Epigallocatechin Gallate Ameliorates Granulosa Cell Developmental via the Eukaryotic Initiation Factor 2 Alpha/Activating Transcription Factor 4 Pathway in Hyperthyroid Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animal Treatments
2.3. Granulosa Cell Isolation and Culture
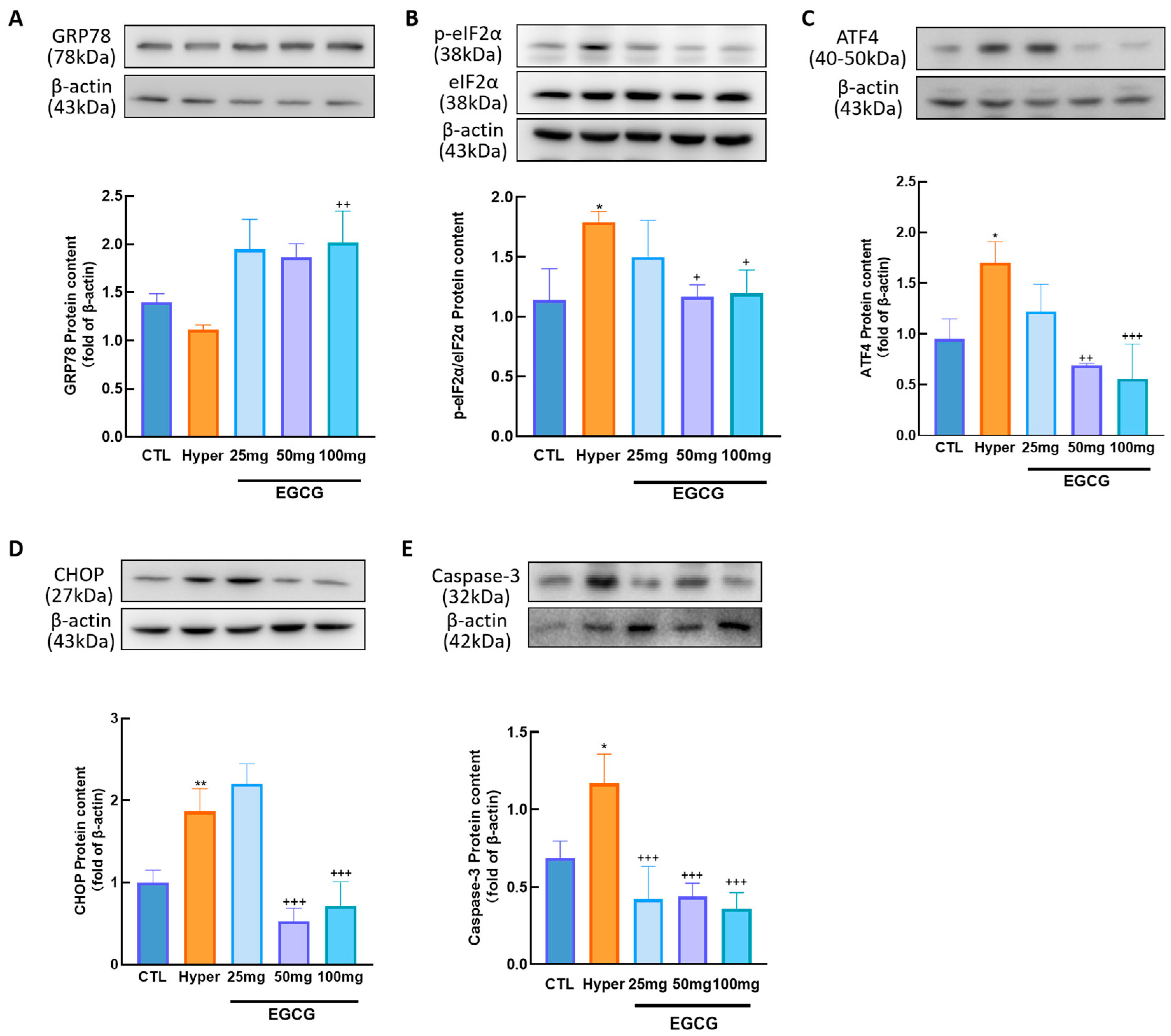
2.4. Protein Extraction and Western Blotting
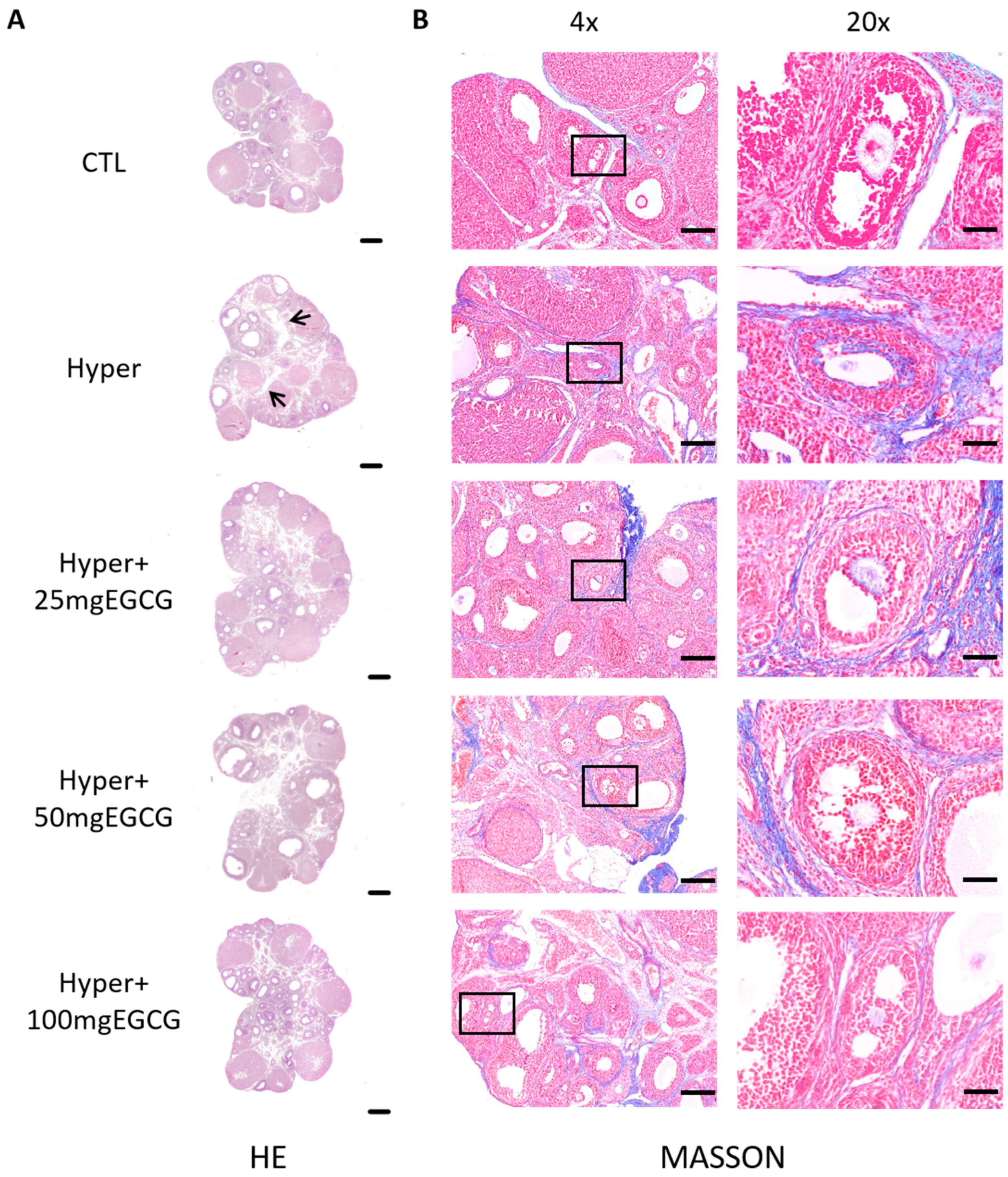
2.5. Hematoxylin-Eosin (HE) Staining
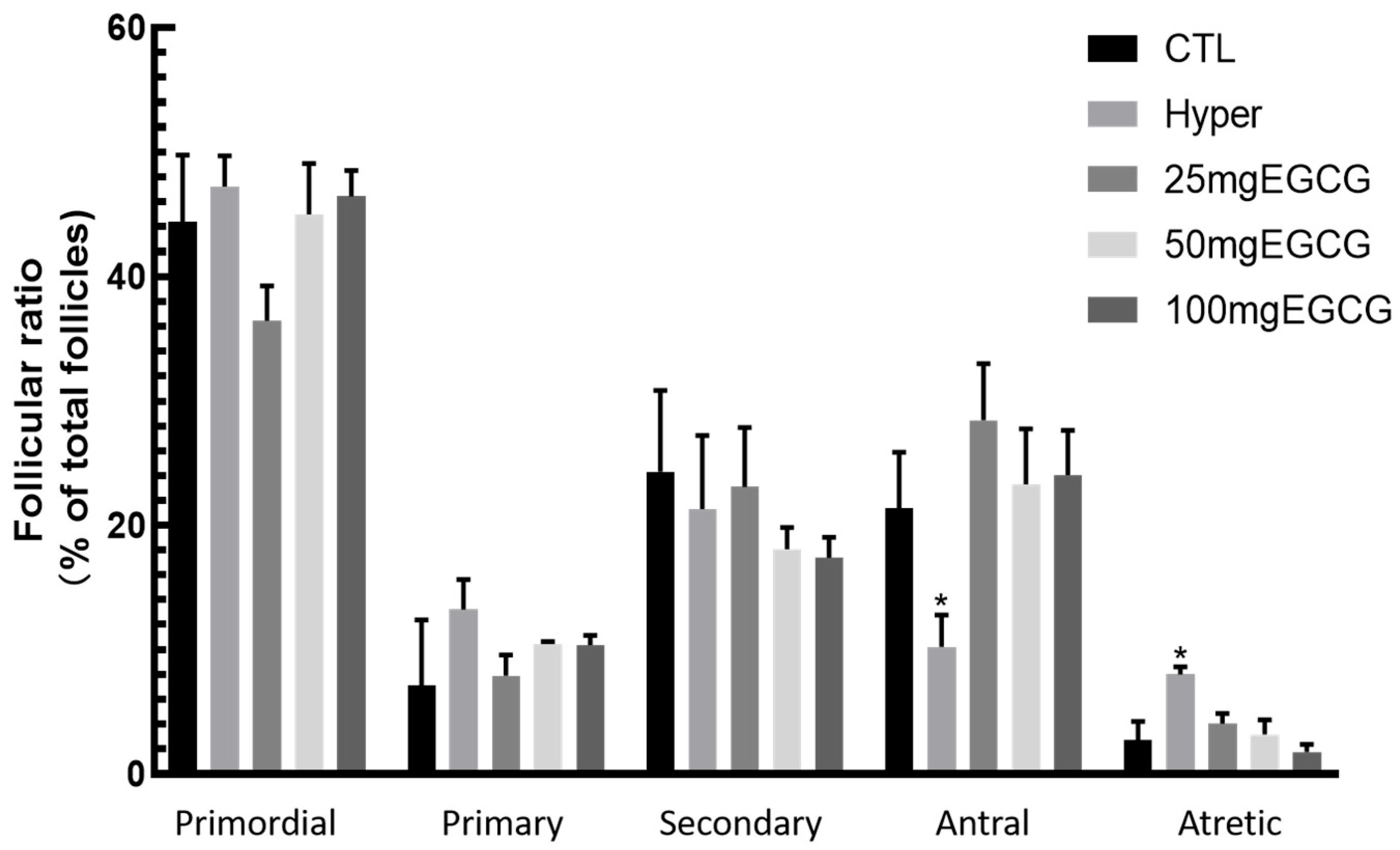
2.6. Follicle Counting Methodology
2.7. Masson Trichrome Staining
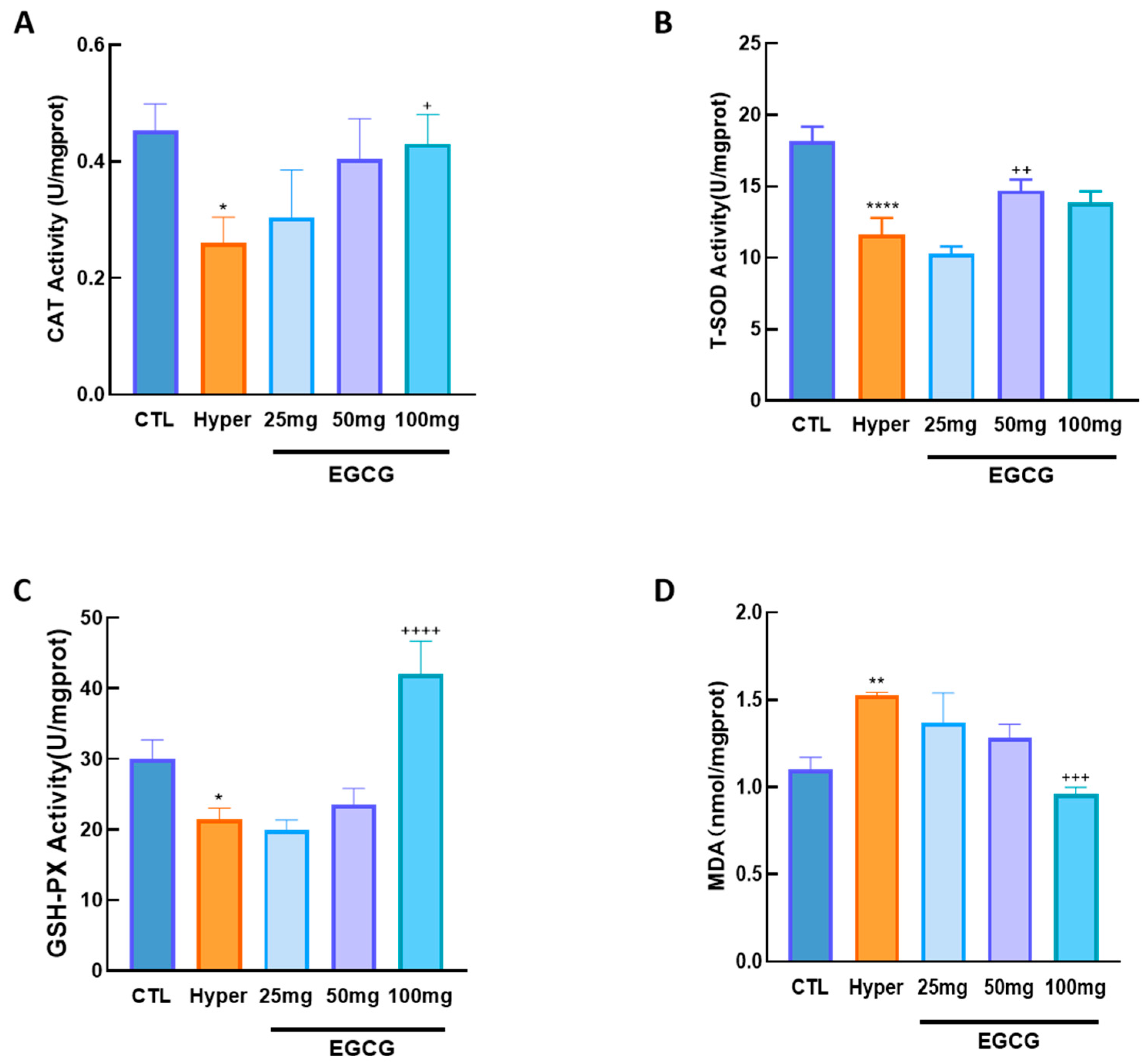
2.8. Quantification of Oxidative Stress Markers
2.8.1. SOD Activity Assay
2.8.2. GSH-PX Activity Assay
2.8.3. CAT Activity Assay
2.8.4. MDA Content Determination
2.9. ROS Detection
2.10. Analysis of Cell Viability
2.11. EdU Detection
2.12. TUNEL Assay
2.13. Statistical Analysis
3. Results
3.1. Effects of EGCG on the Ovarian Structure of Hyperthyroid Rats
3.2. Effects of EGCG on Ovarian OS
3.3. Effects of EGCG on Ovarian ERS
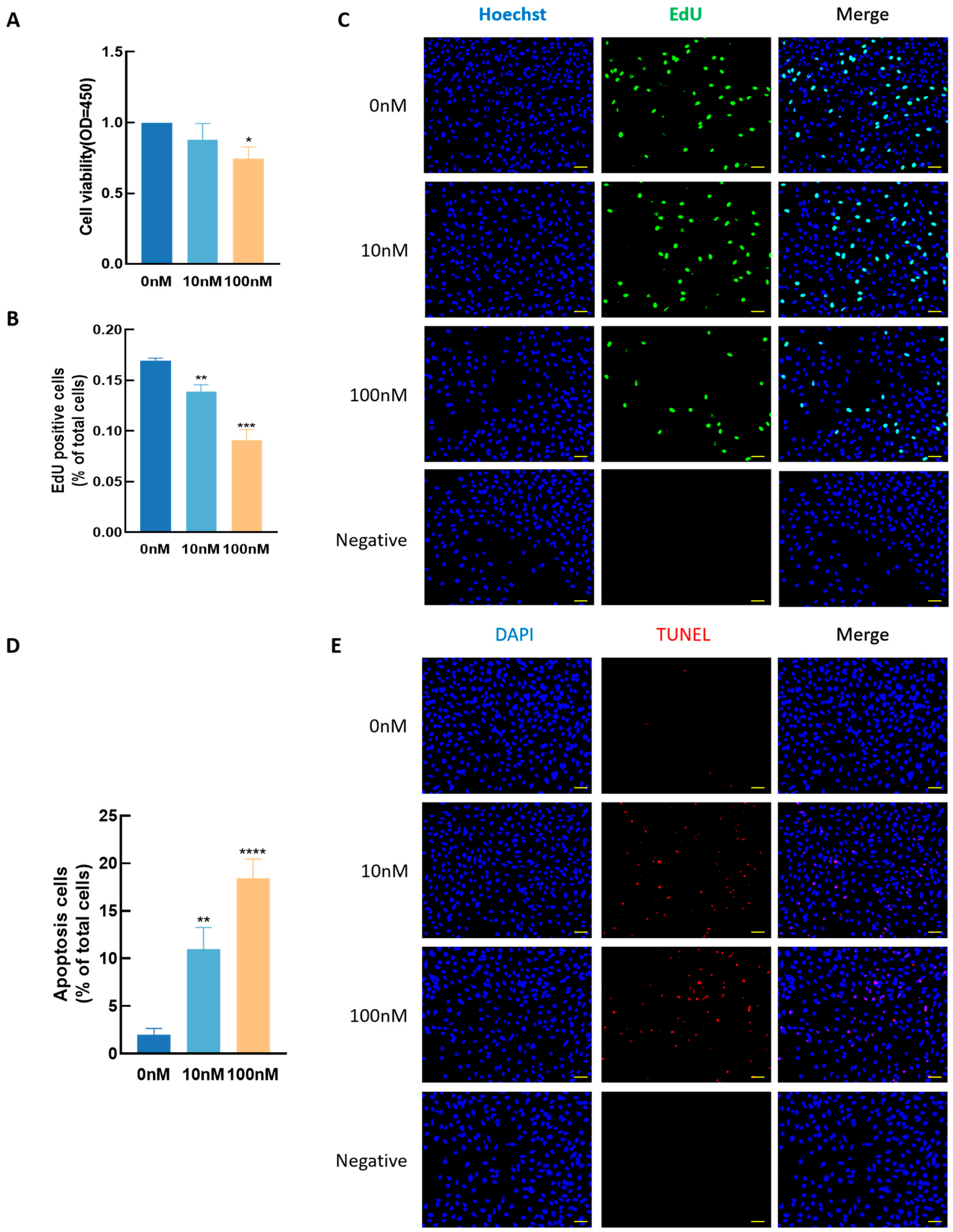
3.4. Effect of T3 GCs Growth
3.5. Effects of EGCG on GC Growth
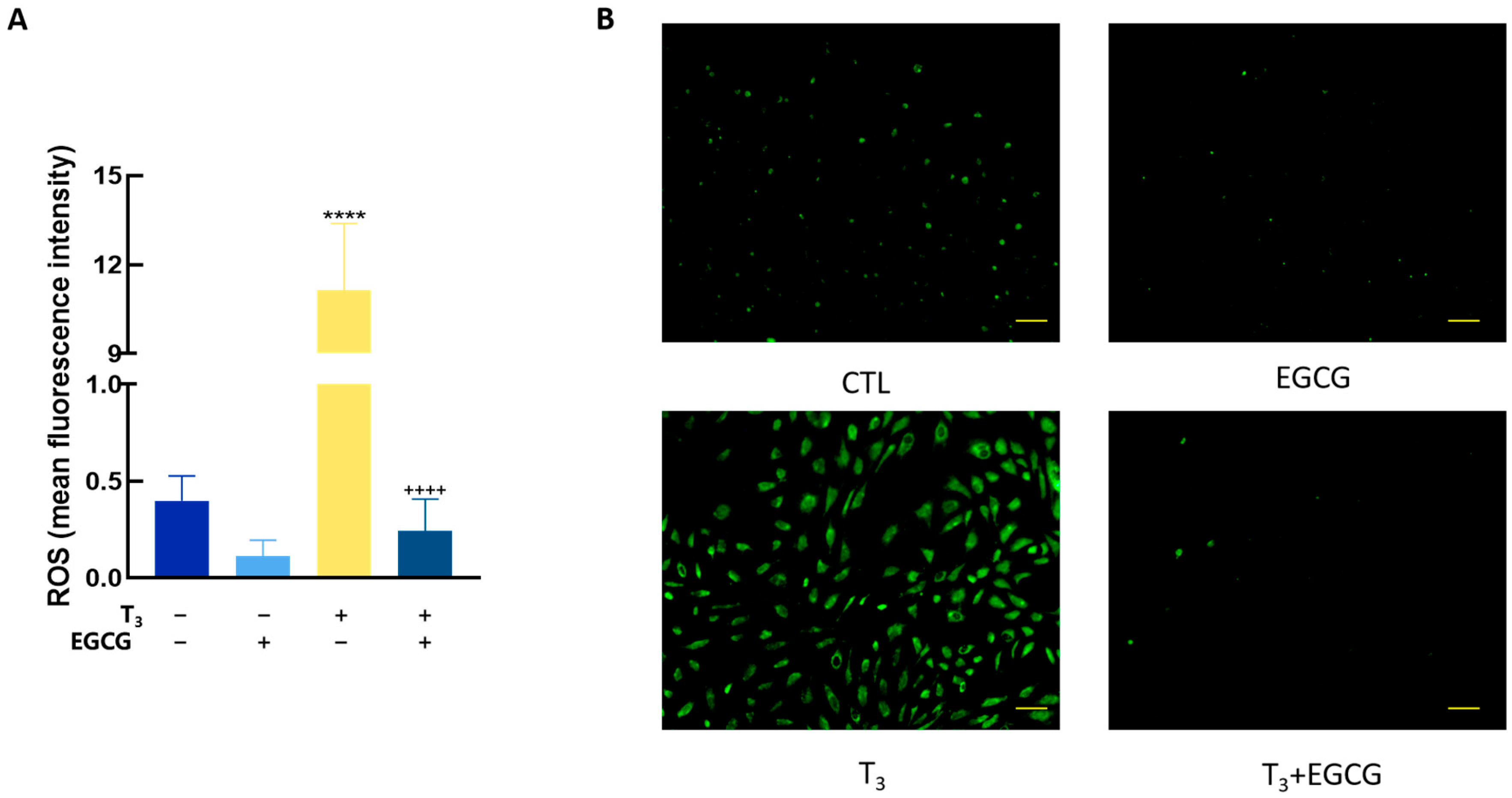
3.6. EGCG Decreased T3-Induced ROS in GCs
3.7. EGCG Decreased T3-Induced ERS in GCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, C.J.; Bevilacqua, C.; Prevedel, R. Mechanical mapping of mammalian follicle development using Brillouin microscopy. Commun. Biol. 2021, 4, 1133. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef]
- Akande, E.O.; Hockaday, T.D. Plasma luteinizing hormone levels in women with thyrotoxicosis. J. Endocrinol. 1972, 53, 173–174. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shi, Y.; Yu, X.; Zhao, Y.; Wei, B.; Huo, M.; Lu, L.; Li, L.; Gao, Q.; Sun, M. THRA1/PGC-1α/SIRT3 pathway regulates oxidative stress and is implicated in hypertension of maternal hypothyroid rat offspring. Hypertens. Res. 2024, 48, 1080–1098. [Google Scholar] [CrossRef]
- Barreiro Arcos, M.L. Role of thyroid hormones-induced oxidative stress on cardiovascular physiology. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130239. [Google Scholar] [CrossRef]
- Sultana, D.R.; Shahin, A.D.; Md Jawadul, H. Measurement of oxidative stress and total antioxidant capacity in hyperthyroid patients following treatment with carbimazole and antioxidant. Heliyon 2022, 8, e08651. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Videla, L.A.; Fernández, V.; Cornejo, P.; Vargas, R.; Carrasco, J.; Fernández, J.; Varela, N. Causal role of oxidative stress in unfolded protein response development in the hyperthyroid state. Free Radic. Biol. Med. 2015, 89, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Zhang, C. Roles of Grp78 in Female Mammalian Reproduction. Adv. Anat. Embryol. Cell Biol. 2017, 222, 129–155. [Google Scholar] [CrossRef]
- Pae, M.; Wu, D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: Mechanisms and applications. Food Funct. 2013, 4, 1287–1303. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, A.; Lv, D.; Cong, L.; Sun, Z.; Liu, L. EGCG activates Keap1/P62/Nrf2 pathway, inhibits iron deposition and apoptosis in rats with cerebral hemorrhage. Sci. Rep. 2024, 14, 31474. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Mikuriya, H.; Tayama, K.; Takahashi, H.; Nagasawa, A.; Yano, N.; Yuzawa, K.; Ogata, A.; Aoki, N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch. Toxicol. 2001, 75, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef]
- Hegde, S.; Poojary, K.K.; Rasquinha, R.; Crasta, D.N.; Gopalan, D.; Mutalik, S.; Siddiqui, S.; Adiga, S.K.; Kalthur, G. Epigallocatechin-3-gallate (EGCG) protects the oocytes from methyl parathion-induced cytoplasmic deformities by suppressing oxidative and endoplasmic reticulum stress. Pestic. Biochem. Physiol. 2020, 167, 104588. [Google Scholar] [CrossRef]
- Xu, K.; Tian, Y.; Weng, X.; Hu, X.; Heng, D.; Xia, G.; Zhang, C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2020, 379, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell. Biochem. 2018, 448, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, N.; Gan, X.; Chen, L.; Wang, R.; Liang, R.; Jian, J. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 119, 154999. [Google Scholar] [CrossRef]
- Ma, X.; Weng, X.; Hu, X.; Wang, Q.; Tian, Y.; Ding, Y.; Zhang, C. Roles of different n-3/n-6 PUFA ratios in ovarian cell development and steroidogenesis in PCOS rats. Food Funct. 2019, 10, 7397–7406. [Google Scholar] [CrossRef]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef]
- Yoshida, M.; Sanbuissyo, A.; Hisada, S.; Takahashi, M.; Ohno, Y.; Nishikawa, A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J. Toxicol. Sci. 2009, 34 (Suppl. S1), Sp189–Sp197. [Google Scholar] [CrossRef]
- Andersen, S.L.; Olsen, J.; Wu, C.S.; Laurberg, P. Spontaneous abortion, stillbirth and hyperthyroidism: A danish population-based study. Eur. Thyroid. J. 2014, 3, 164–172. [Google Scholar] [CrossRef]
- Zheng, K.; Sulieman, F.J.; Li, J.; Wei, Q.; Xu, M.; Shi, F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod. Biol. 2015, 15, 27–33. [Google Scholar] [CrossRef]
- Kitajima, M.; Murakami, N.; Kitajima, Y.; Kajimura, I.; Matsumura, A.; Matsumoto, K.; Harada, A.; Miura, K. Accumulation of fibrosis and altered perifollicular stromal differentiation in vitrified-thawed human ovarian tissue xenografted to nude mice. Reprod. Med. Biol. 2022, 21, e12451. [Google Scholar] [CrossRef]
- Halliwell, B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat. Res. 1999, 443, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Kobayashi, N.; Orisaka, M.; Cao, M.; Kotsuji, F.; Leader, A.; Sakuragi, N.; Tsang, B.K. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 2009, 150, 5566–5574. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, G.; Tsang, B.K. Interactions of thyroid hormone and FSH in the regulation of rat granulosa cell apoptosis. Front. Biosci. (Elite Ed) 2011, 3, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Mogulkoc, R.; Baltaci, A.K.; Oztekin, E.; Aydin, L.; Tuncer, I. Hyperthyroidism causes lipid peroxidation in kidney and testis tissues of rats: Protective role of melatonin. Neuro Endocrinol. Lett. 2005, 26, 806–810. [Google Scholar] [PubMed]
- Jena, S.; Chainy, G.B. Regulation of expression of antioxidant enzymes by vitamin E and curcumin in L-thyroxine-induced oxidative stress in rat renal cortex. Mol. Biol. Rep. 2011, 38, 1047–1054. [Google Scholar] [CrossRef]
- Das, K.; Chainy, G.B. Thyroid hormone influences antioxidant defense system in adult rat brain. Neurochem. Res. 2004, 29, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Roy, A.; Bhanja, S.; Chainy, G.B. Hypothyroidism impairs antioxidant defence system and testicular physiology during development and maturation. Gen. Comp. Endocrinol. 2008, 156, 63–70. [Google Scholar] [CrossRef]
- Luo, D.; Fan, N.; Zhang, X.; Ngo, F.Y.; Zhao, J.; Zhao, W.; Huang, M.; Li, D.; Wang, Y.; Rong, J. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. Elife 2022, 11, e72182. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Gopal, U.; Austin, R.C.; Pizzo, S.V. Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders. IUBMB Life 2021, 73, 843–854. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, C. Effects of thyroid hormone on ovarian cell apoptosis in the rat. Reprod. Fertil. Dev. 2020, 32, 1060–1066. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Facchinetti, M.M. Heme-Oxygenase-1. Antioxid. Redox Signal 2020, 32, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xiang, J.; Shan, W.; Li, M.; Zhou, W.; Han, X.; Chen, F. Epigallocatechin-3-Gallate Inhibits Ethanol-Induced Apoptosis Through Neurod1 Regulating CHOP Expression in Pancreatic β-Cells. Anat. Rec. 2016, 299, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Harini, L.; Krishnakumar, V.; Kannan, V.R.; Sundar, K.; Kathiresan, T. Insights on the involvement of (-)-epigallocatechin gallate in ER stress-mediated apoptosis in age-related macular degeneration. Apoptosis An. Int. J. Program. Cell Death 2017, 22, 72–85. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, M. Epigallocatechin Gallate Reduces Amyloid β-Induced Neurotoxicity via Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol. Nutr. Food Res. 2018, 62, e1700890. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, R.; Sun, K.; Wang, X.; Wang, J.; Yang, C.S.; Zhang, J. Synergistic toxicity of epigallocatechin-3-gallate and diethyldithiocarbamate, a lethal encounter involving redox-active copper. Free Radic. Biol. Med. 2017, 113, 143–156. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, M.; Feng, H.; Yao, Y.; Chen, R.; Yang, Y.; Zhang, C. Epigallocatechin Gallate Ameliorates Granulosa Cell Developmental via the Eukaryotic Initiation Factor 2 Alpha/Activating Transcription Factor 4 Pathway in Hyperthyroid Female Rats. Antioxidants 2025, 14, 1092. https://doi.org/10.3390/antiox14091092
Sun Y, Wu M, Feng H, Yao Y, Chen R, Yang Y, Zhang C. Epigallocatechin Gallate Ameliorates Granulosa Cell Developmental via the Eukaryotic Initiation Factor 2 Alpha/Activating Transcription Factor 4 Pathway in Hyperthyroid Female Rats. Antioxidants. 2025; 14(9):1092. https://doi.org/10.3390/antiox14091092
Chicago/Turabian StyleSun, Ying, Mingqi Wu, Haoyuan Feng, Yilin Yao, Rui Chen, Yanzhou Yang, and Cheng Zhang. 2025. "Epigallocatechin Gallate Ameliorates Granulosa Cell Developmental via the Eukaryotic Initiation Factor 2 Alpha/Activating Transcription Factor 4 Pathway in Hyperthyroid Female Rats" Antioxidants 14, no. 9: 1092. https://doi.org/10.3390/antiox14091092
APA StyleSun, Y., Wu, M., Feng, H., Yao, Y., Chen, R., Yang, Y., & Zhang, C. (2025). Epigallocatechin Gallate Ameliorates Granulosa Cell Developmental via the Eukaryotic Initiation Factor 2 Alpha/Activating Transcription Factor 4 Pathway in Hyperthyroid Female Rats. Antioxidants, 14(9), 1092. https://doi.org/10.3390/antiox14091092





