibacterial Activity. J. Agric. Food Chem. 2019, 67, 11508–11517. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Xu, Y.; Zhang, B.; Li, J.; Zhong, W.; Ma, F. Degradation of polymeric polyproanthocyanidins from black chokeberry by microwave-assisted nucleophilic technique of sulfite/catechin: Reaction kinetics, antioxidation and structural analysis. Food Chem. 2023, 408, 135220. [Google Scholar] [CrossRef]
- Morales, C.F.; Osorio, F.A. Food-grade microwave-assisted depolymerization of grape seed condensed tannins: Optimizing the reaction using gallic acid as a nucleophile. Polymers 2025, 17, 682. [Google Scholar] [CrossRef]
- Kuo, E.; McClements, D.J. Food colloid-based delivery systems for tackling age-related macular degeneration by enhancing carotenoid bioavailability: A review. Food Hydrocoll. Health 2022, 2, 100093. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.; Zi, Y.; Zhang, Y. Fabrication, characterization, and in vitro digestion of bamboo leaf extract loaded liposomes. Food Struct. 2021, 30, 100238. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2020, 130, 108967. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Quilaqueo, M.; Venegas, O.; Ahumada, M.; Silva, W.; Osorio, F.; Giménez, B. Design of dipalmitoyl lecithin liposomes loaded with quercetin and rutin and their release kinetics from carboxymethyl cellulose edible films. J. Food Eng. 2018, 224, 165–173. [Google Scholar] [CrossRef]
- Prabhakar, P.; Tripathy, S.; Verma, D.K.; Singh, S.; Thakur, M.; Singh, A.K.; Aguilar, C.N. Trends and advances in liposome formulation technology with an emphasis on ensuring safety and quality in food and drug applications. Food Biosci. 2025, 69, 106913. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Monasterio, A.; Cantero-López, P.; Osorio, F.A. Combining edible coatings technology and nanoencapsulation for food application: A brief review with an emphasis on nanoliposomes. Food Res. Int. 2021, 145, 110402. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins—A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Eskandari, V.; Sadeghi, M.; Hadi, A. Physical and chemical properties of nano-liposome, application in nano medicine. J. Comput. Appl. Mech. 2021, 52, 751–767. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the bioaccessibility and bioavailability of carotenoids by means of nanostructured delivery systems: A comprehensive review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Hong, S.C.; Hong, C.R.; Kim, M.; Kang, Y.J.; Jung, Y.H.; Park, K.M.; Chang, P.S. Process optimization for microfluidic preparation of liposomes using food-grade components. Food Chem. 2024, 451, 139437. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; López-Giraldo, L.J.; Alvarez-Rivera, G.; Ibánez, E.; Herrero, M. Insight of stability of procyanidins in free and liposomal form under an in vitro digestion model: Study of bioaccessibility, kinetic release profile, degradation, and antioxidant activity. J. Agric. Food Chem. 2019, 67, 1990–2003. [Google Scholar] [CrossRef]
- Xu, X.; Tian, F.; Pan, Y.; Zhang, T.; Deng, L.; Jiang, H.; Liu, W. Emerging mechanistic insights into liposomal stability: Full process management from production and storage to food application. Chem. Eng. J. 2025, 159552. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Mayer, I. Determination of molecular structures of condensed tannins from plant tissues using HPLC-UV combined with thiolysis and MALDI-TOF mass spectrometry. Bio-protocol 2016, 6, e1975. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar] [CrossRef]
- Monasterio, A.; Núñez, E.; Verdugo, V.; Osorio, F.A. Stability and biaxial behavior of fresh cheese coated with nanoliposomes encapsulating grape seed tannins and polysaccharides using immersion and spray methods. Polymers 2024, 16, 1559. [Google Scholar] [CrossRef]
- Díaz, H.S.; Ríos-Gallardo, A.; Ortolani, D.; Díaz-Jara, E.; Flores, M.J.; Vera, I.; Río, R.D. Lipid-encapsuled grape tannins prevent oxidative-stress-induced neuronal cell death, intracellular ROS accumulation and inflammation. Antioxidants 2022, 11, 1928. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine–rutin complex as a potential nanocarrier for food applications. J. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf. B Biointerfaces 2019, 183, 110460. [Google Scholar] [CrossRef]
- Vidal, C.; Lopez-Polo, J.; Osorio, F.A. Physical properties of cellulose derivative-based edible films elaborated with liposomes encapsulating grape seed tannins. Antioxidants 2024, 13, 989. [Google Scholar] [CrossRef]
- Castillo, M.; Borrell, A. General European legislation for food contact materials. Curr. Anal. Chem. 2018, 14, 358–366. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Soto, A.; Zamorano, M.; Silva-Weiss, A.; Oyarzun-Ampuero, F.A.; Brossard, N.; Osorio, F.A. Effect of the incorporation of liposomes loaded with rutin on the transport properties of edible film produced with hydroxypropyl methylcellulose: An in vitro release study. LWT 2024, 191, 115583. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-Doulabi, B. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer–Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Al Sawaftah, N.; Paul, V.; Awad, N.; Husseini, G.A. Modeling of anti-cancer drug release kinetics from liposomes and micelles: A review. IEEE Trans. Nanobiosci. 2021, 20, 565–576. [Google Scholar] [CrossRef]
- Porbaha, P.; Ansari, R.; Kiafar, M.R.; Bashiry, R.; Khazaei, M.M.; Dadbakhsh, A.; Azadi, A. A comparative mathematical analysis of drug release from lipid-based nanoparticles. AAPS PharmSciTech 2024, 25, 208. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Recio, I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Brodkorb, A. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Chen, S.; Zhao, J.; Lei, Y.; Geng, L. Nano-resveratrol liposome: Physicochemical stability, in vitro release, and cytotoxicity. Appl. Biochem. Biotechnol. 2023, 195, 5950–5965. [Google Scholar] [CrossRef]
- Mohseni, M.; Mousavi, M.; Kiani, H.; Homayoonfal, M. Chitosan-coated nanoliposomes as vehicles for L-citrulline: Preparation, characterization, antioxidant properties, and nanovesicles behavior. Carbohydr. Polym. Technol. Appl. 2025, 11, 100925. [Google Scholar] [CrossRef]
- Ahmoda, R.A.; Milošević, M.; Marinković, A.; Jovanović, A.A. Nano-liposomal carrier as promising dermal delivery platform for Fumaria officinalis L. bioactives. Pharmaceutics 2025, 17, 782. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-drug delivery systems entrapping natural bioactive compounds for cancer: Recent progress and future challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Monasterio, A.; Osorio, F.A. Physicochemical properties of nanoliposomes encapsulating grape seed tannins formed with ultrasound cycles. Foods 2024, 13, 414. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, W.; Ye, Y.; Cui, W.; Dong, L.; Yao, Y.; Li, K.; Han, J.; Liu, W. Novel nanoliposome codelivered DHA and anthocyanidin: Characterization, in vitro infant digestibility, and improved cell uptake. J. Agric. Food Chem. 2021, 69, 9395–9406. [Google Scholar] [CrossRef] [PubMed]
- Toro-Uribe, S.; Ibáñez, E.; Decker, E.A.; McClements, D.J.; Zhang, R.; López-Giraldo, L.J.; Herrero, M. Design, fabrication, characterization, and in vitro digestion of alkaloid-, catechin-, and cocoa extract-loaded liposomes. J. Agric. Food Chem. 2018, 66, 12051–12065. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Haikou, M.; Theodoropoulou, M.; Tsakiroglou, C.; Antimisiaris, S.G. The effect of added liposomes on the rheological properties of a hydrogel: A systematic study. J. Colloid Interface Sci. 2008, 317, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duša, F.; Witos, J.; Ruokonen, S.K.; Wiedmer, S.K. Determination of the main phase transition temperature of phospholipids by nanoplasmonic sensing. Sci. Rep. 2018, 8, 14815. [Google Scholar] [CrossRef]
- Jing, Y.; Trefna, H.; Persson, M.; Kasemo, B.; Svedhem, S. Formation of supported lipid bilayers on silica: Relation to lipid phase transition temperature and liposome size. Soft Matter 2014, 10, 187–195. [Google Scholar] [CrossRef]
- Carvalho, G.S.G.; Reis, A.V.F.; Crisóstomo, L.C.C.F.; Dos Santos, A.M.; Araújo, T.G.; Chorilli, M.; Petrilli, R.; Eloy, J.O. Liposomes containing sorbitan monolaurate incorporated in hydroxyethylcellulose hydrogels for topical delivery of 5-fluorouracil: Rheological characterization, skin penetration and cytotoxicity study. J. Pharm. Sci. 2025, 114, 103868. [Google Scholar] [CrossRef]
- Sharifian Gh, M. Recent experimental developments in studying passive membrane transport of drug molecules. Mol. Pharm. 2021, 18, 2122–2141. [Google Scholar] [CrossRef]
- Cavalcanti, R.R.; Lira, R.B.; Riske, K.A. Membrane fusion biophysical analysis of fusogenic liposomes. Langmuir 2022, 38, 10430–10441. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, Y.; Shaddel, R.; Salamatian, M.; Szumny, A. Nanoliposomal encapsulation of Capparis spinosa extract and its application in jelly formulation. Molecules 2024, 29, 2804. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid peroxidation and antioxidant protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, K.; Beg, S.; Anwar, F.; Kumar, V. Liposomes as topical drug delivery systems: State of the arts. In Biomedical Applications of Nanoparticles; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 149–161. [Google Scholar] [CrossRef]
- Phuntsho, S. Impact of Lipid Structural Variations on Bilayer Properties: A Coarse-Grained Molecular Dynamics Study. arXiv 2024, arXiv:2412.09312. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Yazid, A.M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical modelling of drug release. Res. Eng. Struct. Mater. 2020, 6, 4. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Sun, K.; Zhang, R.; Wang, S.; Geng, L. Study of release kinetics and degradation thermodynamics of ferric citrate liposomes. Chem. Phys. Lipids 2019, 225, 104811. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull modeling of controlled drug release from Ag-PMA nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, H.; Hanafi-Bojd, M.Y.; Iranshahy, M.; Zarban, A.; Raissi, H. The combination of polyphenols and phospholipids as an efficient platform for delivery of natural products. Sci. Rep. 2023, 13, 2501. [Google Scholar] [CrossRef]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of drug release. In The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics; Springer International Publishing: Cham, Switzerland, 2022; pp. 613–621. [Google Scholar] [CrossRef]
- Shibusawa, Y.; Shoji, A.; Yanagida, A.; Shindo, H.; Tagashira, M.; Ikeda, M.; Ito, Y. Determination of log Po/w for catechins and their isomers, oligomers, and other organic compounds by stationary phase controlled high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2819–2837. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-products of agri-food industry as tannin-rich sources: A review of tannins’ biological activities and their potential for valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, A.; Zhang, T.; Pan, Y.; Jiang, H.; Deng, L.; Qin, Y.; Li, J.; Han, J.; Liu, W. The novel lactoferrin and DHA-codelivered liposomes with different membrane structures: Fabrication, in vitro infant digestion, and suckling pig intestinal organoid absorption. Food Chem. 2024, 441, 138346. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Gerlei, M.; Jeandel, C.; Sahaka, M.; Carrière, F.; Linder, M. In vitro gastrointestinal digestion of marine oil emulsions and liposomal solutions: Fate of LC-PUFAs upon lipolysis. Food Funct. 2024, 15, 11291–11304. [Google Scholar] [CrossRef]
- Xu, X.; Tian, M.; Deng, L.; Jiang, H.; Han, J.; Zhen, C.; Liu, W. Structural degradation and uptake of resveratrol-encapsulated liposomes using an in vitro digestion combined with Caco-2 cell absorption model. Food Chem. 2023, 403, 133943. [Google Scholar] [CrossRef]
- Yi, X.; Chen, Y.; Gao, X.; Gao, S.; Xia, G.; Shen, X. Enhancement of digestive stability in curcumin-loaded liposomes via glycolipids: An analysis in vitro and in vivo. Food Res. Int. 2025, 208, 116255. [Google Scholar] [CrossRef]
- Pan, L.; Li, H.; Hou, L.; Chang, Z.; Li, Y.; Li, X. Gastrointestinal digestive fate of whey protein isolate coated liposomes loading astaxanthin: Lipolysis, release, and bioaccessibility. Food Biosci. 2022, 45, 101464. [Google Scholar] [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From waste to health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef]
- Pan, L.; Meng, H.; Li, J.; Liu, Z.; Zhang, D.; Liu, Z.; Zhao, Q.; Xu, F. Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study. Molecules 2024, 29, 1687. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Wei, Y.; Fei, T.; Hu, X.; Wang, L. Enhancing the stability and bio-accessibility of carotenoids extracted from canistel (Lucuma nervosa) via liposomes encapsulation. LWT 2024, 204, 116455. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Gómez-Lázaro, L.; Martín-Sabroso, C.; Aparicio-Blanco, J.; Torres-Suárez, A.I. Assessment of in vitro release testing methods for colloidal drug carriers: The lack of standardized protocols. Pharmaceutics 2024, 16, 103. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]

| Characterization | ST | STD |
|---|---|---|
| TPC (GAeq mg/g) | 241.7 ± 13.2 a | 287.4 ± 7.96 b |
| Inhibition ABTS•+ (%) | 89.53 ± 7.96 a | 90.47 ± 2.83 a |
| Inhibition DPPH• (%) | 88.71 ± 6.32 a | 89.62 ± 6.49 a |
| Principal structure | Polymers with mDP * 8 a | Epicatechin monomers b |
| HPD (nm) | 16,860 ± 1073 a | 6.2 ± 1.07 b |
| Parameter | LT | LTD |
|---|---|---|
| EE (%) | 99.65 ± 0.01 *,a | 83.11 ± 1.46 **,b |
| LC (%) | 99.46 ± 0.01 *,a | 97.24 ± 0.05 **,a |
| HPD (nm) | 235.3 ± 21.4 a | 101.3 ± 7.8 b |
| ζ (mV) | −56.0 ± 1.3 a | −50.7 ± 1.1 b |
| PDI | 0.24 ± 0.10 a | 0.26 ± 0.13 a |
| Korsmeyer–Peppas | Higuchi | Weibull | ||||||
|---|---|---|---|---|---|---|---|---|
| Molecule | R2 | KR | n | R2 | KH | R2 | α | β |
| GA-LT | 0.9979 * | 0.247 | 0.763 ± 0.055 | 0.6781 | 0.233 | 0.9653 | 2.2 ± 0.1 | 0.91 ± 0.13 |
| Cat-LT | 0.9823 * | 0.247 | 0.703 ± 0.032 | 0.7776 | 0.207 | 0.9767 | 6.6 ± 0.7 | 0.04 ± 0.01 |
| Ep-LT | 0.9738 * | 0.268 | 0.656 ± 0.054 | 0.7518 | 0.208 | 0.9604 | 7.0 ± 1.9 | 0.04 ± 0.01 |
| AG-LTD | 0.9399 | 0.265 | 0.714 ± 0.056 | 0.7334 | 0.241 | 0.9863 * | 3.7 ± 0.4 | 0.14 ± 0.02 |
| Ep-LTD | 0.9264 | 0.351 | 0.484 ± 0.027 | 0.7691 | 0.202 | 0.9903 * | 5.8 ± 1.7 | 0.07 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, C.F.; Zamorano, M.; Brossard, N.; Rosenkranz, A.; Osorio, F.A. Depolymerization and Nanoliposomal Encapsulation of Grape Seed Condensed Tannins: Physicochemical Characterization, Stability, In Vitro Release and Bioaccessibility. Antioxidants 2025, 14, 1123. https://doi.org/10.3390/antiox14091123
Morales CF, Zamorano M, Brossard N, Rosenkranz A, Osorio FA. Depolymerization and Nanoliposomal Encapsulation of Grape Seed Condensed Tannins: Physicochemical Characterization, Stability, In Vitro Release and Bioaccessibility. Antioxidants. 2025; 14(9):1123. https://doi.org/10.3390/antiox14091123
Chicago/Turabian StyleMorales, Carolina F., Marcela Zamorano, Natalia Brossard, Andreas Rosenkranz, and Fernando A. Osorio. 2025. "Depolymerization and Nanoliposomal Encapsulation of Grape Seed Condensed Tannins: Physicochemical Characterization, Stability, In Vitro Release and Bioaccessibility" Antioxidants 14, no. 9: 1123. https://doi.org/10.3390/antiox14091123
APA StyleMorales, C. F., Zamorano, M., Brossard, N., Rosenkranz, A., & Osorio, F. A. (2025). Depolymerization and Nanoliposomal Encapsulation of Grape Seed Condensed Tannins: Physicochemical Characterization, Stability, In Vitro Release and Bioaccessibility. Antioxidants, 14(9), 1123. https://doi.org/10.3390/antiox14091123








