Physiological Mechanisms of the Enhanced UV-B Radiation Triggering Plant-Specific Peroxidase-Mediated Antioxidant Defences
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site and Plant Material
2.2. Methods
2.2.1. Experimental Design
2.2.2. Sampling and Sample Pre-Treatment
2.2.3. Detection of Pulp Quality Indicators
2.2.4. Detection of Malondialdehyde Content and Relative Conductivity of Pulp
2.2.5. Detection of Hormone Content in the Pulp
2.2.6. Detection of POD Activity and Lignin Content
2.2.7. Screening and qPCR Verification of Differentially Expressed Genes
2.2.8. Statistical Analysis
3. Results
3.1. Effect of Enhanced UV-B Radiation Treatment on Mango Fruit Size and Peel Colouration
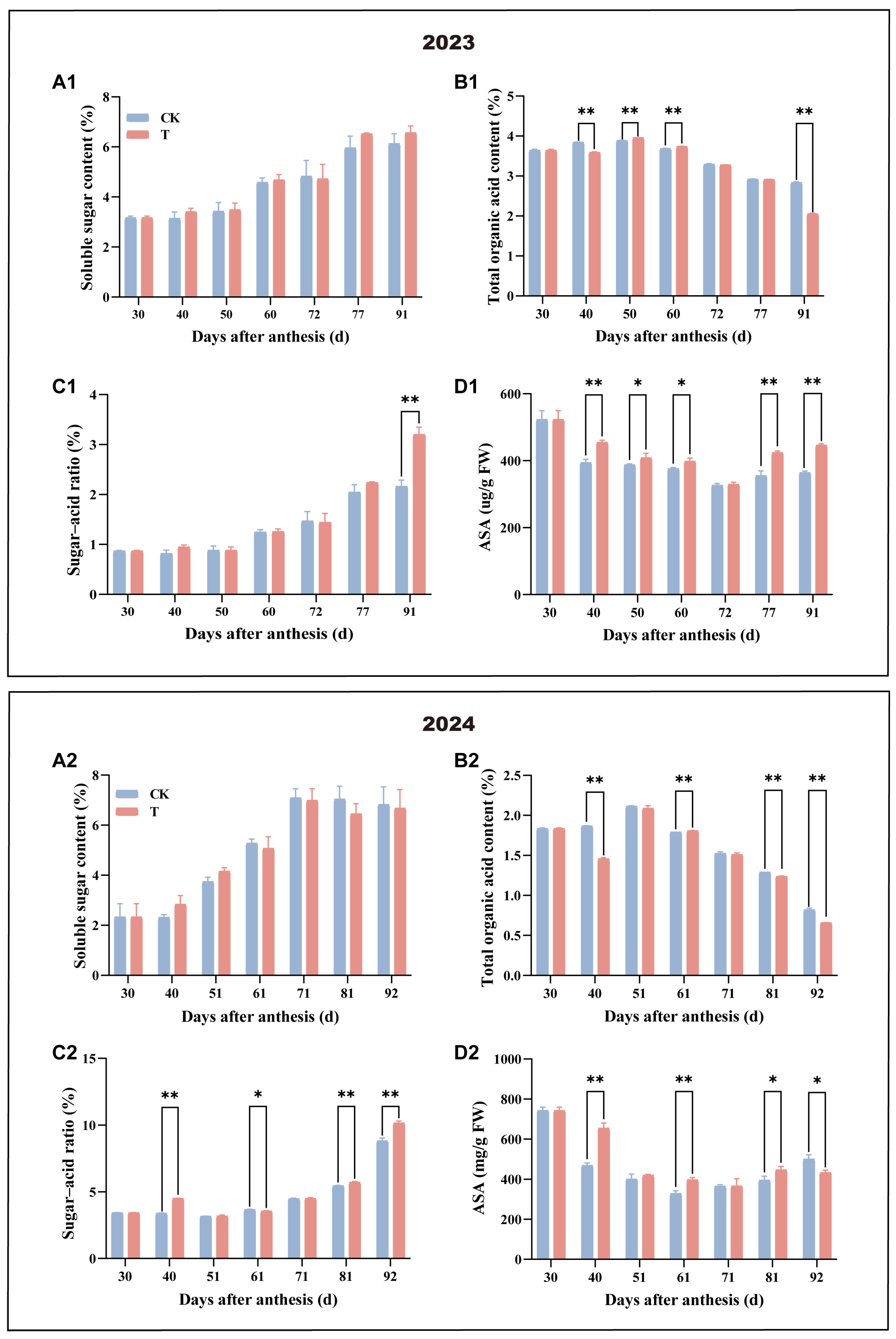
3.2. Effect of Enhanced UV-B Radiation Treatment on the Main Nutritional and Flavour Qualities of Mango Pulp
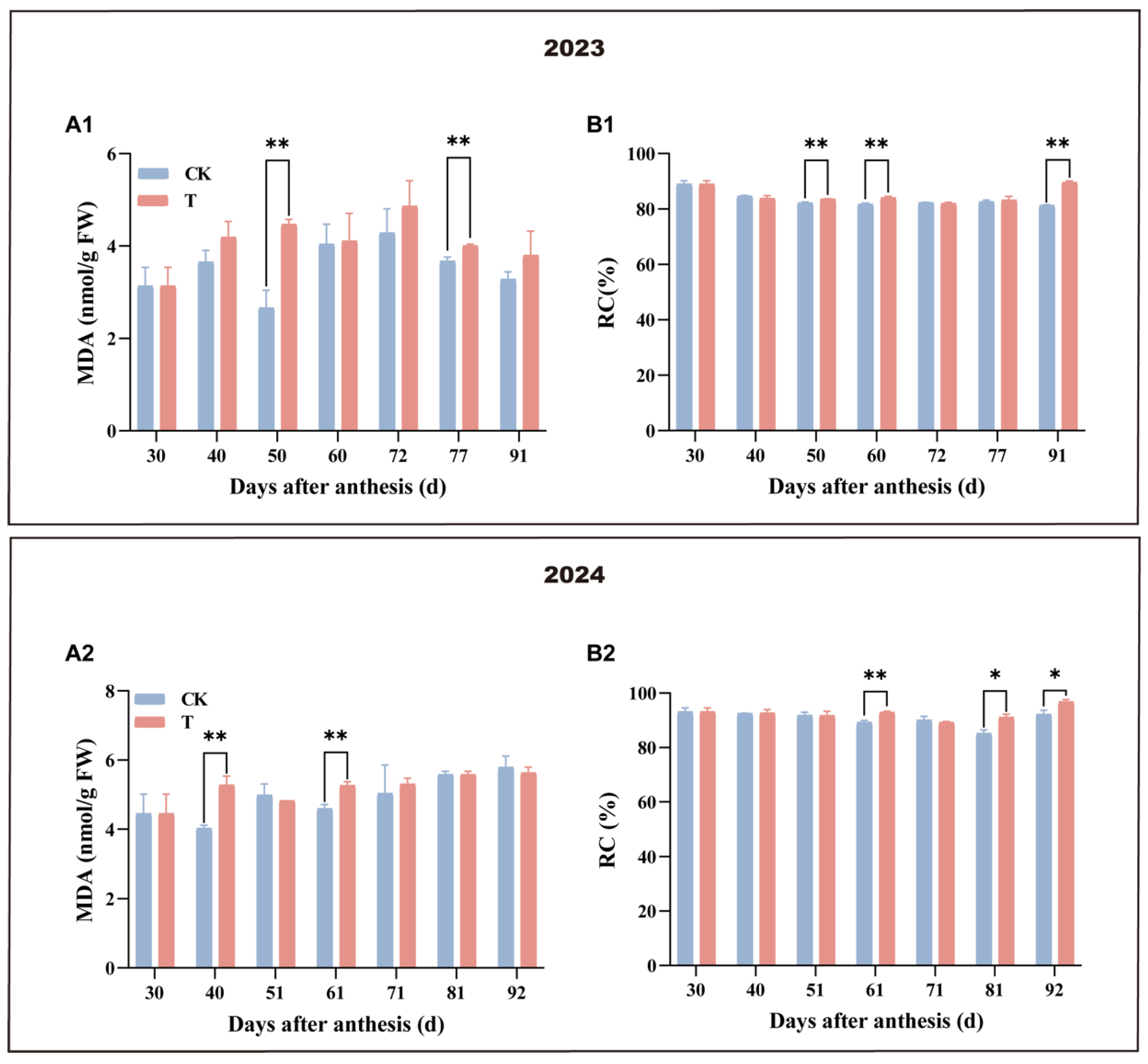
3.3. Effect of Enhanced UV-B Radiation Treatment on the Physiological Damage of Mango Pulp
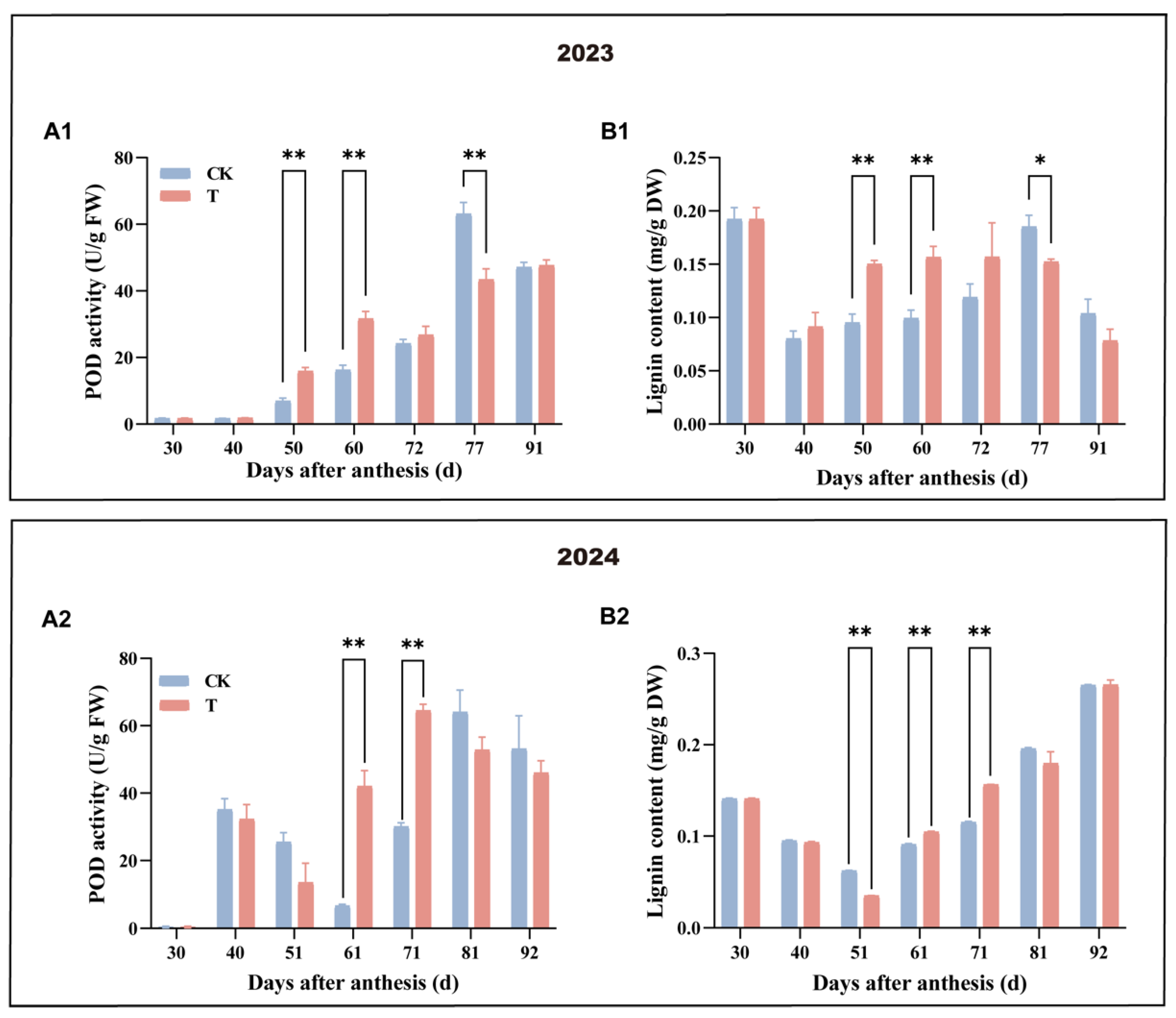
3.4. Effect of Enhanced UV-B Radiation Treatment on the POD Activity and Lignin Content of Pulp
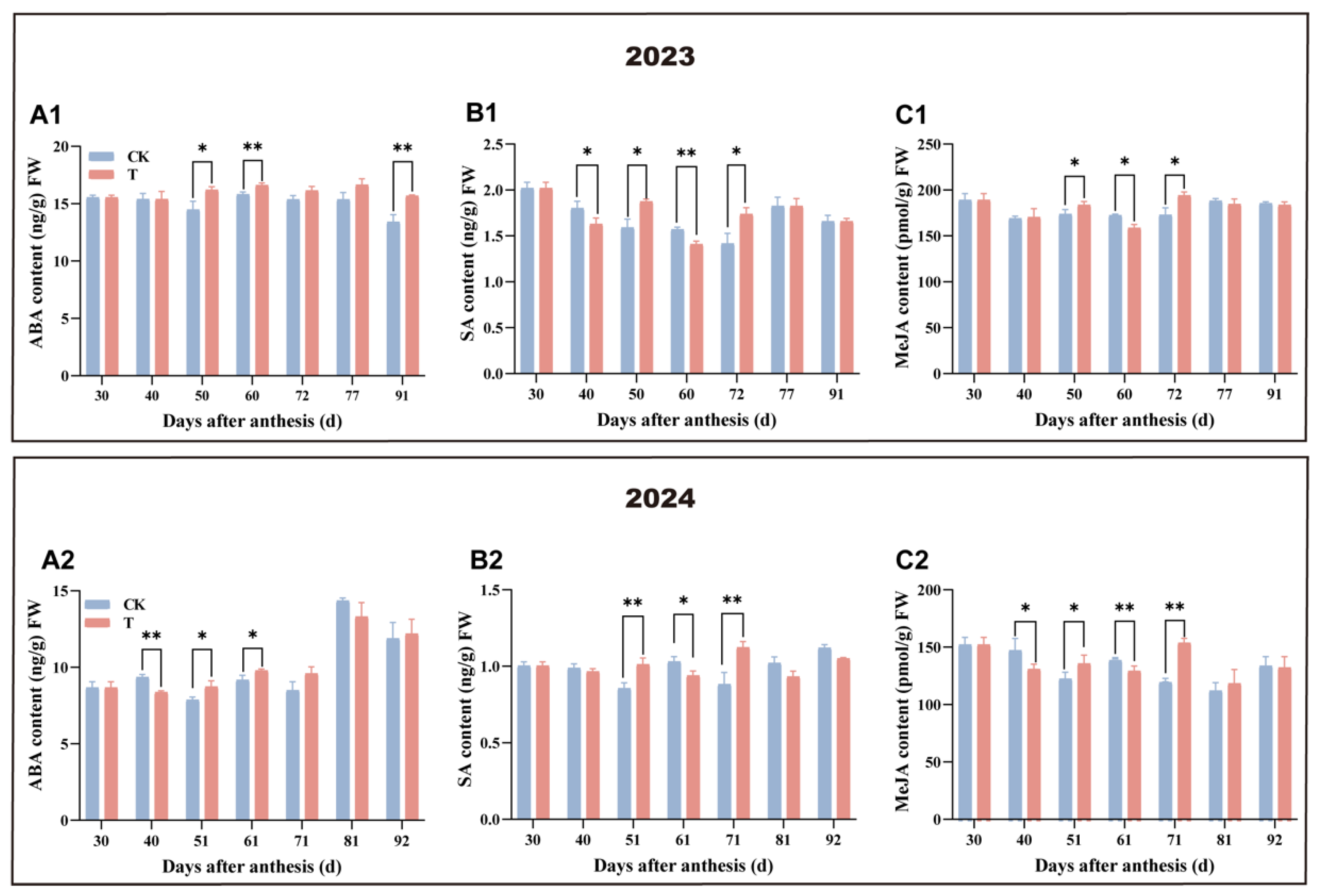
3.5. Effect of Enhanced UV-B Radiation Treatment on the Changes in Hormone Contents of Pulp
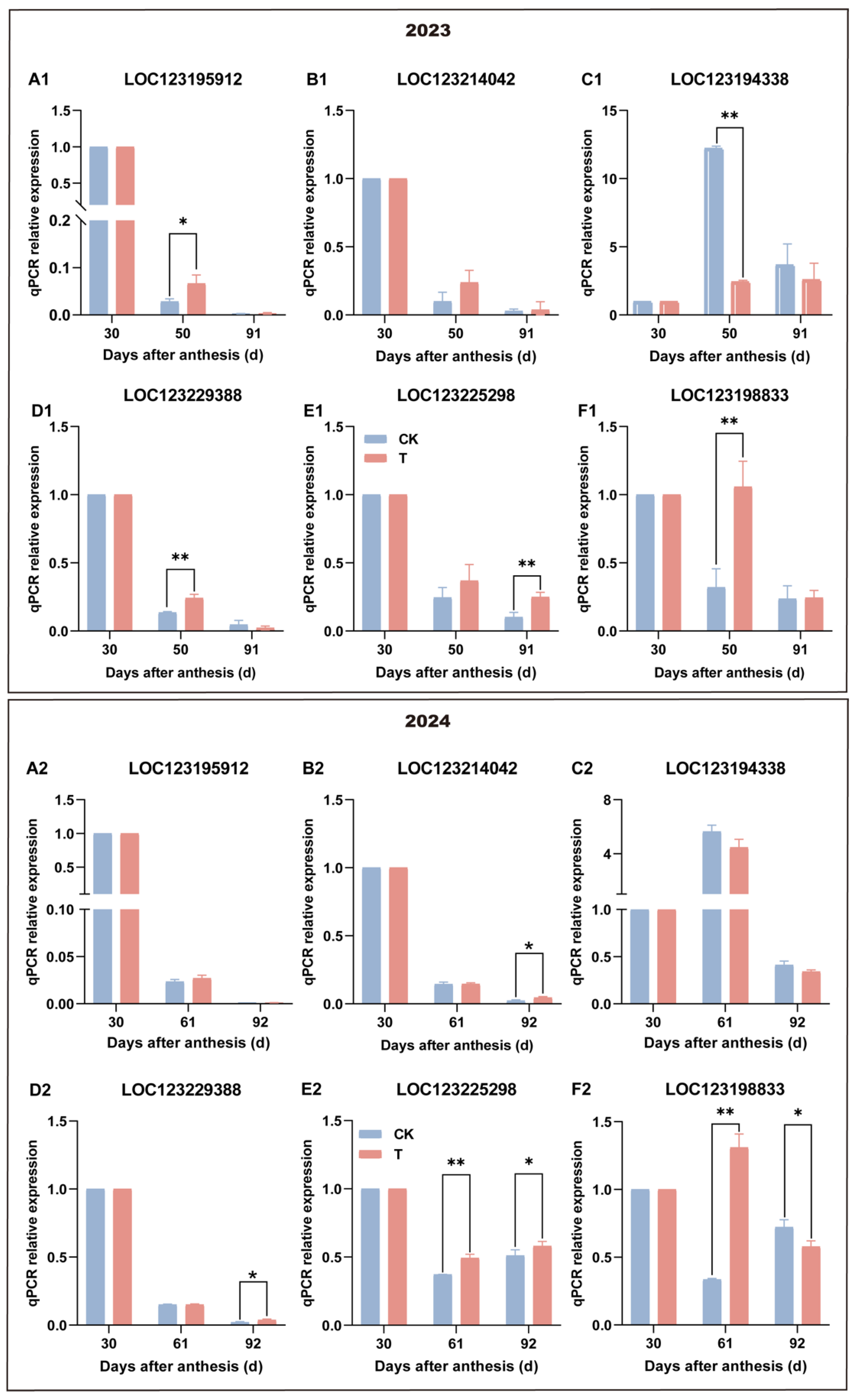
3.6. Effect of Enhanced UV-B Radiation Treatment on POD Expression of Pulp
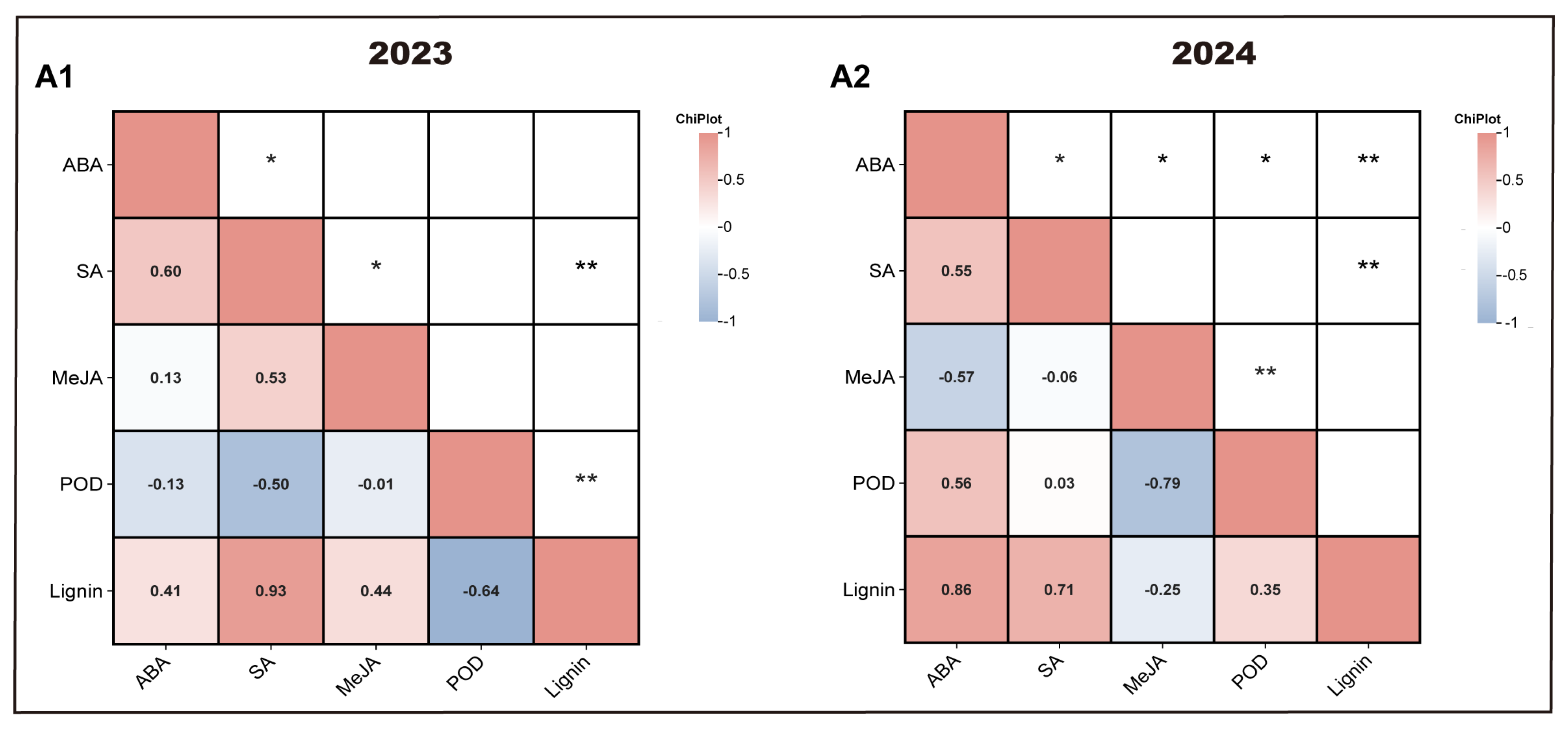
3.7. Multivariate Linear Correlation Analysis
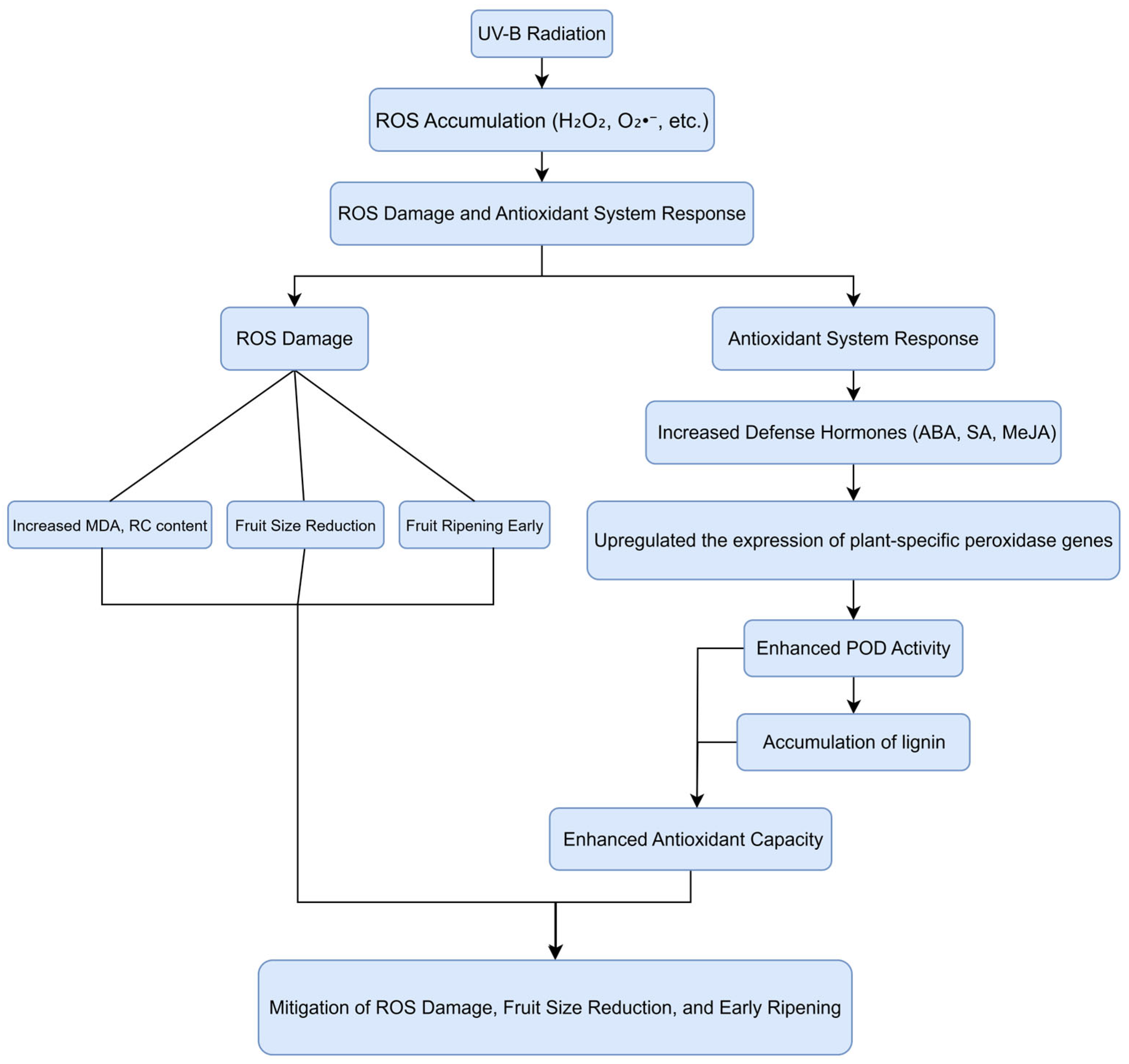
4. Discussion
4.1. The Effect of Enhanced UV-B Radiation Treatment on the Fruit of ‘Tainongyihao’ Mango
4.2. The Mechanism of POD Activity Regulated by UV-B Radiation Treatment
4.3. The Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef]
- Podolec, R.; Demarsy, E.; Ulm, R. Perception and signaling of ultraviolet-B radiation in plants. Annu. Rev. Plant Biol. 2021, 72, 793–822. [Google Scholar] [CrossRef]
- Hossain, F. Fate and transport of stack emissions in the environment and potential reduction of pollutants in the context of global warming. J. Environ. Eng. 2023, 149, 04022080. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Yuan, X.; Xu, Z. Simultaneous harmless ionization of CFC and resource utilization of waste solar panel through one-pot hydrothermal treatment. J. Hazard. Mater. 2023, 441, 129918. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Song, W.; Zheng, L. Pre-harvest UVB irradiation enhances the phenolic and flavonoid content, and antioxidant activity of green-and red-leaf lettuce cultivars. Horticulturae 2023, 9, 695. [Google Scholar] [CrossRef]
- Shoaib, N.; Pan, K.; Mughal, N.; Raza, A.; Liu, L.; Zhang, J.; Wu, X.; Sun, X.; Zhang, L.; Pan, Z. Potential of UV-B radiation in drought stress resilience: A multidimensional approach to plant adaptation and future implications. Plant Cell Environ. 2024, 47, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fotopoulos, V.; Nahar, K.; Fujita, M. Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Sun, C.; Zhao, C.; Wang, G.; Mao, Q.; Han, R. Cerium Oxide Nanoparticles Alleviate Enhanced UV-B Radiation-Induced Stress in Wheat Seedling Roots by Regulating Reactive Oxygen Species. Phyton Int. J. Exp. Bot. 2025, 94, 455–479. [Google Scholar] [CrossRef]
- Li, G.; Shen, X.; Sun, X.; Wang, J.; Yuan, Q. Research progress in recombinant expression and application of peroxidases. Synth. Biol. J. 2024, 5, 1498–1517. [Google Scholar]
- Li, S.; Zheng, H.; Sui, N.; Zhang, F. Class III peroxidase: An essential enzyme for enhancing plant physiological and developmental process by maintaining the ROS level: A review. Int. J. Biol. Macromol. 2024, 283, 137331. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.D.; Costa, J.H.; Germano, T.A.; Rocha, R.d.O.; Ramos, M.V.; Bezerra, L.P. Class III plant peroxidases: From classification to physiological functions. Int. J. Biol. Macromol. 2024, 263, 130306. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Tian, X.; Hu, M.; Yang, J.; Yin, Y.; Fang, W. Ultraviolet-B Radiation Stimulates Flavonoid Biosynthesis and Antioxidant Systems in Buckwheat Sprouts. Foods 2024, 13, 3650. [Google Scholar] [CrossRef]
- Zhou, Z.; Chu, X.; Cheng, Y.; Hu, B.; Li, Q.; Peng, W.; Yang, Q.; Zhang, X.; Li, Y. UV-B pre-irradiation-promoted flavonoid accumulation enhances cold tolerance in tea plants by mediating CsHY5 pathways. Sci. Hortic. 2025, 345, 114145. [Google Scholar] [CrossRef]
- Lu, Y.; Gharib, A.; Chen, R.; Wang, H.; Tao, T.; Zuo, Z.; Bu, Q.; Su, Y.; Li, Y.; Luo, Y. Rice melatonin deficiency causes premature leaf senescence via DNA methylation regulation. Crop J. 2024, 12, 721–731. [Google Scholar] [CrossRef]
- Wang, H.; Kang, Y.; Yang, N.; Li, H.; Huang, S.; Liang, Z.; Zeng, G.; Huang, Y.; Li, W.; Zheng, M. Inhibition of UV-B stress in lettuce through enzyme-like Scutellaria baicalensis carbon dots. Ecotoxicol. Environ. Saf. 2022, 246, 114177. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Li, Y.; Ma, L.; Bu, N.; Ma, C. Changes in photosynthesis, antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to UV-B radiation and/or Cd. Plant Soil 2012, 352, 377–387. [Google Scholar] [CrossRef]
- Rybus-Zajac, M.; Kubis, J. Effect of UV-B radiation on antioxidative enzyme activity in cucumber cotyledons. Acta Biol. Cracoviensia. Ser. Bot. 2010, 52, 97–102. [Google Scholar] [CrossRef]
- Cantarello, C.; Volpe, V.; Azzolin, C.; Bertea, C. Modulation of enzyme activities and expression of genes related to primary and secondary metabolism in response to UV-B stress in cucumber (Cucumis sativus L.). J. Plant Interact. 2005, 1, 151–161. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Wang, Z.; Wang, J. Current status of tropical fruit production, preservation, and processing. Trop. Agric. Sci. 2023, 43, 80–88. (In Chinese) [Google Scholar]
- Chen, T.; Peng, J.; Qian, M.; Shui, X.; Du, J.; Liu, F.; Zhou, K. The Effects of Enhanced Ultraviolet-B Radiation on Leaf Photosynthesis and Submicroscopic Structures in Mangifera indica L. cv. ‘Tainong No 1’. Horticulturae 2023, 9, 83. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Zhu, J.; Yue, K.; Zhou, K.J.H. Characteristics of mango leaf photosynthetic inhibition by enhanced UV-B radiation. Horticulturae 2021, 7, 557. [Google Scholar] [CrossRef]
- Deshmukh, P.; Sairam, R.K.; Shukla, D. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol. 1991, 34, 89–91. [Google Scholar]
- Tahir, H.; Sajjad, M.; Qian, M.; Haq, M.Z.U.; Tahir, A.; Farooq, M.A.; Wei, L.; Shi, S.; Zhou, K.; Yao, Q. Glutathione and Ascorbic Acid Accumulation in Mango Pulp Under Enhanced UV-B Based on Transcriptome. Antioxidants 2024, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Qian, M.; Zhou, K. Identification and Bioinformatics Analysis of Mango Peroxidase Gene Family. Chin. J. Trop. Crops 2024, 45, 2500–2514. (In Chinese) [Google Scholar]
- Zhao, Z.; Wang, C.; Yu, X.; Tian, Y.; Wang, W.; Zhang, Y.; Bai, W.; Yang, N.; Zhang, T.; Zheng, H.; et al. Auxin regulates source-sink carbohydrate partitioning and reproductive organ development in rice. Proc. Natl. Acad. Sci. USA 2022, 119, e2121671119. [Google Scholar] [CrossRef]
- Wei, L.; Jiao, J.; Shi, S.; Gao, Y.; Jiang, C.; Wang, Y.; Tahir, H.; Sajjad, M.; Zhou, K.J.H.P.J. Metabolomic-Transcriptomic Analysis Unravels Flavonoids Accumulation Mechanism in ‘Tainong 1’mango Pulp Under Enhanced UV-B Radiation; Elsevier BV: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Shao, S.; Ling, W.; Gao, Y.; Chen, J.; Jia, J.; Kai, Z. The response of antioxidant enzyme and lignin synthesis system of ‘Tai Nong No.1’ mango pulp to enhanced UV-B radiation treatment. J. Fruit Sci. 2025. (In Chinese) [Google Scholar] [CrossRef]
- Shui, X.; Chen, T.-t.; Qian, M.-j.; Peng, J.-j.; Du, J.-j.; Zhou, K.-b.; Liu, F. The antioxidant response mechanism of flavonoids in ‘Tainong 1’mango pulp under enhanced UV-B radiation. Cogent Food Agric. 2024, 10, 2301273. [Google Scholar] [CrossRef]
- Wang, H.; Yue, K.; Guo, Y.-J.; Yang, C.-K.; Zhou, K.-B. Characteristics of antioxidant responses of Mangifera indica leaves by enhanced UV-B radiation. J. Trop. Subtrop. Bot. 2020, 28, 70–77. [Google Scholar]
- Wang, H.; Yue, K.; Yang, C.-K.; Guo, Y.-J.; Zhou, K.-B. Effects of Mango Trees Fruit Quality and Antioxidant Under Different UV-B Radiation Treatments. Chin. J. Trop. Crops 2020, 41, 275–283. (In Chinese) [Google Scholar]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV light blocker—A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Wong, T.M.; Sullivan, J.H.; Eisenstein, E. Acclimation and compensating metabolite responses to UV-B radiation in natural and transgenic Populus spp. defective in lignin biosynthesis. Metabolites 2022, 12, 767. [Google Scholar] [CrossRef]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Cavagnaro, J.B.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet—Absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef]
- Duan, B.; Xuan, Z.; Zhang, X.; Korpelainen, H.; Li, C. Interactions between drought, ABA application and supplemental UV-B in Populus yunnanensis. Physiol. Plant. 2008, 134, 257–269. [Google Scholar] [CrossRef]
- Chi, H.; Yue, M.; Liu, X. Physiological effects on UV-B resistance of wheat (Triticum aestivum L.) seedlings mediated by jasmonic acid. Plant Sci. J. 2011, 29, 718–726. [Google Scholar]
- Mariotti, L.; Huarancca Reyes, T.; Ramos-Diaz, J.M.; Jouppila, K.; Guglielminetti, L. Hormonal regulation in different varieties of chenopodium quinoa Willd. exposed to short acute UV-B irradiation. Plants 2021, 10, 858. [Google Scholar] [CrossRef]


| Gene ID | Forward Primer Sequences (5′ to 3′) | Reverse Primer Sequences (5′ to 3′) | Expected Amplicon Size |
|---|---|---|---|
| LOC123195912 | GGCGATGAAGACCCGTCT | ACTCCCGGGGTCCATCTC | 108 |
| LOC123214042 | GTTGCACAAGCTTCCGGC | TCTTGGGCATCGACGTCG | 107 |
| LOC123194338 | TTCGCTTGCTGGGGATGG | CCCCTGTCAAGGCAACGA | 137 |
| LOC123229388 | GCTGCTCGTGACTCCGTT | TGCGGCGCTTAAACTTGC | 93 |
| LOC123225298 | CTTCTGCAAGGTGGCCCA | TCTCGGCCGTAGCTTCCT | 85 |
| LOC123198833 | CATGGACCCCGTCACACC | TGGCCCAGGCTTTTACGG | 104 |
| Actin | ATCTGCTGAAGGTGCTGAG | CCAAGCAGCATGAGATCAA | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wei, L.; Jiang, C.; Shi, S.; Jiao, J.; Tahir, H.; Qian, M.; Zhou, K. Physiological Mechanisms of the Enhanced UV-B Radiation Triggering Plant-Specific Peroxidase-Mediated Antioxidant Defences. Antioxidants 2025, 14, 957. https://doi.org/10.3390/antiox14080957
Gao Y, Wei L, Jiang C, Shi S, Jiao J, Tahir H, Qian M, Zhou K. Physiological Mechanisms of the Enhanced UV-B Radiation Triggering Plant-Specific Peroxidase-Mediated Antioxidant Defences. Antioxidants. 2025; 14(8):957. https://doi.org/10.3390/antiox14080957
Chicago/Turabian StyleGao, Yijia, Ling Wei, Chenyu Jiang, Shaopu Shi, Jiabing Jiao, Hassam Tahir, Minjie Qian, and Kaibing Zhou. 2025. "Physiological Mechanisms of the Enhanced UV-B Radiation Triggering Plant-Specific Peroxidase-Mediated Antioxidant Defences" Antioxidants 14, no. 8: 957. https://doi.org/10.3390/antiox14080957
APA StyleGao, Y., Wei, L., Jiang, C., Shi, S., Jiao, J., Tahir, H., Qian, M., & Zhou, K. (2025). Physiological Mechanisms of the Enhanced UV-B Radiation Triggering Plant-Specific Peroxidase-Mediated Antioxidant Defences. Antioxidants, 14(8), 957. https://doi.org/10.3390/antiox14080957








