Complement Receptor 3 Regulates Microglial Exosome Release and Related Neurotoxicity via NADPH Oxidase in Neuroinflammation Associated with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animals
2.4. Extraction and Identification of Exosomes
2.5. Western Blot Analysis
2.6. MTT Assay
2.7. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.8. Cell Transfection
2.9. Superoxide Detection
2.10. Immunocytochemistry
2.11. Statistical Analysis
3. Results
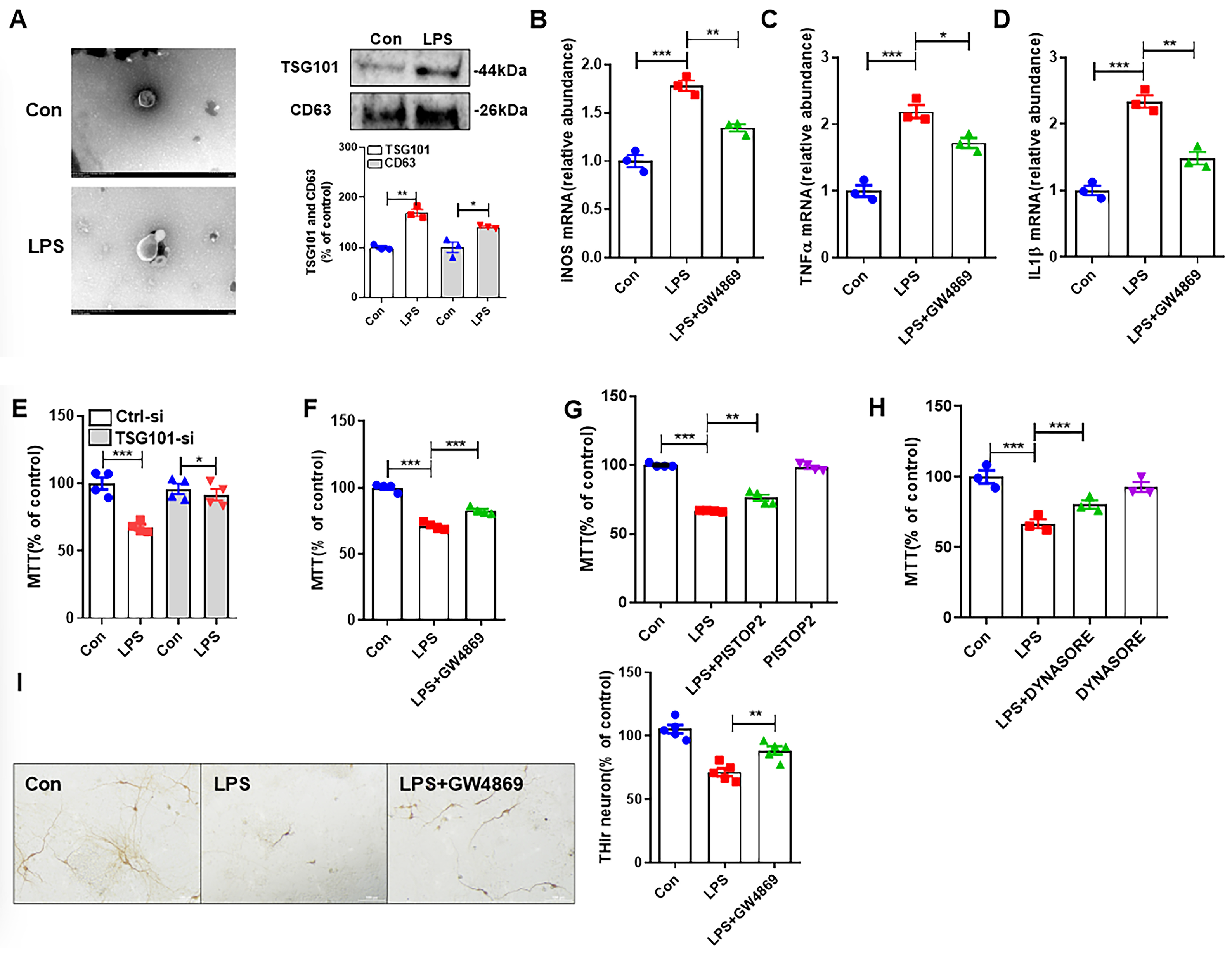
3.1. LPS-Induced M1 Microglia Damages Neurons Through Release of Exosomes
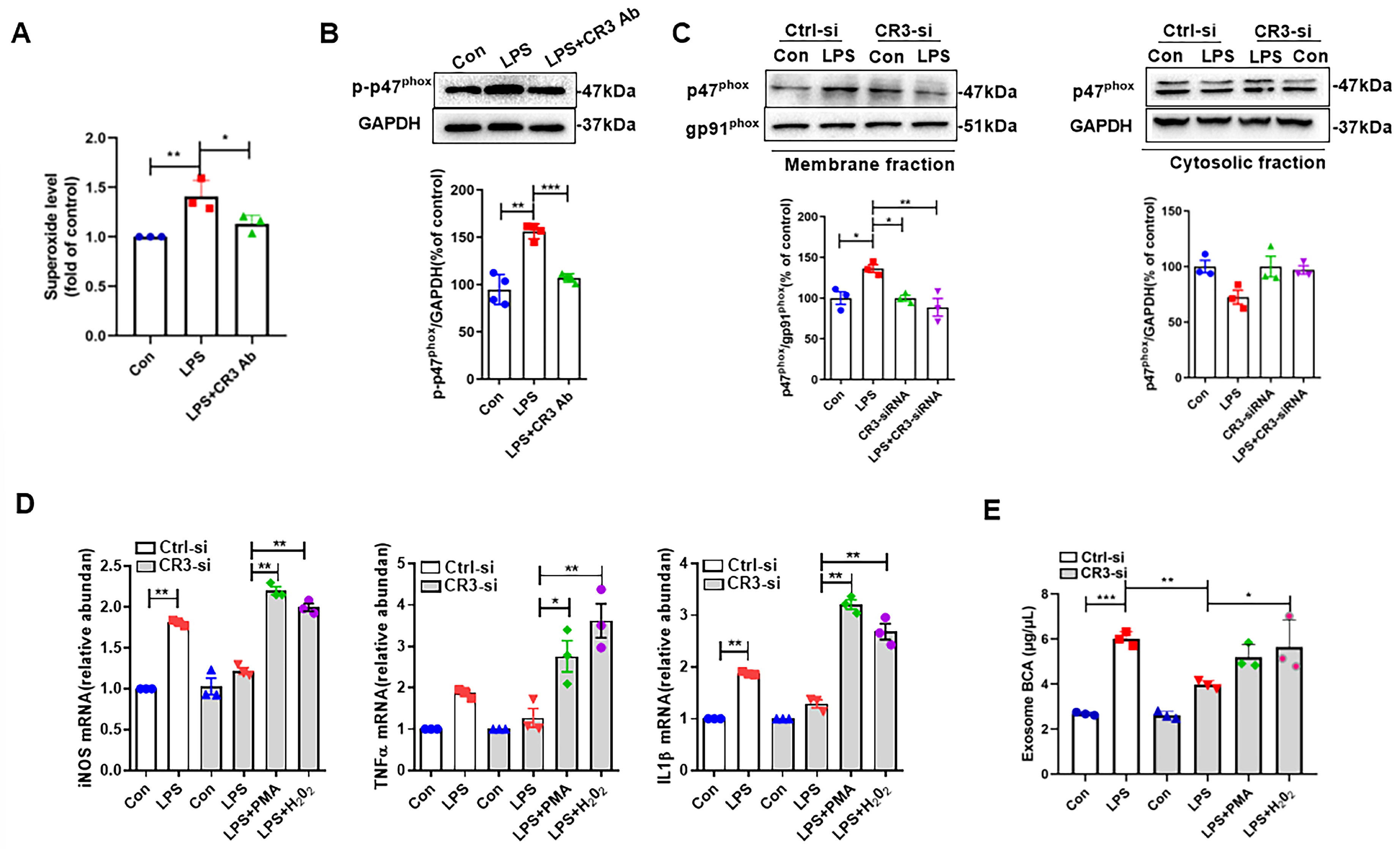
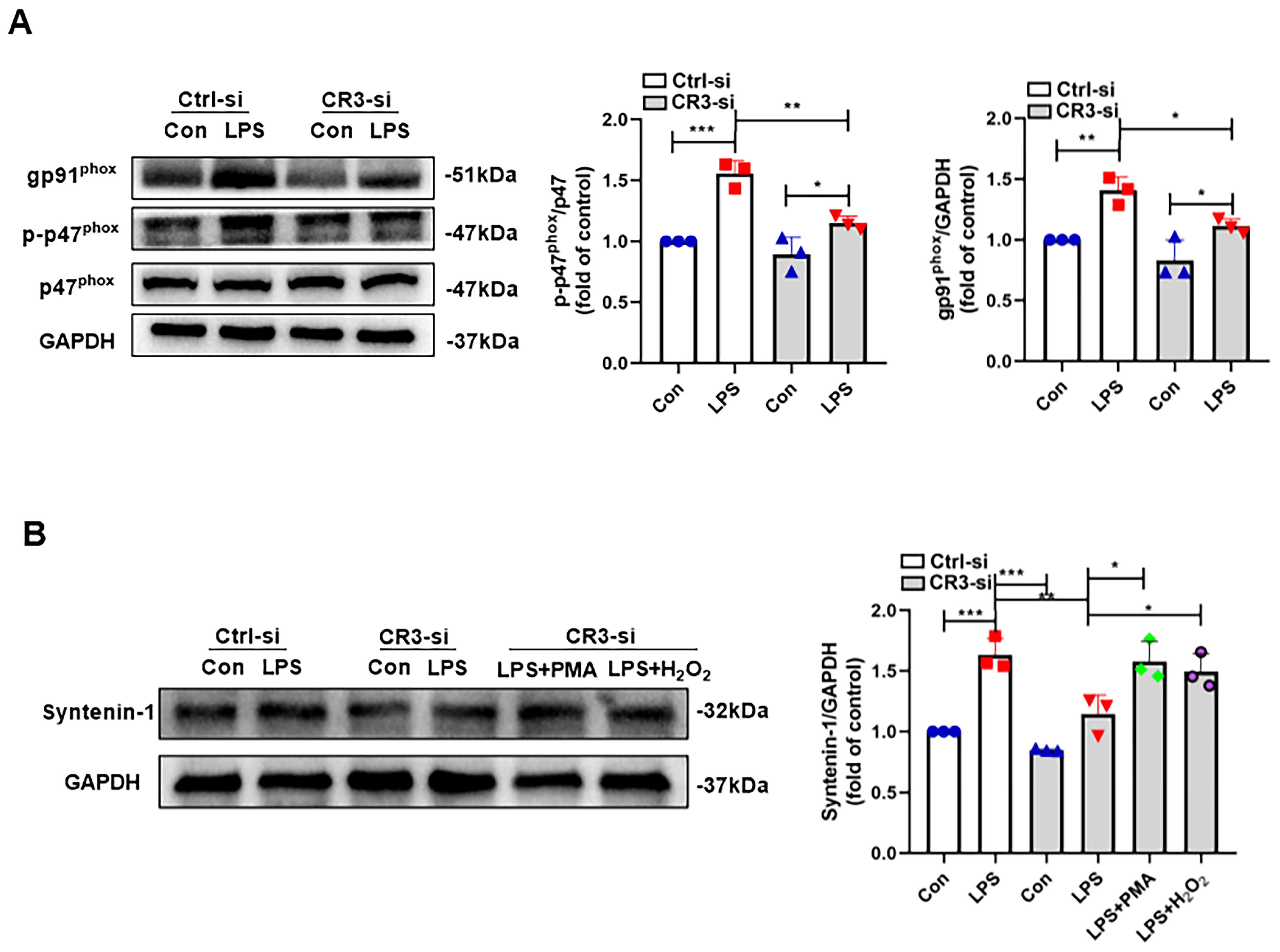
3.2. CR3 Mediates M1 Microglia Exosome Release via NOX2
3.3. CR3-NOX2 Mediates M1 Microglial Exosome Release Through Syntenin-1
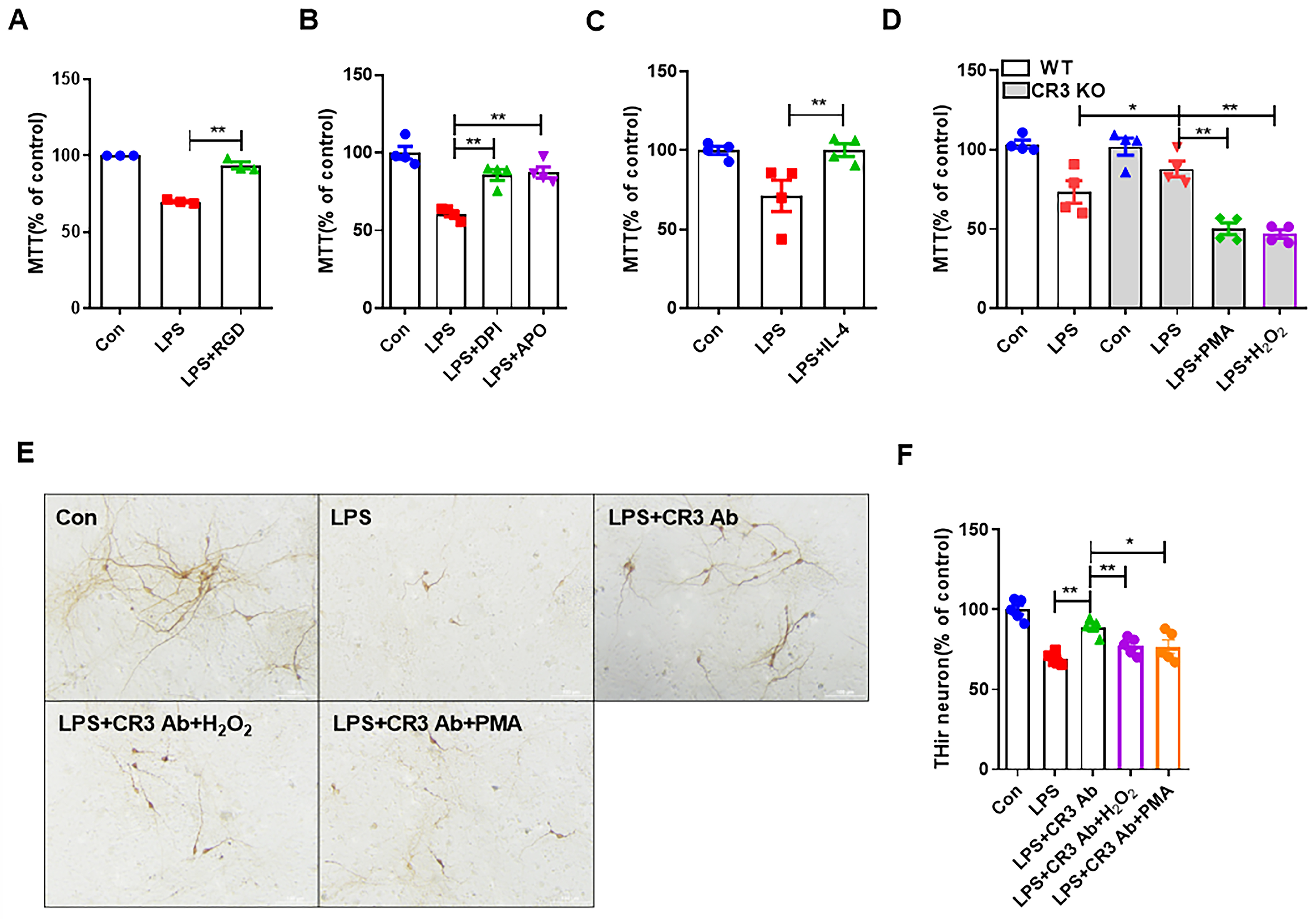
3.4. CR3 Mediates M1 Microglia-Induced Neurotoxicity via NOX2
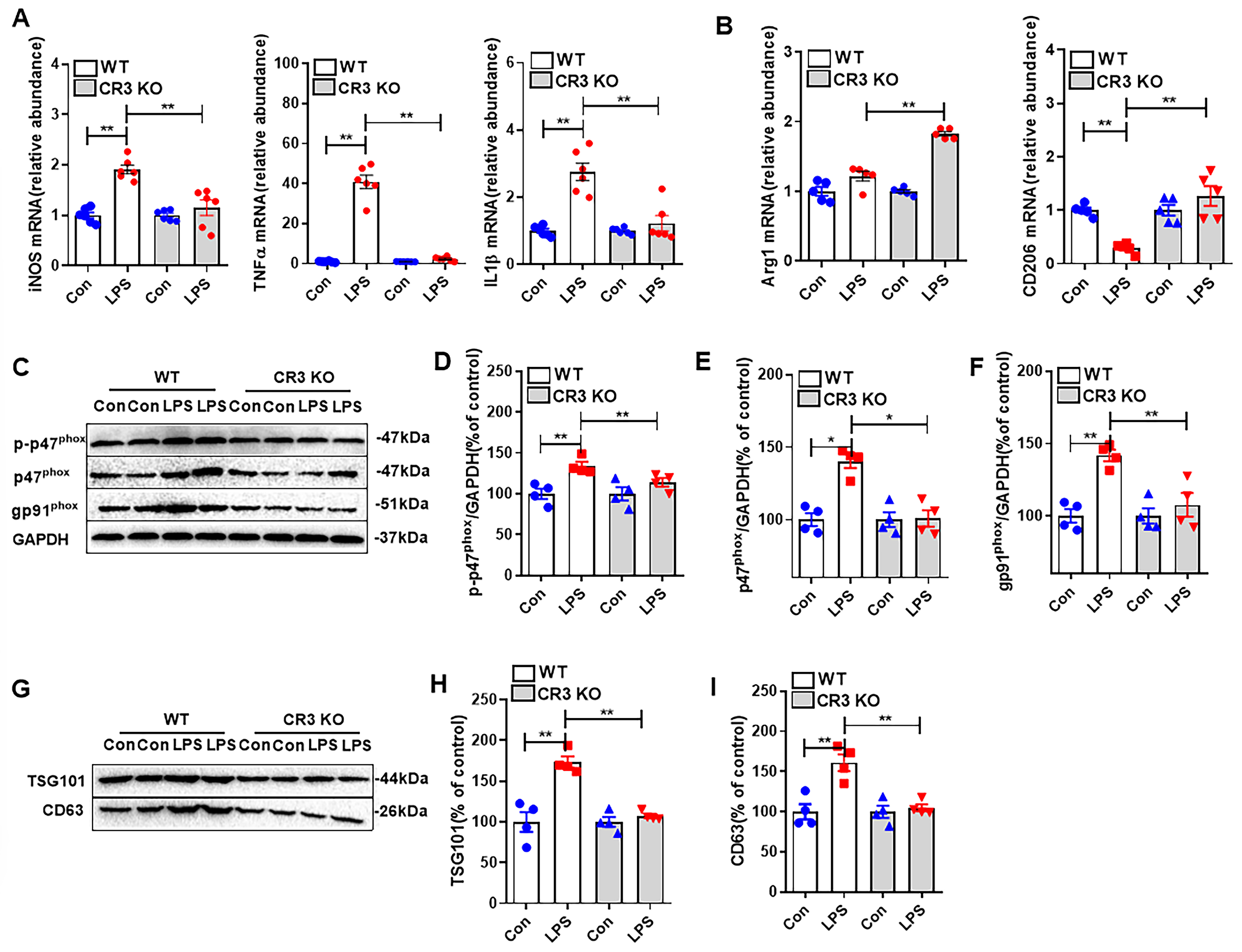
3.5. CR3 Mediates Microglial M1 Polarization, NOX2 Activation, and Exosome Release in LPS-Treated Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Lal, R.; Singh, A.; Watts, S.; Chopra, K. Experimental models of Parkinson’s disease: Challenges and Opportunities. Eur. J. Pharmacol. 2024, 980, 176819. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, H.; Wei, J. Beyond the Brain: Exploring the multi-organ axes in Parkinson’s disease pathogenesis. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Liu, J.; Lv, X.; Ye, T.; Zhao, M.; Chen, Z.; Zhang, Y.; Yang, W.; Xie, H.; Zhan, L.; Chen, L.; et al. Microbiota-microglia crosstalk between Blautia producta and neuroinflammation of Parkinson’s disease: A bench-to-bedside translational approach. Brain Behav. Immun. 2024, 117, 270–282. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhang, Y.; Li, Z.; Zhao, Z.; Jiang, W.; Zhao, J.; Hou, L.; Wang, Q. Microglial CR3 promotes neuron ferroptosis via NOX2-mediated iron deposition in rotenone-induced experimental models of Parkinson’s disease. Redox Biol. 2024, 77, 103369. [Google Scholar] [CrossRef]
- Han, Q.Q.; Shen, S.Y.; Liang, L.F.; Chen, X.R.; Yu, J. Complement C1q/C3-CR3 signaling pathway mediates abnormal microglial phagocytosis of synapses in a mouse model of depression. Brain Behav. Immun. 2024, 119, 454–464. [Google Scholar] [CrossRef]
- Wang, S.; Chu, C.H.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.S.; Zhang, J. α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, S.H.; Zhao, Z.; Tu, D.; Song, S.; Wang, Y.; Wang, Q.; Feng, J.; Hong, J.S. Complement C3 Enhances LPS-Elicited Neuroinflammation and Neurodegeneration Via the Mac1/NOX2 Pathway. Mol. Neurobiol. 2023, 60, 5167–5183. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, C.; Bai, X.; Sun, J.; Wang, K.; Meng, Q.; Li, Y.; Hu, G.; Hu, R.; Cai, Q.; et al. A potential mechanism clue to the periodic storm from microglia activation and progressive neuron damage induced by paraquat exposure. Environ. Toxicol. 2024, 39, 1874–1888. [Google Scholar] [CrossRef]
- Si, X.L.; Fang, Y.J.; Li, L.F.; Gu, L.Y.; Yin, X.Z.; Jun, T.; Yan, Y.P.; Pu, J.L.; Zhang, B.R. From inflammasome to Parkinson’s disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson’s disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Cui, Z.; Luo, Q.; Jiang, Z.; Huang, Y.; Jiang, J.; Qiu, J.; Li, Y.; Yu, K.; et al. M1 Microglia-derived Exosomes Promote Activation of Resting Microglia and Amplifies Proangiogenic Effects through Irf1/miR-155-5p/Socs1 Axis in the Retina. Int. J. Biol. Sci. 2023, 19, 1791–1812. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Z.; Liu, J.; Ma, Y.; Zhang, X.; Hou, L.; Wang, Q.; Jiang, W.; Wang, Q. CD11b-NOX2 mutual regulation-mediated microglial exosome release contributes to rotenone-induced inflammation and neurotoxicity in BV2 microglia and primary cultures. Free. Radic. Biol. Med. 2024, 224, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Pradhan, A.K.; Bhoopathi, P.; Shen, X.N.; August, L.A.; Windle, J.J.; Sarkar, D.; Furnari, F.B.; Cavenee, W.K.; Das, S.K.; et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Murack, M.; Smith, K.B.; Traynor, O.H.; Pirwani, A.F.; Gostlin, S.K.; Mohamed, T.; Tata, D.A.; Messier, C.; Ismail, N. Environmental enrichment alters LPS-induced changes in BDNF and PSD-95 expressions during puberty. Brain Res. 2023, 1806, 148283. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, X.; Qiu, T.; An, Y.; Zhang, G.; Li, Q.; Jiang, L.; Yang, G.; Cao, J.; Sun, X.; et al. Exosomal miR-181a-2-3p derived from citreoviridin-treated hepatocytes activates hepatic stellate cells trough inducing mitochondrial calcium overload. Chem.-Biol. Interact. 2022, 358, 109899. [Google Scholar] [CrossRef]
- Dashtaki, M.E.; Tabibkhooei, A.; Parvizpour, S.; Soltani, R.; Ghasemi, S. Isolation of Cells and Exosomes from Glioblastoma Tissue to Investigate the Effects of Ascorbic Acid on the c-Myc, HIF-1α, and Lnc-SNHG16 Genes. Int. J. Mol. Cell. Med. 2023, 12, 135–143. [Google Scholar]
- Lee, K.M.; Seo, E.C.; Lee, J.H.; Kim, H.J.; Hwangbo, C. The Multifunctional Protein Syntenin-1: Regulator of Exosome Biogenesis, Cellular Function, and Tumor Progression. Int. J. Mol. Sci. 2023, 24, 9418. [Google Scholar] [CrossRef]
- Yildirim-Balatan, C.; Fenyi, A.; Besnault, P.; Gomez, L.; Sepulveda-Diaz, J.E.; Michel, P.P.; Melki, R.; Hunot, S. Parkinson’s disease-derived α-synuclein assemblies combined with chronic-type inflammatory cues promote a neurotoxic microglial phenotype. J. Neuroinflamm. 2024, 21, 54. [Google Scholar] [CrossRef]
- Darwish, S.F.; Elbadry, A.M.M.; Elbokhomy, A.S.; Salama, G.A.; Salama, R.M. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Front. Aging 2023, 4, 1231706. [Google Scholar] [CrossRef]
- Greter, M.; Merad, M. Regulation of microglia development and homeostasis. Glia 2013, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; West, A.B. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Nayernia, Z.; Jaquet, V.; Krause, K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014, 20, 2815–2837. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Bi, Y.; Qiao, X.; Cai, Z.; Zhao, H.; Ye, R.; Liu, Q.; Gao, L.; Liu, Y.; Liang, B.; Liu, Y.; et al. Exosomal miR-302b rejuvenates aging mice by reversing the proliferative arrest of senescent cells. Cell Metab. 2025, 37, 527–541.e6. [Google Scholar] [CrossRef]
- Sonwane, S.; Telrandhe, U.; Chambhare, N.; Vaidya, S. Unraveling exosome-mediated cancer therapy resistance: Pathways and therapeutic challenges. J. Egypt. Natl. Cancer Inst. 2025, 37, 30. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jo, H.Y.; Kim, D.H.; Oh, Y.J.; Kim, M.; Na, S.; Song, H.Y.; Lee, H.J. Exosome-Mediated Mitochondrial Regulation: A Promising Therapeutic Tool for Alzheimer’s Disease and Parkinson’s Disease. Int. J. Nanomed. 2025, 20, 4903–4917. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.P.; Li, Y.X.; Zhu, X.H.; Du, X.G.; Zhou, M.; Li, W.B.; Deng, H.Y. Effect of Regulatory Network of Exosomes and microRNAs on Neurodegenerative Diseases. Chin. Med. J. 2018, 131, 2216–2225. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; Del Rosario, I.; Paul, K.C.; Wong, D.Y.; Folle, A.D.; Markovic, D.; Palma, J.A.; et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Zhang, W. Adaptor proteins in lymphocyte activation. Curr. Opin. Immunol. 2003, 15, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, N.V.; Villuri, B.K.; Nagarajan, B.; Lewicki, S.; Das, S.K.; Fisher, P.B.; Desai, U.R. Design and Synthesis of Small Molecule Probes of MDA-9/Syntenin. Biomolecules 2024, 14, 1287. [Google Scholar] [CrossRef]
- Tomlinson, P.R.; Zheng, Y.; Fischer, R.; Heidasch, R.; Gardiner, C.; Evetts, S.; Hu, M.; Wade-Martins, R.; Turner, M.R.; Morris, J.; et al. Identification of distinct circulating exosomes in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 353–361. [Google Scholar] [CrossRef]
- Friand, V.; David, G.; Zimmermann, P. Syntenin and syndecan in the biogenesis of exosomes. Biol. Cell 2015, 107, 331–341. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Roucourt, B.; Meeussen, S.; Bao, J.; Zimmermann, P.; David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015, 25, 412–428. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, X.; Zhao, H.; Deng, Y.; Zeng, W.; Wu, J.; Chen, Y. Research on the TSPAN6 regulating the secretion of ADSCs-Exos through syntenin-1 and promoting wound healing. Stem Cell Res. Ther. 2024, 15, 430. [Google Scholar] [CrossRef]
- Stevens, A.O.; He, Y. Allosterism in the PDZ Family. Int. J. Mol. Sci. 2022, 23, 1454. [Google Scholar] [CrossRef]
- Faure, A.J.; Domingo, J.; Schmiedel, J.M.; Hidalgo-Carcedo, C.; Diss, G.; Lehner, B. Mapping the energetic and allosteric landscapes of protein binding domains. Nature 2022, 604, 175–183. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, X.; Xu, J.; Luo, R.; Li, S.; Su, H.; Wang, Q.; Hou, L. Complement Receptor 3 Regulates Microglial Exosome Release and Related Neurotoxicity via NADPH Oxidase in Neuroinflammation Associated with Parkinson’s Disease. Antioxidants 2025, 14, 963. https://doi.org/10.3390/antiox14080963
Ma Y, Zhang X, Xu J, Luo R, Li S, Su H, Wang Q, Hou L. Complement Receptor 3 Regulates Microglial Exosome Release and Related Neurotoxicity via NADPH Oxidase in Neuroinflammation Associated with Parkinson’s Disease. Antioxidants. 2025; 14(8):963. https://doi.org/10.3390/antiox14080963
Chicago/Turabian StyleMa, Yu, Xiaomeng Zhang, Jiaqi Xu, Runnan Luo, Sheng Li, Hong Su, Qingshan Wang, and Liyan Hou. 2025. "Complement Receptor 3 Regulates Microglial Exosome Release and Related Neurotoxicity via NADPH Oxidase in Neuroinflammation Associated with Parkinson’s Disease" Antioxidants 14, no. 8: 963. https://doi.org/10.3390/antiox14080963
APA StyleMa, Y., Zhang, X., Xu, J., Luo, R., Li, S., Su, H., Wang, Q., & Hou, L. (2025). Complement Receptor 3 Regulates Microglial Exosome Release and Related Neurotoxicity via NADPH Oxidase in Neuroinflammation Associated with Parkinson’s Disease. Antioxidants, 14(8), 963. https://doi.org/10.3390/antiox14080963






