Abstract
Tomatoes are among the most consumed vegetables within the Mediterranean diet, recognized as one of the healthiest dietary patterns in the world. This study evaluated the effects of four fertilization treatments on the antioxidant and metabolic profiles of four local tomato varieties: Cornabel (V1), Cuban Pepper (V2), Corno Andino (V3), and Roli Rosa (V4). Treatment 1 (T1) used 1.28 kg/m2 of commercial woody compost (C/N ratio 13), while Treatment 2 (T2) served as the control, initially without fertilization. Treatments 3 and 4 (T3, T4) incorporated ramial chipped wood (RCW) at 15 kg/m2 and 7.5 kg/m2, respectively, without tillage. Each treatment × variety combination included four biological replicates (n = 4). Untargeted metabolomic profiling via UHPLC-QToF and statistical analyses identified 163 compounds, 37 of which showed significant varietal differences (p < 0.05). The flavonoid eriodictyol was more abundant in the Pebroter varieties (V1, V2, V3), whereas luteolin-8-O-glucoside (orientin) predominated in V4, likely due to differential expression of biosynthetic genes. RCW (T3) yielded higher levels of antioxidant compounds like phenolic acids, and flavonoids compared to compost and control; however, no dose–response was observed using different doses of RCW (T3 and T4). These findings demonstrate that RCW may modulate the antioxidant metabolite profile of tomatoes, highlighting its potential as a sustainable fertilization strategy for enhancing antioxidant compounds.
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most widely consumed fresh vegetables worldwide. According to the Food and Agriculture Organization (FAO), approximately 170 million tons of fresh and processed tomatoes are produced globally each year, cultivated on approximately 5 million hectares of farmland [1]. Tomatoes are a key component of the Mediterranean diet, considered one of the healthiest dietary patterns and recognized for its role in preventing chronic diseases [2,3], and represent a major dietary source of bioactive compounds with strong antioxidant properties. They are particularly rich in carotenoids, such as lycopene and β-carotene, as well as polyphenols, flavonoids, and ascorbic acid, all of which contribute to their high antioxidant activity [4]. These compounds play a crucial role in neutralizing reactive oxygen species, thereby reducing oxidative stress and supporting the prevention of chronic diseases, including cardiovascular disorders and certain types of cancer [2,5]. Importantly, the antioxidant potential of tomatoes is determined not only by individual metabolites but also by the synergistic interactions among them, which can be effectively captured using metabolomic approaches [6,7]. Therefore, evaluating how agricultural practices, such as sustainable fertilization, impact the antioxidant profile of tomatoes is important both for promoting sustainability and for enhancing their antioxidant content.
Tomato yield and quality depend strongly on soil conditions and fertilization practices [8,9]. Under the pressures of climate change, European agricultural soils face declining quality, and there is an urgent need to adapt agricultural practices to achieve the United Nations Sustainable Development Goals for 2030 [10]. Approximately half of the soils in Europe are estimated to have a low content of organic matter [11], which significantly reduces their capacity to retain water and essential nutrients, or to store carbon. The Mediterranean region is particularly susceptible to soil degradation [12], exhibiting the lowest levels of soil organic matter [13] and highest erosion rates in the EU [14], as well as severe salinization problems [15]. Additionally, overuse of organic or chemical fertilizers has led to excessive nitrogen and phosphorus levels in soils [16], contributing to environmental degradation and raising public health concerns [17]. In response, sustainable agricultural practices—such as organic and regenerative farming—have been developed as viable solutions to reduce the adverse effects of synthetic fertilizers and pesticides on human and environmental health [18].
Organic and sustainable farming methods include the use of cover crops, green manures, and ramial wood by-products (i.e., chipped branches without foliage) as exogenous, plant-derived organic amendments [19]. These inputs contribute to soil organic matter, which not only enhances nutrient availability but also promotes root development, soil biodiversity, and microbial activity [20]. Studies have shown that organic fertilization can match the effectiveness of inorganic fertilizers in improving crop yield and quality [21], reinforcing its central role in organic farming [22]. Moreover, by simultaneously meeting the nutritional needs of plants and suppressing pest populations, organic fertilizers play a dual role in sustainable agriculture. Common sources include composted livestock manures, plant residues, and certain types of industrial waste [23].
Wood by-products, in particular, have been shown to enhance soil physicochemical and biological properties, including organic matter content, microbial activity, water retention, and nutrient availability [24]. Although the high C/N ratio of this type of fertilizer can initially lead to nitrogen immobilization, and temporarily reduce crop growth, improved yields have been observed during subsequent cropping. This delayed benefit is attributed to increased nitrogen release following the prolonged decomposition of the ramial woody residues in the soil [25]. Additionally, the mobilization of nutrients retained in the soil, such as nitrogen and phosphorus, largely depends on plant requirements and interactions with soil organisms [26]. Moreover, Pérez-Llorca et al. (2025) found that RCW applications increased soil organic carbon and microbial biomass [27], indicating improved soil health and nutrient cycling [28]. These findings emphasize the importance of understanding the effects of RCW on crop quality, particularly in terms of antioxidant composition, which remains underexplored.
Although ramial chipped wood (RCW) has been studied as a fertilizer in tomato cultivation, most research has focused on its effects on yield, with limited attention given to its impact on the phytochemical and antioxidant composition of the fruit [25]. Antioxidant compounds such as phenolics, vitamin C, and carotenoids are fundamental to the nutritional quality and health-promoting properties of tomatoes, and their levels may be strongly influenced by agricultural practices. To distinguish these complex metabolic changes, untargeted metabolomics provides a comprehensive approach for profiling antioxidant-related metabolites and their variation across treatments. Based on previous findings, we hypothesize that the incorporation of RCW may initially lead to a slight reduction in tomato yield during the first growing season due to temporary nitrogen immobilization [25], while simultaneously inducing moderate nutritional stress capable of modifying the metabolomic profile of the fruit. Therefore, this study evaluates both the agronomic performance and the antioxidant-related phytochemical profiles of four widely consumed traditional local tomato varieties: Cornabel, Cuban Pepper, Corno Andino, and Roli Rosa. Using a controlled experimental design, we compared the effects of RCW application at two different concentrations (15 kg/m2 and 7.5 kg/m2) with those of a standard commercial organic compost and nitrogen-rich organic pellets.
2. Materials and Methods
2.1. Chemicals and Reagents
Water, acetonitrile and methanol (all LC-MS grade, LiChrosolv®, Merck, Darmstadt, Germany) were purchased from VWR (Bruchsal, Germany). 98–Formic acid was obtained from Honeywell (Morristown, NJ, USA). Ammonium formate ≥99% was purchased from Sigma-Aldrich (Merck, Darmstadt, Germany).
2.2. Field Experiment and Plant Material
The study was carried out at Masia Cal Notari, an organic horticultural farm located in the peri-urban area of Barcelona (Sant Boi de Llobregat, Barcelona, Spain, 41°19′4.8″ N, 2°3′3.6″ E, 2 m.a.s.l.). The soil is classified as silty clay loam (sand: clay ratio 5.5:28.5) with 36.17% calcium carbonate, and the area has a typical Mediterranean climate. During the experimental period, seasonal mean temperatures were recorded as follows: May 2021 (start of the experiment) 17.0 ± 0.4 °C, summer 2021 24.0 ± 2.5 °C, autumn 2021 17.2 ± 1.8 °C, winter 2021–2022 9.6 ± 1.0 °C, spring 2022 15.2 ± 1.6 °C, and summer 2022 25.7 ± 2.7 °C. Corresponding cumulative precipitation values for these periods were 32, 23, 179, 21, 138, and 92 mm, respectively. All meteorological information was obtained from the Servei Meteorològic de Catalunya (https://meteo.cat/, accessed on 23 May 2024). Baseline soil properties were assessed before the experiment, showing a soil organic carbon content of 2.15%, total nitrogen of 0.19%, and a C/N ratio of 11.2.
The tomatoes analyzed in this study belong to two major varietal groups traditionally cultivated in Catalunya: Pebroter and Rosa. The Pebroter varieties Cornabel, Cuban Pepper, and Corno Andino are characterized by long, heavy, fleshy, horn-shaped fruits with a slightly sweet taste. In contrast, Roli Rosa, a representative of the Rosa variety, produces large, pink fruits highly appreciated for their distinctive flavor and texture, with sweetness prevailing over acidity. The Rosa variety has widespread market acceptance due to its superior quality [29].
2.3. Experimental Design
The experiment was conducted to evaluate the effects of different organically managed agronomic systems on tomato cultivation. In May 2021, four soil treatments were applied to the four tomato varieties [Cornabel (V1), Cuban Pepper (V2), Corno Andino (V3), and Roli Rosa (V4)], with four replicates per treatment. In treatment 1 (T1), 1.28 kg/m2 of woody residue compost (C/N ratio = 13) was incorporated into the soil. Treatment 2 (T2) served as the control, with no fertilizer applied. Treatments 3 and 4 (T3 and T4) involved the application of ramial chipped wood (C/N ratio = 40.5) at rates of 15 kg/m2 and 7.5 kg/m2, respectively. The RCW material was sourced from carbon-rich pruning residues collected in the municipality of Sant Boi de Llobregat.
All amendments were incorporated into the top 20 cm of soil using a rotary tiller (rotovator). All plots were rotary cultivated at the time of application. Treatments were randomly assigned to 16 plots, each measuring 1.5 × 7.5 m, separated by 1 m, and arranged along two 2 m-wide pathways to minimize positional or microclimatic effects. The soil surface was covered with biodegradable plastic, and a drip irrigation system was installed, providing the same water amounts to all plots to ensure uniform soil moisture across treatments.
In May 2022, prior to tomato planting, compost was reapplied to the T1 plots. Additionally, 210 kg of a commercial nitrogen-rich organic fertilizer (C/N ratio = 4) was added to the T2 plots, and 120 kg of the same fertilizer was applied to both T3 and T4. At that time, T1 and T2 plots were tilled once using the rotary cultivator, following common organic farming practices. In contrast, T3 and T4 plots were left untilled, based on the assumption that microbial mobilization of soil nitrogen would compensate for the lack of mechanical incorporation. Each of the 16 plots contained three plants per variety, spaced 45 cm apart.
Between the initial treatments and the enrichment of soil with nitrogen, different crops were cultivated. Sweet potatoes (Ipomoea batatas cv Beauregard) were grown from May to October 2021, followed by a combination of spinach (Spinacia oleracea L.) and fava beans (Vicia faba L) from October 2021 to May 2022. These crops were used as a complementary strategy for soil regeneration, aimed at improving tomato production and quality. The complete crop rotation and experiment management are detailed in Table 1.

Table 1.
Crop rotation and management carried out in the experiments.
This experiment was designed to compare the use of RCW as an organic amendment under no-tillage management with two conventional fertilization practices: plant-based compost (characterized by higher nutrient availability due to pre-decomposition) and organic pellet N fertilizer (characterized by slow-release N content). Both conventional systems (T1 and T2) were applied under tillage. This approach enables evaluation of how these different organic management strategies affect tomato yield and antioxidant-related phytochemical composition.
2.4. Tomato Sampling
During the August 2022 harvest, tomato samples were collected from the three plants of each variety within each treatment replicate. Fruits from the same variety and plot were pooled to obtain one composite sample per treatment-variety combination (n = 4 biological replicates). The samples were immediately transferred from the farm and frozen at −80 °C. Subsequently, samples from each treatment replicate were thawed, homogenized using a hand blender and lyophilized overnight (Telstar, Cryodos 50–2009, Terrassa, Spain). The lyophilized samples were stored in a freezer, then milled with a ball mill (15 s at 30 Hz; MM 500, Retsch, Haan, Germany) and stored again at −80 °C. A pooled quality control sample was prepared by combining material from a representative selection of all study samples.
2.5. Methanolic Extraction
Extraction was carried out according to Beer et al. (2024) [30], with some modifications. Briefly, 50 ± 0.5 mg of each sample was weighed in 2 mL tubes. Then, 500 μL of ice-cold methanol (MeOH) was added, followed by 20 µL of an internal standard mix (pool of eighteen compounds), whose details and corresponding chemical classes are provided in Table S1. The mixture was homogenized using a vortex for 5 s, then incubated in a thermal shaker (Ditabis HLC (Pforzheim, Germany), with 2 mL tube attachments) at 35 °C and 1400 rpm for 15 min. After incubation, samples were immediately placed on ice and centrifuged at 4 °C and 16,100 g for 5 min. Supernatants were filtered using a polytetrafluoroethylene (PTFE) syringe filter (0.2 µm, 4 mm). After vortexing for 5 s to ensure homogenization, 80 µL of the filtrate was transferred into an HPLC amber vial.
2.6. Metabolomic Profiling by High-Resolution Mass Spectrometry (UHPLC-QToF-MS)
Metabolomic analysis was performed using a 1290 Infinity LC system (Agilent Technologies, Waldbronn, Germany) coupled with a Triple TOF 5600 mass spectrometer (AB Sciex, Darmstadt, Germany). Separation was carried out on a Waters Acquity UPLC HSS T3 Premier column (2.1 × 150 mm; 1.8 µm) equipped with a VanGuard Acquity HSS T3 Premier pre-column (2.1 × 5 mm; 1.8 µm). Aqueous ammonium formate buffer (2 mM with 0.05 vol% formic acid) was used as eluent A and pure acetonitrile as eluent B at a total flow rate of 0.4 mL/min. The elution gradient was programmed as follows: 0.0–1.0 min, isocratic with 3% B; 1.0–14.0 min from 3 to 99% B; 14.0–20.0 min, isocratic with 99% B; 20.0–20.5 min, from 95 to 3% B; 20.5–25.5, isocratic with 3% B. The column oven was set at 40 °C and the injection volume was 2 μL. Mass spectrometry (MS) was performed in both positive and negative electrospray ionization (ESI) modes, selecting the following ionization source parameters: curtain gas 45 psi; ion spray voltage 5500 V and −4500 V; ion source gas-1 and gas-2 60 psi; and ion source gas-2 temperature 550 °C. The declustering potential was set to 70 V and −70 V, respectively. MS full scans were recorded over an m/z range of 100–1500 with an accumulation time of 150 ms and collision energies of 10 V and −10 V, respectively. MS/MS spectra were recorded in high sensitivity mode over an m/z range of 50–1500 with an accumulation time of 25 ms, a collision energy voltage of 35 V and −35 V, respectively, and a collision energy spread of 15 V.
All samples were freshly prepared on the respective measurement day. After the blanks and EQC samples were injected, the study samples were analyzed in randomized order in blocks of eight, each consisting of one PostCal sample, two QC samples, and five study samples, followed by an automatic mass calibration. The internal standard mix was injected into the solvent at the beginning of the sequence (before the first EQC sample) and at the end (after the last QC sample) to evaluate instrument performance. The analyses were performed using UPLC-QToF-MS under data-dependent acquisition (DDA) mode, first in positive and subsequently in negative ionization polarity. According to the COSMOS Metabolomics Standards Initiative [31], compounds identified by untargeted QTOF analysis reached level 2 annotation (putatively annotated compounds).
2.7. Post-Acquisition Data Analysis
Raw data files (.wiff and .wiff.scan) were converted into ABF format using the Reifycs ABF Converter and subsequently processed with MS-DIAL software (version 4.9) [32]. Automatic peak detection and compound annotation were carried out through spectral matching against a database developed at the Max Rubner-Institut. The mass range 80–1500 m/z was searched for peaks, with a minimum peak height threshold of 1000 cps in both ESI+ and ESI− modes. For peak centroiding, mass tolerances were set at 0.01 for MS and 0.05 Da for MS/MS. Retention time was included in the calculation of the total identification score. Mass accuracy tolerance for identification was set to 0.015 Da. Identification was based on accurate mass, ion species, and retention time, and a total identification score was calculated in MS-DIAL. A score threshold of 70% was applied, considering common ion adducts relevant to metabolomics. Gap filling was performed using the peak finder algorithm, with a 5 ppm tolerance for m/z values to recover missing peaks. To minimize false-positive identifications, all features detected in blank samples were excluded from the dataset prior to annotation. Additionally, only compounds with an identification score ≥ 90% were retained for further analysis, following COSMOS level 2 (putative) identification criteria.
2.8. Statistics
One-way ANOVA followed by post hoc analysis for tomato yields was performed using RStudio (version: 2025.05.0+496). Multivariate statistical analyses were conducted using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca, accessed on 24 October 2025) [33]. Unsupervised principal component analysis (PCA) was used to evaluate variation between tomato varieties in all treatments. Variable Importance in Projection (VIP) scores were used to identify marker compounds. Fold change (FC) analysis of VIP markers (ANOVA, p < 0.05) was performed for each Pebroter tomato variety (V1, V2, V3) compared to V4, retaining significant compounds with a |Log2FC value| > ±2. In addition, Student’s t-tests and supervised orthogonal projections to latent structures discriminant analysis (OPLS-DA) were conducted using two classification criteria (T1 and T3), applied separately to each variety. VIP scores greater than 2 were considered indicative of marker compounds. Further FC analysis (Log2FC) of these VIP markers (t-test, p < 0.05) was performed for treatments T1 and T3 to identify significant marker compounds.
3. Results and Discussion
3.1. Tomato Yield
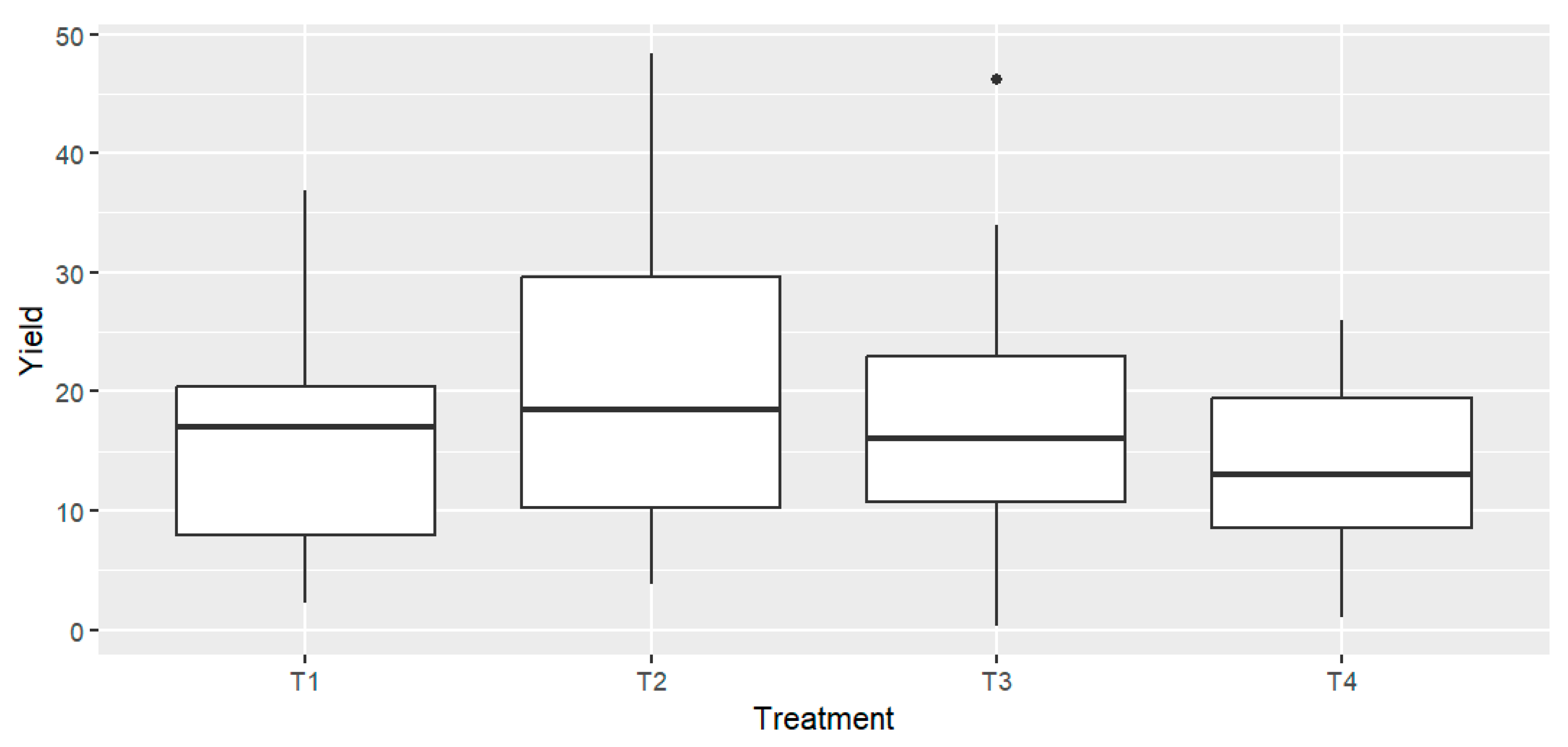
At harvest, the total tomato yield from each treatment was collected and weighed. The average yield from four replicates per treatment was calculated and expressed in tons per hectare (Tn/ha) (Table 2). One-way ANOVA indicated no statistically significant differences in production among the different treatments across all tomato varieties F = 1.23, p = 0.307 (Figure 1). Assumptions of normality and homogeneity of variances were satisfied, as confirmed by Levene’s test (F = 1.03, p = 0.384). A two-way ANOVA was performed to evaluate the effects of fertilization treatment and tomato variety on yield and to assess potential treatment × variety interactions. Results showed no significant effect of treatment on yield (p = 0.052), while a significant effect of variety was observed (p < 0.001). The interaction between treatment and variety was not significant (p = 0.257), indicating that yield differences among treatments were consistent across varieties. Although numerical differences were observed between some treatments (e.g., Cornabel T2 = 32.65 Tn/ha vs. T4 = 16.44 Tn/ha), the overall variation was not statistically significant (Figure 1). However, the average yields revealed a consistent, though moderate, decrease under both high and low doses of RCW compared to the T2. This trend was observed across all four varieties (Table 2).

Table 2.
Average tomato fruit yield for each treatment for the four investigated tomato varieties expressed in Tn/ha.
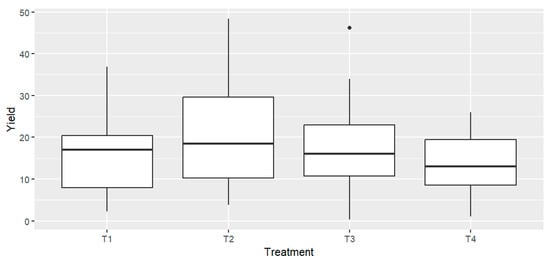
Figure 1.
Tomato yields expressed as box plots for the different treatments. n = 4 biological replicates per treatment-variety.
These findings are consistent with previous research by Soumare et al. (2002), who reported a decline in tomato growth and yield in the first cropping season after RCW application [25]. The reduction was attributed to nitrogen immobilization in the soil, caused by the high C/N ratio of RCW. In the present study, the applied RCW had a C/N ratio of 40.5, which likely induced similar immobilization effects. Notably, Soumare et al. (2002) also observed yield improvements in subsequent cropping, attributed to increased nitrogen availability as the RCW continued to decompose [25].
3.2. Phytochemical Variations Among the Tomato Varieties
Data obtained from UHPLC-QTOF-MS analysis were used for compound annotation with the Max Rubner-Institut in-house library, enabling putative (level 2, COSMOS) identification of metabolites. A total of 32,691 and 21,228 features were detected in positive and negative ionization modes, respectively. Features with identification scores ≥70% were initially retained (242 in positive and 221 in negative mode), while only those with scores ≥90% were included for final interpretation (93 in positive and 70 in negative mode). Background features detected in blanks were removed prior to analysis to minimize false discoveries. Overall, this approach enabled the putative identification of 163 compounds in tomatoes (93 in positive mode and 70 in negative mode). These were predominantly organic and phenolic acids (45), flavonoids (32), amino acids (31), alkaloids and amines (25), carbohydrates (20), fatty acids (4) and six other compounds. A full list of putatively identified compounds is available in the Supplementary Material (Table S2), including retention time, composite mass spectra, total identification score, and peak areas. Only compounds with a total score above 90% (based on retention time and accurate mass) were retained.
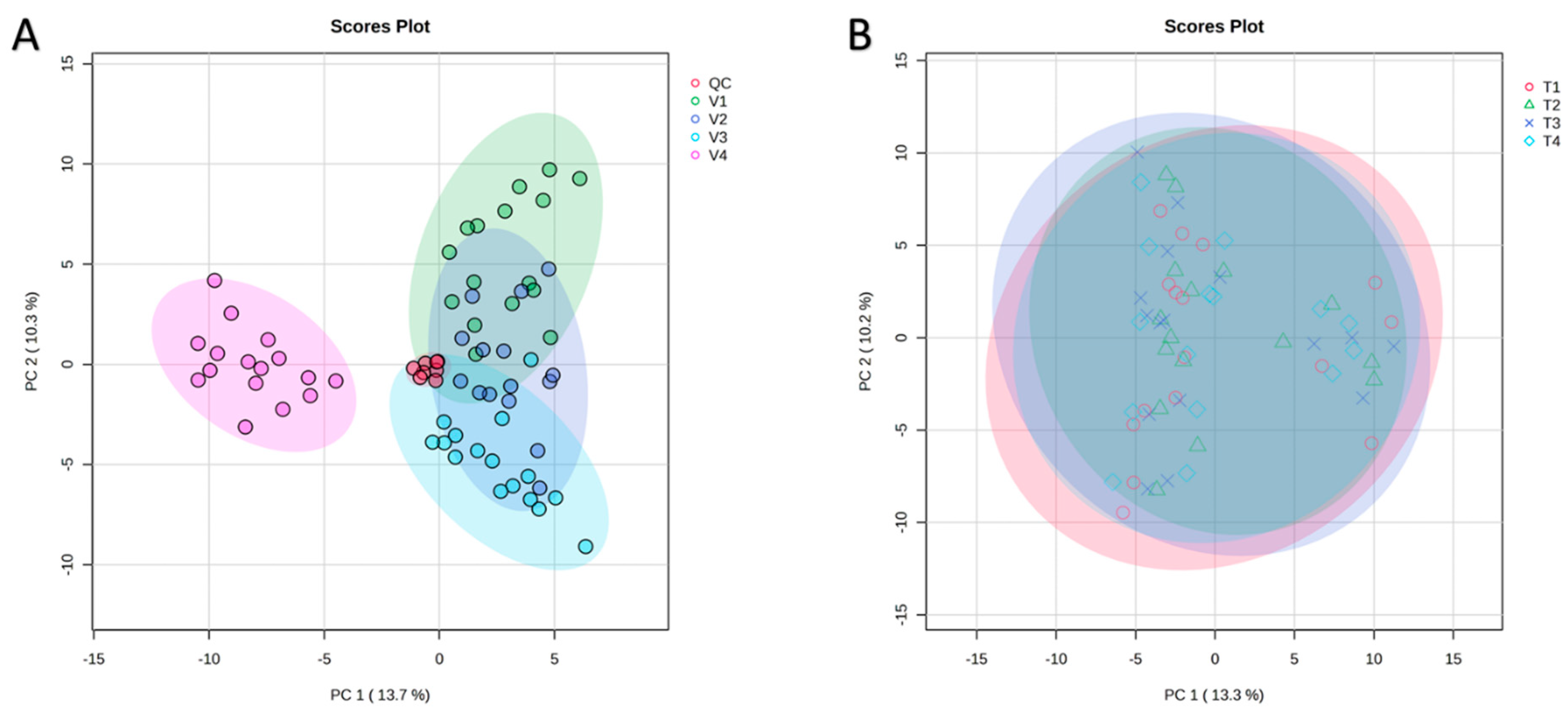
PCA was performed on all samples to assess variation and correlations among the four tomato varieties (Figure 2A). The first two principal components explained 13.7% (PC1) and 10.3% (PC2) of the total variance. In the PCA score plot, V1, V2, and V3 (all belonging to the Pebroter group) formed a tight cluster, whereas V4 (Rosa group) was clearly separated along PC1, reflecting distinct metabolomic profiles between the two varietal groups. The QC samples clustered closely at the center of the PCA plot, demonstrating excellent analytical stability and reproducibility of the UHPLC-QToF-MS measurements. The PERMANOVA test supported these findings, revealing significant differences among varieties (F = 67.506, R2 = 0.8036, p = 0.001, based on 999 permutations).
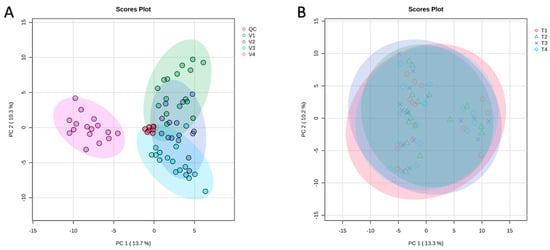
Figure 2.
Principal component analysis (PCA) of (A)—The four varieties (V1: Cornabel, V2: Cuban Pepper, V3: Corno Andino, and V4: Roli Rosa) showing the clear separation of V4 from the Pebroter group; and (B)—The four fertilization treatments in all varieties, showing the four treatments clustered together. n = 4 biological replicates per treatment-variety.
To identify key discriminatory variables among the four tomato varieties, a one-way ANOVA followed by post hoc tests was applied to all identified compounds and their corresponding peak intensities (areas). The analysis revealed significant differences (p < 0.05) among the varieties. Of the 163 identified compounds, 115 showed statistically significant differences between at least two varieties. To ensure statistical robustness, both raw and FDR-corrected p-values were calculated, and interpretation was based on the adjusted results. A complete list of these compounds, along with p-values, false discovery rates, and Fisher’s least significant difference comparisons, is provided in the Supplementary Material (Table S3).
To identify discriminant metabolites, fold change analysis was conducted on the significant features (ANOVA, p < 0.05), comparing each Pebroter variety (V1, V2, V3) to Roli Rosa (V4). Compounds with a |Log2FC value| > +2 were considered significantly different. The results (Table 3) indicate that 37 compounds showed significant variation, primarily flavonoids (25) and phenolic acids (5). These findings are consistent with previous research [4], which identified flavonoids and phenolic acids as potential chemotaxonomic markers to differentiate tomato varieties. Additionally, six alkaloids and amines differed significantly between varieties, whereas no amino acids showed significant variation. This contrasts with a previous report that proposed specific amino acids as biochemical markers to distinguish between 12 Italian tomato varieties [34]. Another study suggested that isotopic analysis of amino acids may serve as a novel tool for authenticating organic tomatoes [35].

Table 3.
Marker compounds from each chemical class with ANOVA p < 0.05. Fold change and Log2FC comparing each Pebroter variety (V1, V2, V3) with the Roli Rosa variety (V4). The compounds were putatively identified based on accurate mass and retention time.
Among the 37 discriminant compounds, 30 exhibited higher intensities in the Pebroter varieties than in Roli Rosa (Log2FC ≥ +2). In contrast, only seven compounds (orientin, phloretin glucoside, kaempferol, quercetin dihydrate, cyanidin, quercetin diglucoside, and cucurbitacin D) were more abundant in Roli Rosa (Log2FC ≤ −2). Although some amines and alkaloids also differed between varieties, their Log2FC values only ranged from 2 to 3. Notably, seven flavonoids showed substantial variation, with Log2FC values ≥ 4, indicating more than a fourfold increase in intensity in the Pebroter varieties: eriodictyol, naringenin glucoside, naringenin chalcone, orobol (isoluteolin), eriodictyol glucoside, phloretin, and apigenin. Their structural similarity (Figure S1) suggests enhanced activity of flavonoid biosynthetic enzymes in Pebroter.
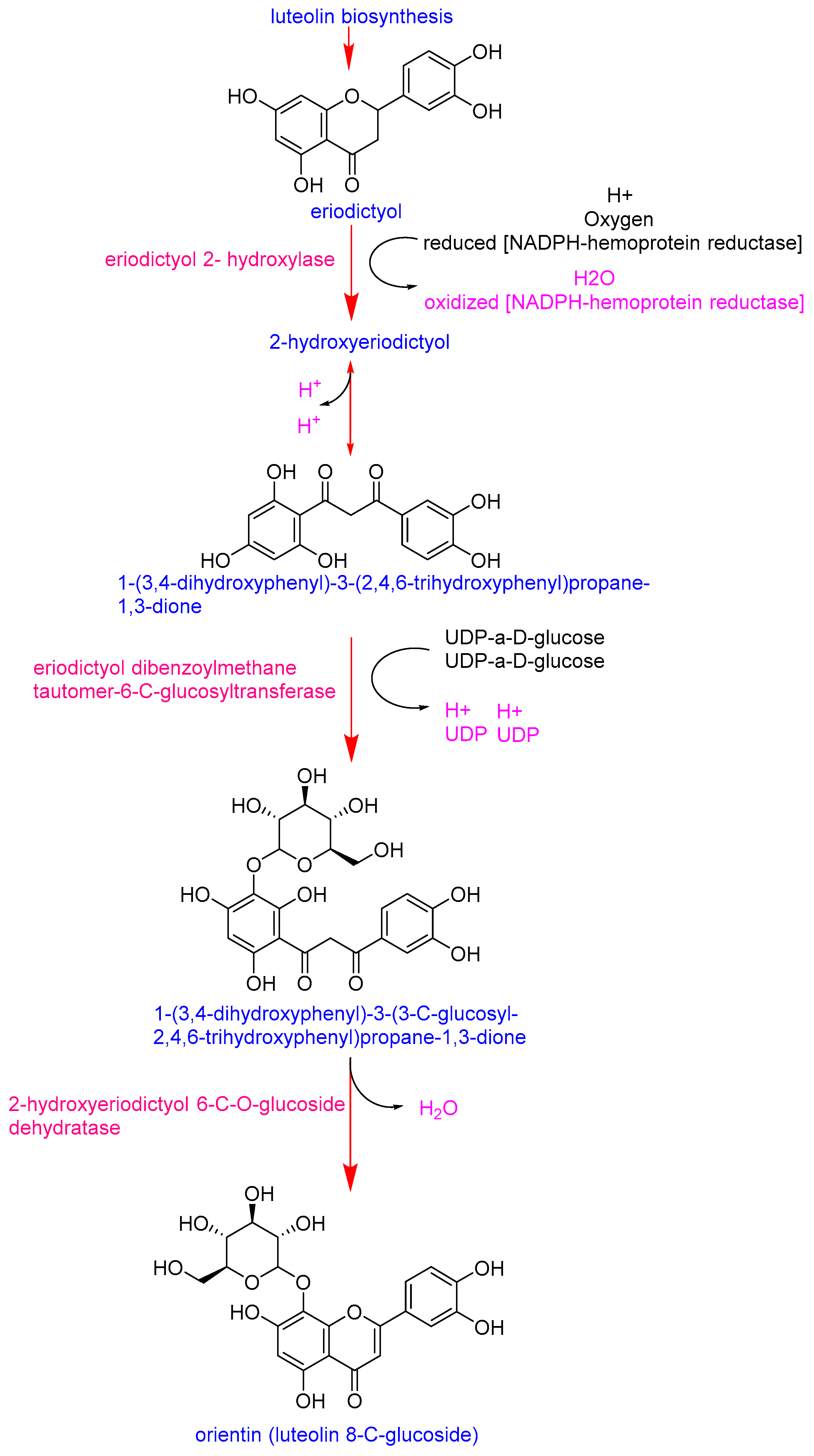
Eriodictyol emerged as the most distinctive marker, with significantly higher intensities in the Pebroter varieties (Log2FC > 5). In contrast, luteolin-8-C-glucoside (orientin) was more abundant in Roli Rosa (Log2FC < −2). Examination of the orientin biosynthetic pathway (Figure 3) shows that its synthesis in plants begins with eriodictyol and involves two key enzymes: eriodictyol 2-hydroxylase and eriodictyol dibenzoylmethane tautomer-6-C-glucosyltransferase [36]. The accumulation of eriodictyol in Pebroter varieties, without conversion to orientin (as observed in Roli Rosa), suggests a deficiency or low expression of the genes encoding these enzymes in Pebroter. Although this metabolic interpretation is consistent with known biosynthetic routes, experimental validation through targeted gene expression or enzyme activity assays would be required to confirm these observations. Quantifying the activity of these two enzymes could therefore provide a useful biochemical marker to differentiate between the two varietal groups. These results are consistent with previous studies in which enzyme profiling effectively distinguished between tomato varieties, reflecting their genetic and metabolic diversity [37].
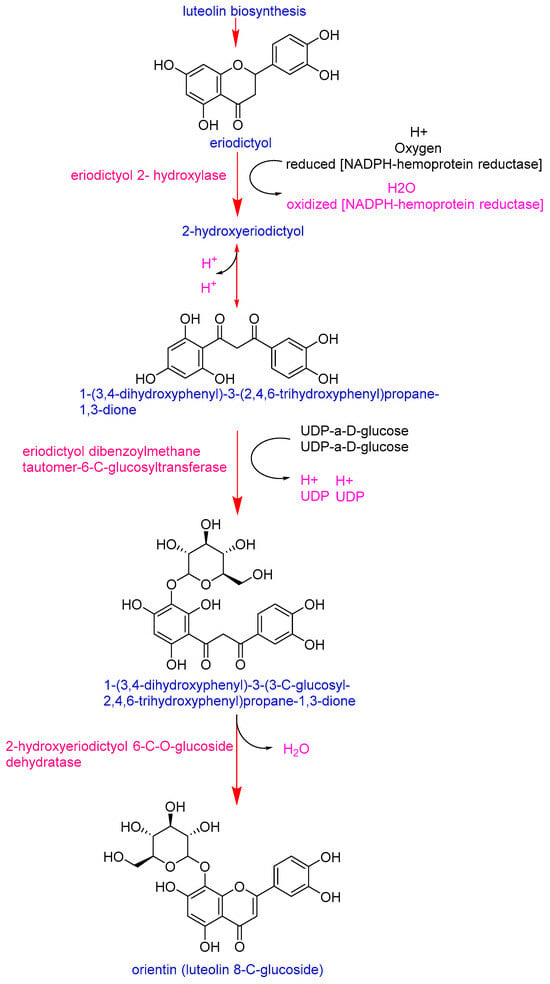
Figure 3.
Biosynthetic pathway of orientin [36].
Furthermore, five phenolic compounds (homovanillic acid, hydroxyphenylacetic acid, hydroxytyrosol, neochlorogenic acid, and chlorogenic acid; Figure S2) showed significant variation between Pebroter and Roli Rosa varieties. Their elevated intensities in Pebroter suggest increased activity or abundance of the enzymes involved in their biosynthetic pathways. Among them, homovanillic acid (Log2FC ≈ 5.2) and hydroxyphenylacetic acid (Log2FC > 4) exhibited the most pronounced differences, with homovanillic acid presenting the largest fold change among phenolic acids.
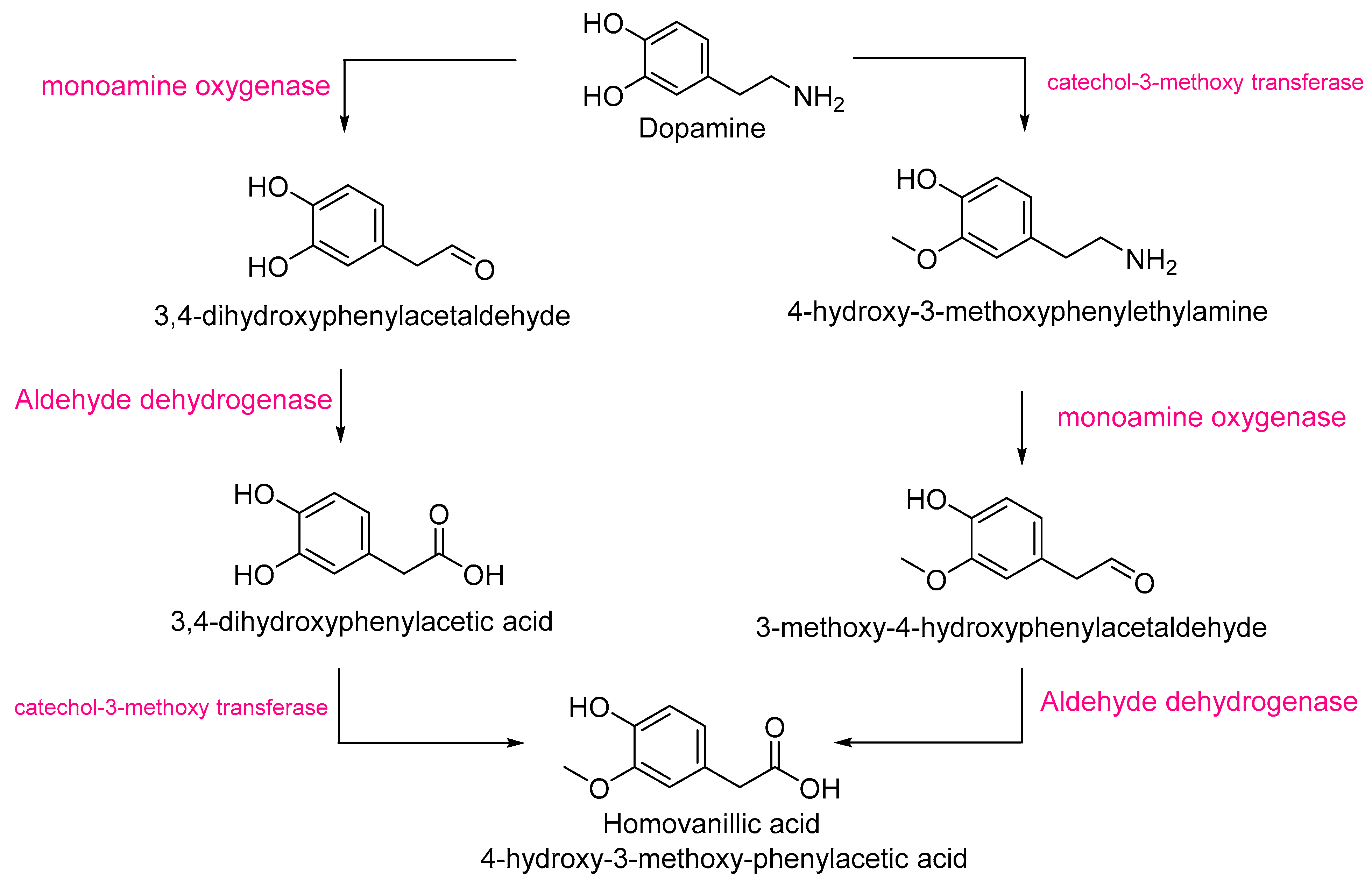
Homovanillic acid is a metabolite of dopamine, which was also significantly more abundant in Pebroter (Log2FC ≈ +2). The biosynthesis of homovanillic acid proceeds via dopamine degradation [38] and involves three enzymes: monoamine oxidase (MAO), catechol-O-methyl transferase (COMT), and aldehyde dehydrogenase (Figure 4). Both MAO [39,40] and COMT [41] have been previously identified in tomato. The higher intensities of homovanillic acid and dopamine in Pebroter could imply enhanced activity or expression of MAO, COMT, and/or aldehyde dehydrogenase compared to Roli Rosa.
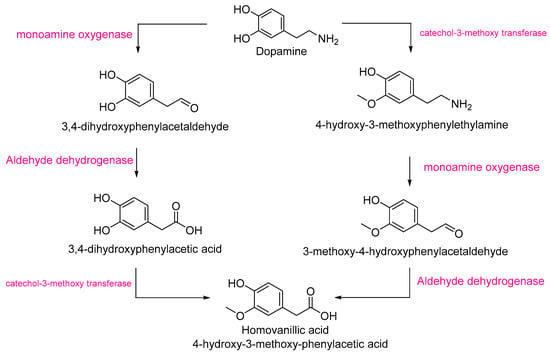
Figure 4.
The homovanillic acid biosynthetic pathway starts from dopamine [38].
3.3. Effects of Fertilization Treatments on the Phytochemical Composition of Tomato Varieties
In contrast to the pronounced varietal differences, PCA revealed no significant separation among the four fertilization treatments across all tomato varieties, with treatments clustering closely together and exhibiting similar distribution patterns (Figure 2B). This observation was supported by one-way ANOVA of the 163 identified compounds, which showed no significant differences in peak intensities (areas) between treatments.
To investigate treatment effects in more detail, we analyzed each variety individually. A t-test comparing the two organic systems with different doses of RCW (T3 vs. T4) revealed no significant differences in compound abundances or peak areas in any variety, indicating that increasing the RCW concentration beyond 7.5 kg/m2 does not influence the phytochemical composition of the tomato fruits. To further examine the effect of using woody residues as an organic fertilizer, OPLS-DA was performed separately for T3 vs. T1 (compost fertilizer with a C/N ratio of 15) and T3 vs. T2.
However, empirical validation using 100 permutation tests indicated that the predictive ability of the OPLS-DA models was not statistically significant for any variety: V1 Q2 p = 0.86, R2Y p = 0.91; V2 Q2 p = 0.93, R2Y p = 0.18; V3 Q2 p = 0.84, R2Y p = 0.54; V4 Q2 p = 0.41, R2Y p = 0.44. The lack of statistically significant Q2 and R2Y values across all varieties indicates that the supervised OPLS-DA models are not overfitted, and therefore, the identified treatment-associated metabolites should be interpreted as preliminary observations. Consequently, while per-variety OPLS-DA identified metabolites that respond to fertilization, these findings are considered exploratory and require confirmation with larger sample sizes.
3.3.1. Effect of Using Ramial Chipped Wood (T3) Versus Commercial Woody Residue Compost (T1) on Tomato Varieties
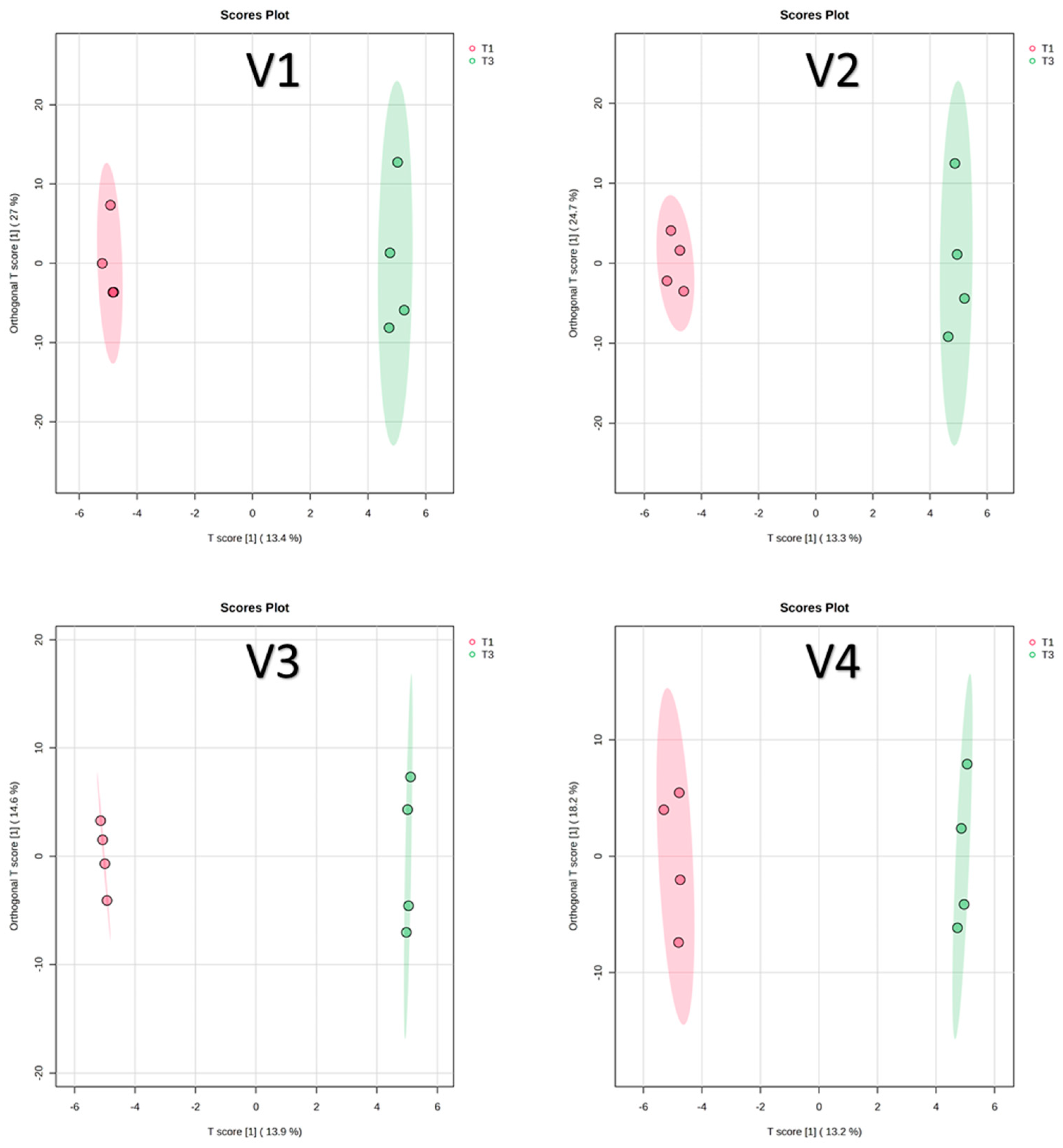
Low fertilization levels are known to affect both tomato yield and phytochemical composition [42]. In this study, a supervised OPLS-DA was conducted to identify key compounds that differentiate between compost (T1) and RCW (T3) treatments within each of the four varieties. The OPLS-DA score plots for T1 versus T3 in all varieties are shown in Figure 5.
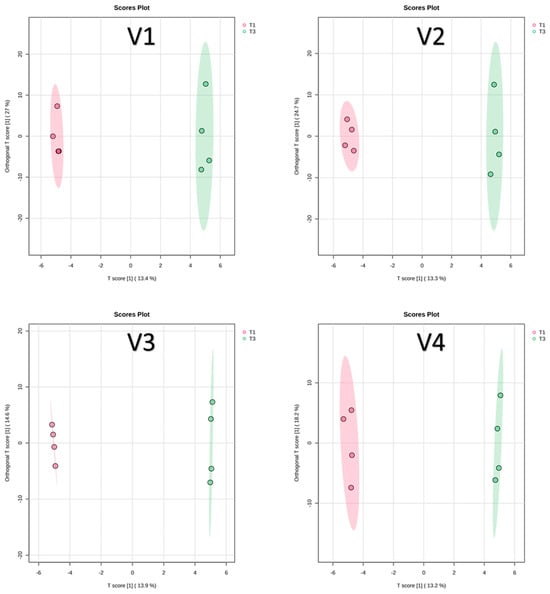
Figure 5.
OPLS-DA score plots for T1 versus T3. V1: Cornabel, V2: Cuban Pepper, V3: Corno Andino, and V4: Roli Rosa. T1: compost fertilizer from woody residues. T3: Ramial chipped wood (15 kg/m2). n = 4 biological replicates per treatment-variety.
In the Cornabel variety (V1), VIP analysis identified 10 marker compounds that significantly distinguished between T1 and T3 (Figure S3). Additionally, Log2FC values were calculated to quantify the differences in marker abundance between treatments (Table 4). Among these, p-coumaric acid showed the highest VIP score (>2.4), indicating greater abundance under the RCW treatment (T3) compared to compost (T1). This likely reflects the lower nitrogen availability in T3 soils and is consistent with previous findings in Cornabel, where replacing nitrogen-rich with nitrogen-poor fertilizers increased p-coumaric acid levels from 0.48 to 0.94 mg/kg [9]. While soil mineral N was not directly measured in this study, the higher p-coumaric acid in T3 may reflect reduced nitrogen availability and stress-induced phenolic synthesis, a common plant defense response [43].

Table 4.
OPLS-DA VIP scores, and Log2fold changes for T1 versus T3, along with the chemical class of each marker compound. The compounds were putatively identified based on accurate mass and retention time.
Similarly, abscisic acid (an organic acid) and trigonelline (the only alkaloid marker identified in Cornabel) showed higher intensities in T3 than in T1. Carbohydrates such as kestose and glucovanillin were also more abundant under RCW treatment, each showing approximately a onefold increase relative to T1. This finding contrasts with previous studies reporting that soluble carbohydrate levels are not affected by increasing the C/N ratio [44].
The Cuban Pepper variety responded differently to RCW treatment compared to Cornabel. Flavonoid intensities decreased, with quercetin diglucoside and luteolin-8-glucoside (orientin) showing significant reductions (Log2FC values of 1.5 and 1.9, respectively). This decline in flavonoid glycosides is consistent with previous reports of decreased rutin and naringenin glucoside in Cuban Pepper under low-nitrogen organic fertilization [9]. Similarly, carbohydrates such as sucrose and sorbitol derivatives also declined under the woody residue treatment (T3). In contrast, the isopentylamine content in T3 was approximately double that of T1. The fatty acid nonanoic acid increased under T3 in Cuban Pepper but decreased in Cornabel. These differing metabolic responses to the same treatment likely reflect genetic differences between the varieties.
The Corno Andino variety responded similarly to Cornabel under T3, though with distinct increases in the flavonoid luteolin and the organic acid succinic acid. In Roli Rosa, six marker compounds had significant VIP scores, predominantly organic acids (pyruvic acid, dihydroxybenzoic acid, homovanillic acid, and adipic acid), which increased by approximately onefold under the compost treatment (T1).
Across all varieties, the high-dose woody residue treatment (T3) consistently increased the abundance of organic acids and amines (Figure S4), whereas flavonoid and carbohydrate responses were more variety-specific. These findings suggest that flavonoid accumulation is more strongly influenced by the tomato variety, whereas organic acids and amines appear more responsive to nutritional stress induced by the application of woody by-products.
3.3.2. Effect of Woody Residue Versus Commercial Nitrogen-Rich Pellets on Tomato Fruit Composition
Scharenbroch & Watson (2014) demonstrated that RCW is an effective, cost-efficient organic amendment for improving soil quality and stimulating tree growth [45]. In this study, we examined whether the application of woody by-products (T3 and T4) influenced the chemical composition of tomato fruits compared to a conventional nitrogen-rich fertilizer (T2). OPLS-DA revealed a clear metabolic separation between T2 and T3 across all four tomato varieties (Figure S5). Potential marker compounds, identified based on VIP(t) scores (>2), are listed in Table 5 and Figure S6 with the corresponding Log2FC values.

Table 5.
OPLS-DA VIP scores, and Log2fold changes for T2 versus T3, along with the chemical class of each marker compound. The compounds were putatively identified based on accurate mass and the retention time.
In the Cornabel variety (V1), treatment with low-nitrogen RCW (T3) led to reduced intensities of several phenolic compounds and organic acids. Specifically, dihydroxyphenyl lactic acid and isoferulic acid decreased by approximately half-fold. Succinic acid, lactic acid and the amino acid gamma-aminobutyric acid (GABA) were also reduced under T3 compared to the nitrogen-rich treatment (T2). In contrast, flavonoids such as iso-schaftosid and eriodictyol glucoside were more abundant under T3.
In the Cuban Pepper variety, a decrease in nitrogen-containing compounds such as malonyl tryptophan and feruloyl tyramine was observed, with an approximate onefold reduction (Log2FC around 0.9) under the high C/N RCW treatment.
In the Corno Andino variety, nine marker compounds showed significant variation between treatments (p-value < 0.05). Among these, two flavonoids (taxifolin and quercetin dihydrate) displayed a notable increase under the low-nitrogen RCW treatment (Log2FC values of −1.7, and −1.5, respectively). In contrast, most nitrogen-containing compounds, including amines and amino acids, decreased in intensity, except for L-homoglutamic acid, which showed a modest half-fold increase.
The Roli Rosa variety showed a similar pattern to Pebroter group, with reduced intensities of phenolic acids (e.g., caffeic acid) and amines (e.g., p-coumaroyl tyramine) (Log2FC values of 0.74 and 1.49, respectively). Notably, L-glutamic acid increased, mirroring the trend observed for L-homoglutamic acid in Corno Andino. These two compounds were the only amino acids that increased in response to wood chip fertilization. As observed for the T1–T3 comparison, permutation testing for the T2–T3 OPLS-DA models showed no statistically significant predictive ability (V1–V4 Q2 p = 0.19–0.92; R2Y p = 0.11–1.00). These findings suggest that the models are not overfitted and that the detected treatment-related metabolic variations should be interpreted as exploratory, requiring confirmation with larger sample sizes.
Overall, consistent with previous findings [46], the RCW treatment tended generally to increased flavonoid levels across all cultivars while decreasing phenolic acids, organic acids, and most nitrogen-containing compounds, except for glutamic acid and its isomer, homoglutamic acid, compared to the unfertilized control.
Finally, post-harvest stability parameters, including physiological loss in weight (PLW), were not evaluated in this study, as all tomato samples were harvested at full maturity, immediately frozen, and analyzed to preserve their native metabolite composition. Consequently, no conclusions can be drawn regarding post-harvest performance or storage behavior under RCW fertilization. Nevertheless, since RCW application may influence the antioxidant profile of tomatoes, future studies should explore its potential effects on post-harvest stability. Moreover, the RCW and compost materials used originated solely from clean plant residues collected from city gardens and forests, minimizing the risk of E. coli or other microbial contamination.
4. Conclusions
In this study, we analyzed four tomato varieties—Cornabel, Cuban Pepper, Corno Andino, and Roli Rosa—under contrasting organic farming practices using untargeted metabolomic profiling via UHPLC-QToF-MS and statistical analyses, with a particular focus on antioxidant-related metabolites. 163 compounds were putatively identified via spectral matching based on accurate mass, ion species, and retention time, using a reference database from the Max Rubner-Institut. Among them, 37 compounds were identified as biomarkers capable of distinguishing between the Pebroter and Roli Rosa varieties: 30 polyphenolic compounds were more abundant in Pebroter, while seven phenolic compounds and amines were enriched in Roli Rosa. Notably, eriodictyol was highly abundant in Pebroter, whereas Roli Rosa accumulated orientin, suggesting lower expression or activity of enzymes responsible for converting eriodictyol to orientin in Pebroter, while further validation through targeted gene expression or enzyme activity analyses is recommended.
Although PCA did not reveal statistically significant differences across fertilization treatments, variety-specific responses were observed. RCW showed tendency for increasing the abundance of several antioxidant compounds and related metabolites such as phenolic and organic acids (e.g., p-coumaric acid, abscisic acid, succinic acid, pyruvic acid, and dihydroxybenzoic acid), compared to commercial compost; however the concentration of RCW had no significant effect on the overall phytochemical composition. RCW also slightly increased levels of nitrogen-containing compounds, such as amines and alkaloids, relative to the compost. Compared to the control, flavonoid levels generally tended to increase under RCW, whereas phenolic acids tended to decline, though these effects were variety-dependent. Importantly, first-season yield reductions, variability among varieties, differences in nitrogen availability, and the use of tillage in T1/T2 versus no tillage in T3/T4 highlight the need for multi-season trials and further studies to confirm the agronomic performance of RCW under diverse management conditions. Moreover, the multivariate models comparing fertilization treatments indicated no separation, suggesting that further investigation is needed to confirm these preliminary metabolic trends. Overall, our findings support the use of RCW as a sustainable organic fertilizer that may influence the antioxidant metabolite profile of tomatoes; further studies with unfertilized controls are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14111330/s1, Figure S1: Chemical structures of the main markers; Figure S2: Phenolic compounds showed significant variation between the Pebroter and Roli Rosa varieties; Figure S3: OPLS-DA VIP scores for the selected compounds in T1 versus T3; Figure S4: Box-plots of organic acids and amines show variations between T1 versus T3; Figure S5: OPLS-DA score plots for T2 versus T3. V1: Cornabel; Figure S6: OPLS-DA VIP score for the selected compounds in T2 versus T3; Table S1: Internal standards used in UHPLC-QToF-MS evaluation of instrument performance; Table S2: List of identified compounds; Table S3: Differentially accumulated metabolites between treatments with corresponding raw and FDR-adjusted p-values and Fisher’s LSD comparisons.
Author Contributions
Methodology, M.M.A.; Validation, J.L.-C., J.G.-C. and S.T.S.; Formal analysis, M.M.A.; Investigation, M.M.A. and S.T.S.; Data curation, M.M.A., C.A.-R. and J.L.-C.; Writing—original draft, M.M.A.; Writing—review & editing, A.V.-Q., S.T.S., R.M.L.-R., M.P. and J.R.; Supervision, A.V.-Q., S.T.S., R.M.L.-R. and M.P.; Project administration, R.M.L.-R., M.P. and J.R.; Funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project HORTBIOSOL (Grant TED2021-130783B-C21), funded by MICIU/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR, and by the project SOILCARE (Grant PID2023-146650OB-I00), funded by MICIU/AEI/10.13039/501100011033 and ERDF/EU, UE. This work was also supported by [PID2020-114022RB-I00], CIBEROBN from the Instituto de Salud Carlos III (ISCIII) of the Ministerio de Ciencia, Innovación y Universidades (AEI/FEDER, UE), Generalitat de Catalunya (GC) [2021-SGR-00334] [2021-SGR-00311], and the INSA-UB Unit of Excellence (María de Maeztu CEX2021-001234-M funded by MICIN/AEI/FEDER, UE).
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
This study did not involve humans.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
Mohamed M. Abuhabib is grateful for the predoctoral scholarship FI-SDUR (REU/551/2022) from the Agency of Management of University and Research Grants (AGAUR), GC. Also Mohamed M. Abuhabib wants to thank INSA-UB for the fund of the research stay at Max-Rubner Institute, Karlsruhe, Germany. Clara Abarca-Rivas thanks MICIU/AEI/10.13039/501100011033 and FSE+ for her doctoral fellowship (PRE2022-104187). Johana González-Coria thanks the National Scholarship Program of Paraguay “Carlos Antonio López” (BECAL-183/2021). R.M.L-R would like to thank the GC for the ICREA academia recognition. We also extend our thanks to the FoodPhyt project, under the umbrella of the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL) (2019–02201) UE.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Geetha, P.; Indhu Rani, C. Post Harvest Technology and Value Addition of Tomatoes. Food Sci. Res. J. 2020, 11, 217–229. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e314. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi De Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Home Cooking and Phenolics: Effect of Thermal Treatment and Addition of Extra Virgin Olive Oil on the Phenolic Profile of Tomato Sauces. J. Agric. Food Chem. 2014, 62, 3314–3320. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Martínez-Huélamo, M.; Jáuregui, O.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Phenolic Profile and Hydrophilic Antioxidant Capacity as Chemotaxonomic Markers of Tomato Varieties. J. Agric. Food Chem. 2011, 59, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Ganugi, P.; Fiorini, A.; Tabaglio, V.; Capra, F.; Zengin, G.; Bonini, P.; Caffi, T.; Puglisi, E.; Trevisan, M.; Lucini, L. The Functional Profile and Antioxidant Capacity of Tomato Fruits Are Modulated by the Interaction between Microbial Biostimulants, Soil Properties, and Soil Nitrogen Status. Antioxidants 2023, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2021, 62, 5658–5677. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, J.C.; Díaz-Flores, P.E.; Zavala-Sierra, D. Organic vs Conventional Fertilization: Soil Nutrient Availability, Production, and Quality of Tomato Fruit. Water Air Soil Pollut. 2022, 233, 87. [Google Scholar] [CrossRef]
- González-Coria, J.; Lozano-Castellón, J.; Jaime-Rodríguez, C.; Olmo-Cunillera, A.; Laveriano-Santos, E.P.; Pérez, M.; Lamuela-Raventós, R.M.; Puig, J.; Vallverdú-Queralt, A.; Romanyà, J. The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties. Antioxidants 2022, 11, 2127. [Google Scholar] [CrossRef]
- Eyhorn, F.; Muller, A.; Reganold, J.P.; Frison, E.; Herren, H.R.; Luttikholt, L.; Mueller, A.; Sanders, J.; Scialabba, N.E.H.; Seufert, V.; et al. Sustainability in Global Agriculture Driven by Organic Farming. Nat. Sustain. 2019, 2, 253–255. [Google Scholar] [CrossRef]
- Andrés, P.; Doblas-Miranda, E.; Rovira, P.; Bonmatí, A.; Ribas, À.; Mattana, S.; Romanyà, J. Research for AGRI Committee. Agricultural Potential in Carbon Sequestration. Humus Content of Land Used for Agriculture and CO2 Storage; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2022; Volume 103, pp. 655–699. [Google Scholar]
- Lahmar, R.; Ruellan, A. Soil Degradation in the Mediterranean Region and Cooperative Strategies. Cah. Agric. 2007, 16, 318–323. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-cobena, A.; Garnier, J.; Vallejo, A. The Potential of Organic Fertilizers and Water Management to Reduce N2O Emissions in Mediterranean Climate Cropping Systems. A Review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Poesen, J.; Lugato, E.; Scarpa, S.; Montanarella, L.; Borrelli, P. A Soil Erosion Indicator for Supporting Agricultural, Environmental and Climate Policies in the European Union. Remote Sens. 2020, 12, 1365. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Seifollahi-aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil Degradation in the European Mediterranean Region: Processes, Status and Consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Maréchal, A.; Panagos, P.; Jones, A.; Arias Navarro, C.; Ballabio, C.; Belitrandi, D.; Breure, T.; De Medici, D.; De Rosa, D.; Fendrich, A.; et al. EUSO Annual Bulletin, EUR 31506 EN; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-68-03434-7. [Google Scholar] [CrossRef]
- Zaman, K.; Moemen, M.A. Energy Consumption, Carbon Dioxide Emissions and Economic Development: Evaluating Alternative and Plausible Environmental Hypothesis for Sustainable Growth. Renew. Sustain. Energy Rev. 2017, 74, 1119–1130. [Google Scholar] [CrossRef]
- Quirós, R.; Halog, A.; Muñoz, P. Environmental Assessment of Two Irrigation Systems in an Organic Tomato Crop System Under Manure Compost Fertilization: A Sustainable Circular Economy Approach. Circ. Econ. Sustain. 2022, 2, 1445–1462. [Google Scholar] [CrossRef]
- Robert, N.; Tanguy, M.; Riss, J.; Gallois, R. Effects of Ramial Chipped Wood Amendments on Weed Control, Soil Properties and Tomato Crop Yield. Acta Hortic. 2014, 1018, 383–390. [Google Scholar] [CrossRef]
- Lal, R. Geoderma Food Security Impacts of the “4 per Thousand” Initiative. Geoderma 2020, 374, 114427. [Google Scholar] [CrossRef]
- Tonfack, L.B.; Bernadac, A.; Youmbi, E.; Mbouapouognigni, V.P.; Ngueguim, M.; Akoa, A. Impact of Organic and Inorganic Fertilizers on Tomato Vigor, Yield and Fruit Composition under Tropical Andosol Soil Conditions. Fruits 2009, 64, 167–177. [Google Scholar] [CrossRef]
- Berner, A.; Hildermann, I.; Fließbach, A.; Pfiffner, L.; Niggli, U.; Mäder, P. Crop Yield and Soil Fertility Response to Reduced Tillage under Organic Management. Soil Tillage Res. 2008, 101, 89–96. [Google Scholar] [CrossRef]
- Yanar, D.; Lu, N.G.Ğ.; Yanar, Y.; Aydin, M.; Çakmak, P. Effect of Different Organic Fertilizers on Yield and Fruit Quality of Indeterminate Tomato (Lycopersicon Esculentum). Sci. Res. Essays 2011, 6, 3623–3628. [Google Scholar] [CrossRef]
- Barthès, B.G.; Manlay, R.J.; Porte, O. Effects of Ramial Wood Amendments on Crops and Soil: A Synthesis of Experimental Results. Cah. Agric. 2010, 19, 280–287. [Google Scholar] [CrossRef]
- Soumare, M.D.; Mnkeni, P.N.S.; Khouma, M. Effects of Casuarina Equisetifolia Composted Litter and Ramial-Wood Chips on Tomato Growth and Soil Properties in Niayes, Senegal. Biol. Agric. Hortic. 2002, 20, 111–123. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Venieraki, A.; Chorianopoulou, S.N.; Katinakis, P. Impact of Elemental Sulfur on the Rhizospheric Bacteria of Durum Wheat Crop Cultivated on a Calcareous Soil. Plants 2019, 8, 379. [Google Scholar] [CrossRef]
- González-Coria, J.; Ioan, M.-D.; Hohmann, P.; Segarra, G.; Pérez-Llorca, M.; Pérez, M.; Vallverdú-Queralt, A.; Romanyà, J. Early-Stage Effects of Carbon-Rich Soil Amendments Stimulate Retention-Related Nitrogen Genes While Maintaining Nitrogen and Yield Levels. Soil Tillage Res. 2025, 254, 106729. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Pérez, M.; Vallverdú-Queralt, A.; Hernández, R.; Chantry, O.; Romanyà, J. Increasing Soil Organic Matter and Short-Term Nitrogen Availability by Combining Ramial Chipped Wood with a Crop Rotation Starting with Sweet Potato. Agric. Ecosyst. Environ. 2025, 392, 109740. [Google Scholar] [CrossRef]
- Carravedo Fantova, M. Variedades Autóctonas de Tomates de Aragón; C.I.T.A.: Zaragoza, Spain, 2006; ISBN 84-7753-436-5. [Google Scholar]
- Beer, F.; Weinert, C.H.; Wellmann, J.; Hillebrand, S.; Ley, J.P.; Soukup, S.T.; Kulling, S.E. Comprehensive Metabolome Characterization of Leaves, Internodes, and Aerial Roots of Vanilla Planifolia by Untargeted LC–MS and GC × GC–MS. Phytochem. Anal. 2025, 36, 30–51. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A Lipidome Atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Ercolano, M.R.; Carli, P.; Soria, A.; Cascone, A.; Fogliano, V.; Frusciante, L.; Barone, A. Biochemical, Sensorial and Genomic Profiling of Traditional Italian Tomato Varieties. Euphytica 2008, 164, 571–582. [Google Scholar] [CrossRef]
- Bontempo, L.; van Leeuwen, K.A.; Paolini, M.; Holst Laursen, K.; Micheloni, C.; Prenzler, P.D.; Ryan, D.; Camin, F. Bulk and Compound-Specific Stable Isotope Ratio Analysis for Authenticity Testing of Organically Grown Tomatoes. Food Chem. 2020, 318, 126426. [Google Scholar] [CrossRef]
- PlantCyc Orientin and Isoorientin Biosynthesis I. Available online: https://pmn.plantcyc.org/pathway?orgid=SWEETCHERRY&id=PWY-7188 (accessed on 13 January 2025).
- Lozano-Castellón, J.; Laveriano-Santos, E.P.; Abuhabib, M.M.; Pozzoli, C.; Pérez, M.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Proven Traceability Strategies Using Chemometrics for Organic Food Authenticity. Trends Food Sci. Technol. 2024, 147, 104430. [Google Scholar] [CrossRef]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Park. Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef] [PubMed]
- Plantcyc Monoamine Oxidase. Available online: https://pmn.plantcyc.org/gene?orgid=TOMATO&id=MONOMER-13652 (accessed on 1 July 2024).
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-Phenylethanol in Roses as the Dominant Floral Scent Compound from L-Phenylalanine by Two Key Enzymes, a PLP-Dependent Decarboxylase and a Phenylacetaldehyde Reductase. Biosci. Biotechnol. Biochem. 2007, 71, 2408–2419. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Tieman, D.M.; Floystad, A.; Taylor, M.G.; Klee, H.J. A Solanum lycopersicum Catechol-O-Methyltransferase Involved in Synthesis of the Flavor Molecule Guaiacol. Plant J. 2012, 69, 1043–1051. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P.; Heeb, A. Influence of Different Types of Fertilisers on the Major Antioxidant Components of Tomatoes. J. Food Compos. Anal. 2006, 19, 20–27. [Google Scholar] [CrossRef]
- Czech, A.; Szmigielski, M.; Sembratowicz, I. Nutritional Value and Antioxidant Capacity of Organic and Conventional Vegetables of the Genus Allium. Sci. Rep. 2022, 12, 18713. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Robin, C. Is the C:N Ratio a Reliable Indicator of C Allocation to Primary and Defence-Related Metabolisms in Tomato? Phytochemistry 2013, 88, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Scharenbroch, B.C.; Watson, G.W. Wood Chips and Compost Improve Soil Quality and Increase Growth of Acer Rubrum and Betula Nigra in Compacted Urban Soil. Arboric. Urban For. 2014, 40, 319–331. [Google Scholar] [CrossRef]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic Characterization and Variability in Leaves, Stems and Roots of Micro-Tom and Patio Tomatoes, in Response to Nitrogen Limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).