Ginseng Nanosizing: The Second Spring of Ginseng Therapeutic Applications
Abstract
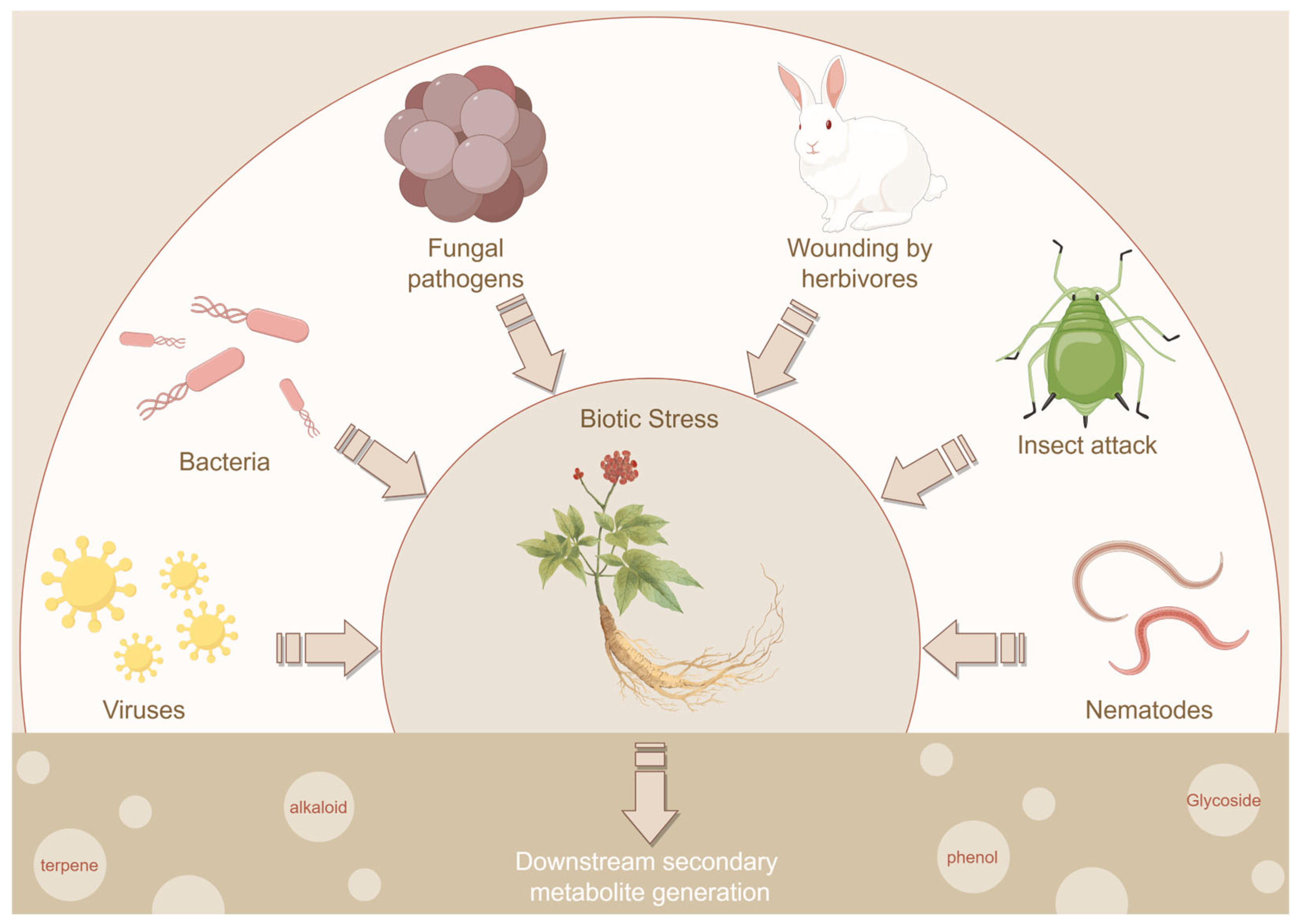
1. Introduction
2. Acquisition of GDVLNs
3. Therapeutic Applications of GDVLNs
3.1. Anti-Tumor Effects
3.2. Anti-Inflammatory and Antioxidant Effects
3.3. Drug Delivery
4. Gintonin: A Potentially Active Substance in GDVLNs
5. Nanosizing of Ginseng Extracts
5.1. Ginsenoside Nanosizing
5.2. Ginsenoside Excipients
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halperin, W.; Jensen, W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultrastruct. Res. 1967, 18, 428–443. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense Against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Börger, V.; Staubach, S.; Dittrich, R.; Stambouli, O.; Giebel, B. Scaled Isolation of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles. Curr. Protoc. Stem Cell Biol. 2020, 55, e128. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, D.H.; Park, S.J.; Kim, J.M.; Ryu, J.H. Ginseng in traditional herbal prescriptions. J. Ginseng Res. 2012, 36, 225–241. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Théry, C.; Welsh, J.A.; Blenkiron, C.; Buzás, E.I.; Di Vizio, D.; Erdbrügger, U.; Falcón-Pérez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of Exosome-Like Vesicles from Plants by Ultracentrifugation on Sucrose/Deuterium Oxide (D2O) Density Cushions. Methods Mol. Biol. 2016, 1459, 259–269. [Google Scholar]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Xu, X.H.; Yuan, T.J.; Dad, H.A.; Shi, M.Y.; Huang, Y.Y.; Jiang, Z.H.; Peng, L.H. Plant Exosomes As Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells In Vitro and In Vivo. Nano Lett. 2021, 21, 8151–8159. [Google Scholar] [CrossRef]
- Han, X.; Wei, Q.; Lv, Y.; Weng, L.; Huang, H.; Wei, Q.; Li, M.; Mao, Y.; Hua, D.; Cai, X.; et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol. Ther. 2022, 30, 327–340. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Lei, X.; Li, H.; Chen, S.; Li, B.; Xia, H.; Li, J.; Guan, F.; Ge, J. Tea leaf exosome-like nanoparticles (TELNs) improve oleic acid-induced fatty metabolic by regulate miRNAs in HepG-2 cells. Bioresour. Bioprocess. 2025, 12, 9. [Google Scholar] [CrossRef]
- Ban, J.J.; Lee, M.; Im, W.; Kim, M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef] [PubMed]
- Gerland, L.; Friedrich, D.; Hopf, L.; Donovan, E.J.; Wallmann, A.; Erdmann, N.; Diehl, A.; Bommer, M.; Buzar, K.; Ibrahim, M.; et al. pH-Dependent Protonation of Surface Carboxylate Groups in PsbO Enables Local Buffering and Triggers Structural Changes. Chembiochem 2020, 21, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-Derived Vesicle-Like Nanoparticles as Promising Biotherapeutic Tools: Present and Future. Adv. Mater. 2023, 35, e2207826. [Google Scholar] [CrossRef]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.S.; Jang, G. Isolation of high-purity and high-stability exosomes from ginseng. Front. Plant Sci. 2022, 13, 1064412. [Google Scholar] [CrossRef]
- Du, J.; Liang, Z.; Xu, J.; Zhao, Y.; Li, X.; Zhang, Y.; Zhao, D.; Chen, R.; Liu, Y.; Joshi, T.; et al. Plant-derived phosphocholine facilitates cellular uptake of anti-pulmonary fibrotic HJT-sRNA-m7. Sci. China Life Sci. 2019, 62, 309–320. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef]
- Feng, W.; Teng, Y.; Zhong, Q.; Zhang, Y.; Zhang, J.; Zhao, P.; Chen, G.; Wang, C.; Liang, X.J.; Ou, C. Biomimetic Grapefruit-Derived Extracellular Vesicles for Safe and Targeted Delivery of Sodium Thiosulfate against Vascular Calcification. ACS Nano 2023, 17, 24773–24789. [Google Scholar] [CrossRef]
- Iriawati, I.; Vitasasti, S.; Rahmadian, F.N.A.; Barlian, A. Isolation and characterization of plant-derived exosome-like nanoparticles from Carica papaya L. fruit and their potential as anti-inflammatory agent. PLoS ONE 2024, 19, e0304335. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- Chernyshev, V.S.; Rachamadugu, R.; Tseng, Y.H.; Belnap, D.M.; Jia, Y.; Branch, K.J.; Butterfield, A.E.; Pease, L.F. 3rd; Bernard, P.S.; Skliar, M. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 2015, 407, 3285–3301. [Google Scholar] [CrossRef]
- Rome, S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L. Reprogramming of macrophages--new opportunities for therapeutic targeting. Curr. Opin. Pharmacol. 2016, 26, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, M.; Weng, L.; Huang, H.; Mao, Y.; Yang, D.A.; Wei, Q.; Zhao, M.; Wei, Q.; Rui, K.; et al. Ginseng-derived nanoparticles reprogram macrophages to regulate arginase-1 release for ameliorating T cell exhaustion in tumor microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 322. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, X.; Wang, X.; Yang, M.; Weng, L.; Li, D.; Liu, T.; Gong, B.; Wang, Z.; Fan, H. Enhanced therapeutic effects of ginseng-derived exosome-like nanoparticles loaded hyaluronic acid injectable hydrogels for breast tumor treatment. Int. J. Biol. Macromol. 2025, 310 Pt 3, 142914. [Google Scholar] [CrossRef]
- Mansor, N.I.; Nordin, N.; Mohamed, F.; Ling, K.H.; Rosli, R.; Hassan, Z. Crossing the Blood-Brain Barrier: A Review on Drug Delivery Strategies for Treatment of the Central Nervous System Diseases. Curr. Drug Deliv. 2019, 16, 698–711. [Google Scholar] [CrossRef]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnology 2023, 21, 253. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Fan, H.; Han, B.; Ye, H.; Ye, X.; Zhang, D.; Ma, F.; Huang, Q.; Cheng, L.; et al. Ginseng exosomal miRNA ameliorates rheumatoid arthritis by mediating KRAS-MAPK signaling. Int. Immunopharmacol. 2025, 161, 115046. [Google Scholar] [CrossRef]
- Mahmoudi, S.K.; Tarzemani, S.; Aghajanzadeh, T.; Kasravi, M.; Hatami, B.; Zali, M.R.; Baghaei, K. Exploring the role of genetic variations in NAFLD: Implications for disease pathogenesis and precision medicine approaches. Eur. J. Med. Res. 2024, 29, 190. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Zhao, Y.; Di Nunzio, G.; Ren, L.; Fan, L.; Zhao, R.; Zhao, D.; Wang, J. Ginseng-derived nanoparticles alleviate alcohol-induced liver injury by activating the Nrf2/HO-1 signalling pathway and inhibiting the NF-κB signalling pathway in vitro and in vivo. Phytomedicine 2024, 127, 155428. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Seo, K.; Yoo, J.H.; Kim, J.; Min, S.J.; Heo, D.N.; Kwon, I.K.; Moon, H.J. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale 2023, 15, 5798–5808. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, S.; Zhu, Y.; Wang, R.; Wang, J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J. Ginseng Res. 2023, 47, 627–637. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, X.; Di Nunzio, G.; Fan, L.; Zhao, Y.; Ren, L.; Zhao, R.; Bian, S.; Liu, M.; et al. Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways. J. Nanobiotechnology 2024, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy. Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef]
- Yang, S.; Lu, S.; Ren, L.; Bian, S.; Zhao, D.; Liu, M.; Wang, J. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J. Ginseng Res. 2023, 47, 133–143. [Google Scholar] [CrossRef]
- Choi, W.; Cho, J.H.; Park, S.H.; Kim, D.S.; Lee, H.P.; Kim, D.; Kim, H.S.; Kim, J.H.; Cho, J.Y. Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J. Ginseng Res. 2024, 48, 211–219. [Google Scholar] [CrossRef]
- Pirsadeghi, A.; Namakkoobi, N.; Behzadi, M.S.; Pourzinolabedin, H.; Askari, F.; Shahabinejad, E.; Ghorbani, S.; Asadi, F.; Hosseini-Chegeni, A.; Yousefi-Ahmadipour, A.; et al. Therapeutic approaches of cell therapy based on stem cells and terminally differentiated cells: Potential and effectiveness. Cells Dev. 2024, 177, 203904. [Google Scholar] [CrossRef]
- Wang, H.; Mu, J.; Chen, Y.; Liu, Y.; Li, X.; Li, H.; Cao, P. Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis. Adv. Sci. 2024, 11, e2308235. [Google Scholar] [CrossRef]
- Yu, Y.L.; Zheng, J.C.; Duan, P.; Cheng, Y.N.; Zhang, H.; Zheng, L.; Yu, Z.R.; Xu, J.M.; Hu, H.X.; Pan, Z.Y. A gelatin methacryloyl (GelMA) treated with gallic acid and coated with specially designed nanoparticles derived from ginseng enhances the healing of wounds in diabetic rats. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133372. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Zimmermann, A.K.; Rivieccio, F.; Visser, C.; Blango, M.G. Host-derived extracellular vesicles for antimicrobial defense. Microlife 2021, 2, uqab003. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; King, S.; Samuels, A.L.; McFarlane, H.E. Subcellular coordination of plant cell wall synthesis. Dev. Cell 2021, 56, 933–948. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Li, X.; Bao, H.; Wang, Z.; Wang, M.; Fan, B.; Zhu, C.; Chen, Z. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: A review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.W.; Lee, B.H.; Kim, H.J.; Hwang, S.H.; Kim, H.K.; Nah, S.Y. Ginseng pharmacology: A new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front. Pharmacol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Zeng, L.; Lv, Y.; Ding, X.; Cheng, Y.; Zou, X. Plant lipid phosphate phosphatases: Current advances and future outlooks. Crit. Rev. Biotechnol. 2023, 43, 384–392. [Google Scholar] [CrossRef]
- Liu, S.; Paknejad, N.; Zhu, L.; Kihara, Y.; Ray, M.; Chun, J.; Liu, W.; Hite, R.K.; Huang, X.Y. Differential activation mechanisms of lipid GPCRs by lysophosphatidic acid and sphingosine 1-phosphate. Nat. Commun. 2022, 13, 731. [Google Scholar] [CrossRef]
- Choi, S.H.; Hong, M.K.; Kim, H.J.; Ryoo, N.; Rhim, H.; Nah, S.Y.; Kang, L.W. Structure of ginseng major latex-like protein 151 and its proposed lysophosphatidic acid-binding mechanism. Acta Crystallogr. D Biol. Crystallogr. 2015, 71 Pt 5, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.Y. Ginseng ginsenoside pharmacology in the nervous system: Involvement in the regulation of ion channels and receptors. Front. Physiol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Shin, T.J.; Choi, S.H.; Cho, H.J.; Lee, B.H.; Pyo, M.K.; Lee, J.H.; Kang, J.; Kim, H.J.; Park, C.W.; et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol. Cells 2012, 33, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, H.J.; Kim, B.R.; Shin, T.J.; Hwang, S.H.; Lee, B.H.; Lee, S.M.; Rhim, H.; Nah, S.Y. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, potentiates ATP-gated P2X1 receptor channel currents. Mol. Cells 2013, 35, 142–150. [Google Scholar] [CrossRef]
- Hong, S.; Lee, R.; Park, G.S.; Han, S.; Shin, J.; Lee, Y.M.; Nah, S.Y.; Oh, J.W. Gintonin-Enriched Panax ginseng Extract Fraction Sensitizes Renal Carcinoma Cells to TRAIL-Induced Apoptosis through DR4/5 Upregulation. Curr. Issues Mol. Biol. 2024, 46, 10880–10895. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, G.S.; Lee, R.; Hong, S.; Han, S.; Lee, Y.M.; Nah, S.Y.; Han, S.G.; Oh, J.W. Gintonin-Enriched Panax ginseng Extract Induces Apoptosis in Human Melanoma Cells by Causing Cell Cycle Arrest and Activating Caspases. Foods 2025, 14, 381. [Google Scholar] [CrossRef]
- Lee, R.; Kim, J.H.; Hwang, H.; Rhim, H.; Hwang, S.H.; Cho, I.H.; Kim, D.G.; Kim, H.C.; Nah, S.Y. Preparation of Red Ginseng Marc-Derived Gintonin and Its Application as a Skin Nutrient. Nutrients 2023, 15, 2574. [Google Scholar] [CrossRef]
- Ray, R.; Rai, V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood 2017, 129, 1177–1183. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, R.; Xiang, W.; Kang, X.; Tang, B.; Li, C.; Gao, L.; Zhang, X.; Zhang, L.; Dai, R.; et al. The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages. Signal Transduct. Target. Ther. 2020, 5, 24. [Google Scholar] [CrossRef]
- Kim, M.; Sur, B.; Villa, T.; Nah, S.Y.; Oh, S. Inhibitory activity of gintonin on inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and collagen-induced arthritis in mice. J. Ginseng Res. 2021, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, H.K.; Jang, M.; Kim, H.J.; Choi, S.H.; Hwang, S.H.; Kim, H.C.; Rhim, H.; Cho, I.H.; Nah, S.Y. Effects of Gintonin-Enriched Fraction in an Atopic Dermatitis Animal Model: Involvement of Autotaxin Regulation. Biol. Pharm. Bull. 2017, 40, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Jang, M.; Choi, S.H.; Kim, H.J.; Jhun, H.; Kim, H.C.; Rhim, H.; Cho, I.H.; Nah, S.Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int. J. Biol. Macromol. 2018, 114, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Choi, S.H.; Choi, J.H.; Oh, J.; Lee, R.M.; Lee, N.E.; Cho, Y.J.; Rhim, H.; Kim, H.C.; Cho, I.H.; et al. Ginseng gintonin attenuates the disruptions of brain microvascular permeability and microvascular endothelium junctional proteins in an APPswe/PSEN-1 double-transgenic mouse model of Alzheimer’s disease. Exp. Ther. Med. 2021, 21, 310. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, N.E.; Cho, H.J.; Lee, R.M.; Rhim, H.; Kim, H.C.; Han, M.; Lee, E.H.; Park, J.; Nah, S.Y. Gintonin facilitates brain delivery of donepezil, a therapeutic drug for Alzheimer disease, through lysophosphatidic acid 1/3 and vascular endothelial growth factor receptors. J. Ginseng Res. 2021, 45, 264–272. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, A.; Li, G.; Liu, Q. Pharmacokinetics and bioavailability study of ginsenoside Rk1 in rat by liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4580. [Google Scholar] [CrossRef]
- Ma, C.; Lin, Q.; Xue, Y.; Ju, Z.; Deng, G.; Liu, W.; Sun, Y.; Guan, H.; Cheng, X.; Wang, C. Pharmacokinetic studies of ginsenosides Rk1 and Rg5 in rats by UFLC-MS/MS. Biomed. Chromatogr. 2021, 35, e5108. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, S.; Wang, J.; Yin, T.; Teng, Y.; Wu, B.; You, M.; Jiang, Z.; Hu, M. Enhancement of oral bioavailability of 20(S)-ginsenoside Rh2 through improved understanding of its absorption and efflux mechanisms. Drug Metab. Dispos. 2011, 39, 1866–1872. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.J.; Wu, X.L.; Zheng, Y.T.; Zhang, J.W.; Ai, H.; Sun, J.G.; Jia, Y.W. Intestinal absorption mechanisms of ginsenoside Rh2: Stereoselectivity and involvement of ABC transporters. Xenobiotica 2010, 40, 602–612. [Google Scholar] [CrossRef]
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. Bioavailability challenges associated with development of saponins as therapeutic and chemopreventive agents. Curr. Drug Targets 2012, 13, 1885–1899. [Google Scholar] [CrossRef]
- Akao, T.; Kanaoka, M.; Kobashi, K. Appearance of Compound K, a Major Metabolite of Ginsenoside Rb1 by Intestinal Bacteria, in Rat Plasma after Oral Administration: Measurement of Compound K by Enzyme Immunoassay. Biol. Pharm. Bull. 1998, 21, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Huang, L.; Song, Y.; Liu, Z.; Liang, L.; Wang, L.; Wang, T. Preparation and pharmacological effects of minor ginsenoside nanoparticles: A review. Front. Pharmacol. 2022, 13, 974274. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Liu, Q.; Wan, J.Y.; Zhao, Y.J.; Guo, R.Z.; Alolga, R.N.; Li, P.; Qi, L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015, 5, 8598. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Hanh, N.T.Y.; Quyen, M.L.; Nguyen, Q.H.; Lien, T.T.P.; Do, K.V. Compound K Production: Achievements and Perspectives. Life 2023, 13, 1565. [Google Scholar] [CrossRef]
- Tran, T.N.A.; Son, J.S.; Awais, M.; Ko, J.H.; Yang, D.C.; Jung, S.K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering 2023, 10, 484. [Google Scholar] [CrossRef]
- Salarian, M.; Samimi, R.; Xu, W.Z.; Wang, Z.; Sham, T.-K.; Lui, E.M.K.; Charpentier, P.A. Microfluidic Synthesis and Angiogenic Activity of Ginsenoside Rg1-Loaded PPF Microspheres. ACS Biomater. Sci. Eng. 2016, 2, 1872–1882. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Subramaniyam, S.; Kim, Y.J.; Kim, Y.C.; Yang, D.C. Ginsenoside compound K-bearing glycol chitosan conjugates: Synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr. Polym. 2014, 112, 359–366. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.W.; Li, X.; Li, Y.F.; Ye, X.M.; Hu, J.N. Glycogen-based pH and redox sensitive nanoparticles with ginsenoside Rh(2) for effective treatment of ulcerative colitis. Biomaterials 2022, 280, 121077. [Google Scholar] [CrossRef]
- Yu, H.; Teng, L.; Meng, Q.; Li, Y.; Sun, X.; Lu, J.; R, J.L.; Teng, L. Development of liposomal Ginsenoside Rg3: Formulation optimization and evaluation of its anticancer effects. Int. J. Pharm. 2013, 450, 250–258. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Sun, Q.; Zhang, Z.; Zhao, M.; Peng, C.; Shi, S. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J. Nanobiotechnology 2021, 19, 322. [Google Scholar] [CrossRef]
- Selvaraj, K.; Yoo, B.K. Curcumin-Loaded Nanostructured Lipid Carrier Modified with Partially Hydrolyzed Ginsenoside. AAPS PharmSciTech 2019, 20, 252. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Xu, Q.; Wu, W.; Liu, Y.; Jiang, Y.; Cai, Q.Q.; Lv, Q.Z.; Li, X.Y. Brain Transport Profiles of Ginsenoside Rb(1) by Glucose Transporter 1: In Vitro and in Vivo. Front. Pharmacol. 2018, 9, 398. [Google Scholar]
- Xiong, J.; Sun, M.; Guo, J.; Huang, L.; Wang, S.; Meng, B.; Ping, Q. Active absorption of ginsenoside Rg1 in vitro and in vivo: The role of sodium-dependent glucose co-transporter 1. J. Pharm. Pharmacol. 2009, 61, 381–386. [Google Scholar] [CrossRef]
- Pliszka, M.; Szablewski, L. Glucose Transporters as a Target for Anticancer Therapy. Cancers 2021, 13, 4184. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef]
- Mehta, P.; Shende, P. Evasion of opsonization of macromolecules using novel surface-modification and biological-camouflage-mediated techniques for next-generation drug delivery. Cell Biochem. Funct. 2023, 41, 1031–1043. [Google Scholar] [CrossRef]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nanomicro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Xu, H.Q.; Chen, S.; Zhang, J.; Yang, S.L.; Cheng, J.L.; Peng, L.H.; Liu, A. Dissolution difference of ginsenosides from ultrafine granular powder and common powder traditional pieces of Panacis Quinquefolii Radix in vitro. Zhongguo Zhong Yao Za Zhi 2015, 40, 2576–2581. [Google Scholar]
- Zuo, S.; Wang, J.; An, X.; Wang, Z.; Zheng, X.; Zhang, Y. Fabrication of Ginsenoside-Based Nanodrugs for Enhanced Antitumor Efficacy on Triple-Negative Breast Cancer. Front. Bioeng. Biotechnol. 2022, 10, 945472. [Google Scholar] [CrossRef]
- Dai, L.; Liu, K.; Si, C.; Wang, L.; Liu, J.; He, J.; Lei, J. Ginsenoside nanoparticle: A new green drug delivery system. J. Mater. Chem. B 2016, 4, 529–538. [Google Scholar] [CrossRef]



| Diseases/Model | Mechanisms/Function | PMID |
|---|---|---|
| Gastric Ulcers | Inhibits the expression of inflammatory cytokines and increases COX-2 and LPA5 receptor expression and PGE2 levels | 38069044 |
| Wound-Healing | Epidermal growth factor receptor activation and heparin-binding EGF-like growth factor release | 37762395 |
| LPA receptor activation and / or VEGF release | 34576317 | |
| Skin ageing | Inhibition of beta-galactosidase overexpression | 37299538 |
| Lung cancer | Inhibition of cancer cell metastasis by maintaining the integrity of cell-cell junctions | 37653930 |
| Epilepsy | Decreased levels of glial cell activation and expression of pro-inflammatory cytokines/enzymes and increased levels of Nrf2 antioxidant response | 37252272 |
| Neuroprotection | Activation of Akt and CREB stimulates dendritic growth of striatal neurons. | 36385759 |
| Activation of the LPA1 receptor-BDNF-TrkB-Akt signaling pathway reduces oxidative stress in neuronal cells | 34299412 | |
| Sarcopenic obesity | Promoting energy expenditure and reducing skeletal muscle atrophy | 35600770 |
| Arthritis | Inhibiting the activation of NF-κB and reducing the expression of inflammatory factors by mediating the phosphorylation of JNK, ERK, and MAPK | 34803427 |
| Cancer cachexia | Reduce TNF-α-induced oxidative stress and muscle atrophy by reducing ROS and inhibiting inflammation-related genes. | 34798386 |
| Sarcopenia | Restoring age-related immune homeostasis by maintaining the T cell compartment and regulating inflammatory biological responses | 34764729 |
| Amyotrophic lateral sclerosis | The level of oxidative stress and the activation of immunoreactive glial cells were reduced, and the expression level of LPA1 receptor was restored | 34025132 |
| Alzheimer’s disease | Attenuated cerebral microvascular permeability and disruption of microvascular endothelial junction proteins | 33717253 |
| Obesity | Decreased expression of pro-adipogenic and adipogenic factors to reduce lipid accumulation and increase lipolysis and thermogenesis | 32679738 |
| Lead poisoning | Inhibition of neuronal apoptosis and attenuation of oxidative stress and inflammation in the brain | 32131481 |
| Heat stress | Reducing oxidative stress and inflammatory damage | 32106493 |
| Hair growth | Stimulated release of vascular endothelial growth factor | 32095099 |
| Mercury poisoning | Reduced methylmercury-induced neurotoxicity and oxidative stress and increased its removal | 32013120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, H.; Ding, X.; Liu, T.; Li, Q.; Li, R.; Yuan, Y.; Yan, X.; Su, J. Ginseng Nanosizing: The Second Spring of Ginseng Therapeutic Applications. Antioxidants 2025, 14, 961. https://doi.org/10.3390/antiox14080961
Wang J, Liu H, Ding X, Liu T, Li Q, Li R, Yuan Y, Yan X, Su J. Ginseng Nanosizing: The Second Spring of Ginseng Therapeutic Applications. Antioxidants. 2025; 14(8):961. https://doi.org/10.3390/antiox14080961
Chicago/Turabian StyleWang, Jian, Huan Liu, Xinshuo Ding, Tianqi Liu, Qianyuan Li, Runyuan Li, Yuan Yuan, Xiaoyu Yan, and Jing Su. 2025. "Ginseng Nanosizing: The Second Spring of Ginseng Therapeutic Applications" Antioxidants 14, no. 8: 961. https://doi.org/10.3390/antiox14080961
APA StyleWang, J., Liu, H., Ding, X., Liu, T., Li, Q., Li, R., Yuan, Y., Yan, X., & Su, J. (2025). Ginseng Nanosizing: The Second Spring of Ginseng Therapeutic Applications. Antioxidants, 14(8), 961. https://doi.org/10.3390/antiox14080961








