The Glutathione Peroxidase Gene Family in Chenopodium quinoa: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the CqGPXs Gene Family in Quinoa
2.2. Evolutionary Relationship of the CqGPXs Gene Family
2.3. Gene Structure and Protein Conserved Motif Analysis
2.4. Chromosomal Location and Gene Duplication Analysis
2.5. Analysis of Cis-Acting Elements in the Promoter Regions
2.6. Analysis of CqGPXs Gene Expression Patterns in Diverse Tissues
2.7. Plant Materials and Stress Treatments
2.8. RNA Extraction and qRT-PCR Analysis
2.9. Enzyme Activity Mensuration
2.10. Heterologous Expression of CqGPX4 and CqGPX15 in E. coli and Stress Tolerance Assay
3. Results
3.1. Identification of CqGPX Genes in Quinoa
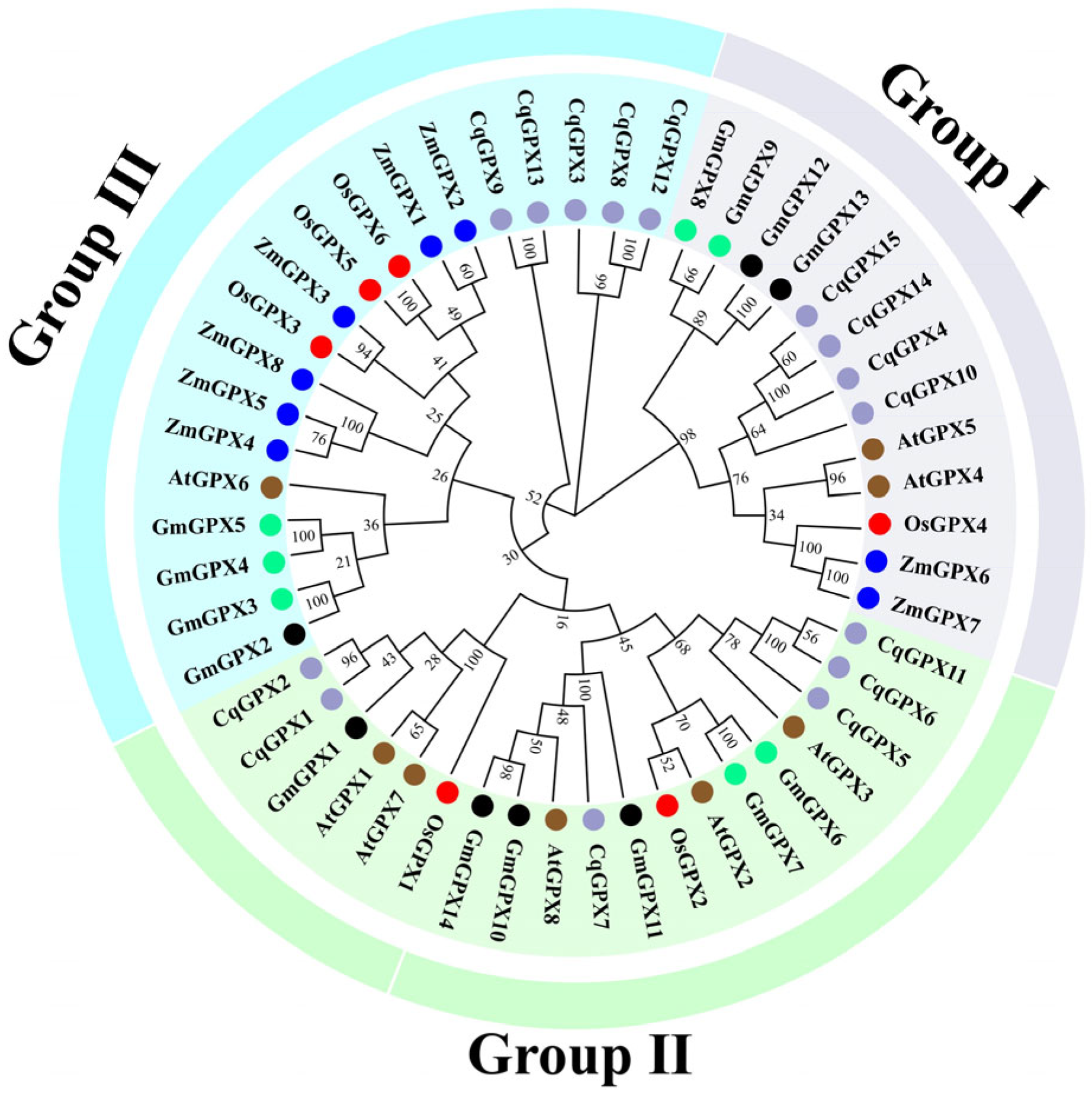
3.2. Phylogenetic Analysis of CqGPX Genes
3.3. Gene Structural and Conserved Domain Analyses of CqGPXs
3.4. Chromosomal Location and Duplication of CqGPX Genes
3.5. Cis-Acting Elements Analysis
3.6. Analysis of CqGPXs Expression Patterns in Tissues
3.7. Analysis of CqGPX Genes Expression Patterns Under Stress Conditions
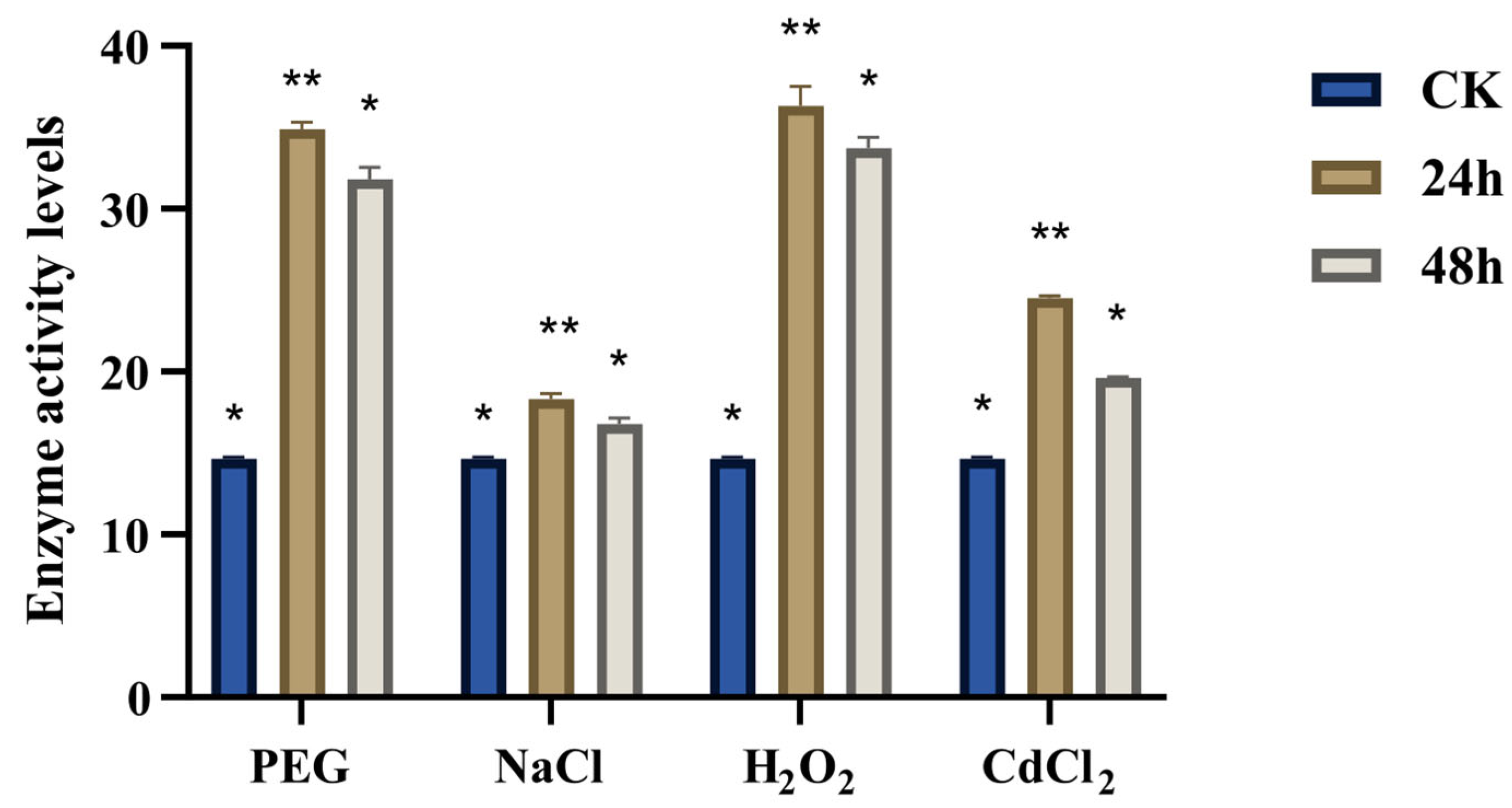
3.8. Determination of the Activity of CqGPX Enzyme Under Stress Conditions
3.9. Overexpression of CqGPX4 and CqGPX15 Enhanced Stress Tolerance in E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, P.K.; Agarwal, P.; Reddy, M.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef]
- Zafar, S.A.; Zaidi, S.S.-e.-A.; Gaba, Y.; Singla-Pareek, S.L.; Dhankher, O.P.; Li, X.; Mansoor, S.; Pareek, A. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J. Exp. Bot. 2020, 71, 470–479. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Afzal, I.; Iqbal, S.; Hafeez, M.B.; Raza, A. Low leaf sodium content improves the grain yield and physiological performance of wheat genotypes in saline-sodic soil. Pesqui. Agropecu. Trop. 2021, 51, e67663. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.A.; Hameed, A.; Ashraf, M.; Khan, A.S.; Li, X.; Siddique, K.H. Agronomic, physiological and molecular characterisation of rice mutants revealed the key role of reactive oxygen species and catalase in high-temperature stress tolerance. Funct. Plant Biol. 2020, 47, 440–453. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Sugimoto, M.; Oono, Y.; Gusev, O.; Matsumoto, T.; Yazawa, T.; Levinskikh, M.A.; Sychev, V.N.; Bingham, G.E.; Wheeler, R.; Hummerick, M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014, 14, 4. [Google Scholar] [CrossRef]
- San Koh, C.; Didierjean, C.; Navrot, N.; Panjikar, S.; Mulliert, G.; Rouhier, N.; Jacquot, J.-P.; Aubry, A.; Shawkataly, O.; Corbier, C. Crystal structures of a poplar thioredoxin peroxidase that exhibits the structure of glutathione peroxidases: Insights into redox-driven conformational changes. J. Mol. Biol. 2007, 370, 512–529. [Google Scholar] [CrossRef]
- Bela, K.; Riyazuddin, R.; Csiszár, J. Plant glutathione peroxidases: Non-heme peroxidases with large functional flexibility as a Core Component of ROS-processing mechanisms and signalling. Antioxidants 2022, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.-P.; Rouhier, N. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef]
- Koua, D.; Cerutti, L.; Falquet, L.; Sigrist, C.J.; Theiler, G.; Hulo, N.; Dunand, C. PeroxiBase: A database with new tools for peroxidase family classification. Nucleic Acids Res. 2009, 37, D261–D266. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Li, W.; Huai, X.; Li, P.; Raza, A.; Mubarik, M.S.; Habib, M.; Fiaz, S.; Zhang, B.; Pan, J.; Khan, R.S.A. Genome-wide characterization of glutathione peroxidase (GPX) gene family in rapeseed (Brassica napus L.) revealed their role in multiple abiotic stress response and hormone signaling. Antioxidants 2021, 10, 1481. [Google Scholar] [CrossRef]
- Attacha, S.; Solbach, D.; Bela, K.; Moseler, A.; Wagner, S.; Schwarzländer, M.; Aller, I.; Müller, S.J.; Meyer, A.J. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 1281–1295. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Zhou, G.; Yue, Z.; Hu, Q.; Chen, Y.; Liu, B.; Qiu, Q.; Wang, Z.; Zhang, J. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 2013, 4, 2797. [Google Scholar] [CrossRef]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A. The importance of Arabidopsis glutathione peroxidase 8 for protecting Arabidopsis plant and E. coli cells against oxidative stress. GM Crops Food 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Passaia, G.; Queval, G.; Bai, J.; Margis-Pinheiro, M.; Foyer, C.H. The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1403–1413. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.-C.; Chen, J.; Miao, C.; Song, C.-P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Bela, K.; Horváth, E.; Rigó, G.; Gallé, Á.; Szabados, L.; Fehér, A.; Csiszár, J. Overexpression of the Arabidopsis glutathione peroxidase-like 5 gene (AtGPXL5) resulted in altered plant development and redox status. Environ. Exp. Bot. 2019, 167, 103849. [Google Scholar] [CrossRef]
- Gaber, A.; Ogata, T.; Maruta, T.; Yoshimura, K.; Tamoi, M.; Shigeoka, S. The involvement of Arabidopsis glutathione peroxidase 8 in the suppression of oxidative damage in the nucleus and cytosol. Plant Cell Physiol. 2012, 53, 1596–1606. [Google Scholar] [CrossRef]
- Wang, K.; Tang, S.-F.; Hou, X. Molecular mechanism investigation on the interactions of copper (II) ions with glutathione peroxidase 6 from Arabidopsis thaliana. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 428–433. [Google Scholar] [CrossRef]
- Chang, C.C.; Slesak, I.; Jordá, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 2009, 150, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Lima-Melo, Y.; Carvalho, F.E.; Martins, M.O.; Passaia, G.; Sousa, R.H.; Neto, M.C.L.; Margis-Pinheiro, M.; Silveira, J.A. Mitochondrial GPX1 silencing triggers differential photosynthesis impairment in response to salinity in rice plants. J. Integr. Plant Biol. 2016, 58, 737–748. [Google Scholar] [CrossRef]
- Passaia, G.; Fonini, L.S.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; de Araujo Mariath, J.E.; Margis, R.; Margis-Pinheiro, M. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Xu, H.; Li, G.; Yu, A.; Yu, X.; Hu, W.; Zheng, X.; Li, S.; Wang, Y.; Hu, Z. Cloning a glutathione peroxidase gene from Nelumbo nucifera and enhanced salt tolerance by overexpressing in rice. Mol. Biol. Rep. 2014, 41, 4919–4927. [Google Scholar] [CrossRef]
- Zhai, C.-Z.; Zhao, L.; Yin, L.-J.; Chen, M.; Wang, Q.-Y.; Li, L.-C.; Xu, Z.-S.; Ma, Y.-Z. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE 2013, 8, e73989. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, X.; Peterson, T. Identification of conserved gene structures and carboxy-terminal motifs in the Myb gene family of Arabidopsis and Oryza sativa L. ssp. indica. Genome Biol. 2004, 5, R46. [Google Scholar] [CrossRef]
- Yi, Y.; Mirosevich, J.; Shyr, Y.; Matusik, R.; George, A.L., Jr. Coupled analysis of gene expression and chromosomal location. Genomics 2005, 85, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Clemente, R.M.; Vives, V.; Zandalinas, S.I.; López-Climent, M.F.; Muñoz, V.; Gómez-Cadenas, A. Biotechnological approaches to study plant responses to stress. BioMed Res. Int. 2013, 2013, 654120. [Google Scholar] [CrossRef] [PubMed]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa willd) to exogenous selenium application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Ruan, M.; Ye, Q.; Yao, Z.; Wang, R.; Zhou, G.; Liu, D.; Wan, H. Comprehensive identification of glutathione peroxidase (GPX) gene family in response to abiotic stress in pepper (Capsicum annuum L.). Gene 2023, 881, 147625. [Google Scholar] [CrossRef]
- Parveen, K.; Saddique, M.A.B.; Ali, Z.; Rehman, S.U.; Khan, Z.; Waqas, M.; Munir, M.Z.; Hussain, N.; Muneer, M.A. Genome-wide analysis of glutathione peroxidase (GPX) gene family in chickpea (Cicer arietinum L.) under salinity stress. Gene 2024, 898, 148088. [Google Scholar] [CrossRef]
- Jana, G.A.; Yaish, M.W. Genome-wide identification and functional characterization of glutathione peroxidase genes in date palm (Phoenix dactylifera L.) under stress conditions. Plant Gene 2020, 23, 100237. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Wang, J.; Yang, W.; Yang, Y. Identification and characterization of the glutathione peroxidase (GPX) gene family in watermelon and its expression under various abiotic stresses. Agronomy 2018, 8, 206. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Ye, S.; Jiang, L.; Liu, S. Genome-wide identification of glutathione peroxidase (GPX) gene family and their response to abiotic stress in cucumber. 3 Biotech 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, J.; Ma, T.; Li, H.; Wang, N.; Li, Z.; Zhang, Z.; Zhou, Y. The glutathione peroxidase gene family in Thellungiella salsuginea: Genome-wide identification, classification, and gene and protein expression analysis under stress conditions. Int. J. Mol. Sci. 2014, 15, 3319–3335. [Google Scholar] [CrossRef]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.N.; Shakeel, A.; Lei, S. Characterization of SOD and GPX gene families in the soybeans in response to drought and salinity stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Peng, X.; Ma, T.; Song, K.; Ji, X.; Xiang, L.; Chen, N.; Zu, R.; Xu, W.; Zhu, S.; Liu, W. Overexpression of NtGPX8a improved cadmium accumulation and tolerance in tobacco (Nicotiana tabacum L.). Genes 2024, 15, 366. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, X.; Gao, A.; Cao, M.; Yang, D.; An, K.; Guo, S.; Yin, H. Genome-Wide Identification and Expression Analysis of 1-Aminocyclopropane-1-Carboxylate Synthase (ACS) Gene Family in Chenopodium quinoa. Plants 2023, 12, 4021. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Cao, M.; Yin, L.; Gao, A.; An, K.; Gao, S.; Guo, S.; Yin, H. Genome-wide identification, expression and interaction analyses of PP2C family genes in Chenopodium quinoa. Genes 2023, 15, 41. [Google Scholar] [CrossRef]
- Jiang, B.; Su, C.; Wang, Y.; Xu, X.; Li, Y.; Ma, D. Genome-wide identification of Glutathione peroxidase (GPX) family genes and silencing TaGPX3.2A reduced disease resistance in wheat. Plant Physiol. Biochem. 2023, 204, 108139. [Google Scholar] [CrossRef]
- Islam, T.; Manna, M.; Reddy, M.K. Glutathione peroxidase of Pennisetum glaucum (PgGPx) is a functional Cd2+ dependent peroxiredoxin that enhances tolerance against salinity and drought stress. PLoS ONE 2015, 10, e0143344. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Miao, X.; Mo, F.; Liu, T.; Chen, Y. Genome-wide analysis and expression profiling of the glutathione peroxidase-like enzyme gene family in Solanum tuberosum. Int. J. Mol. Sci. 2023, 24, 11078. [Google Scholar] [CrossRef]
- Ramos, J.; Matamoros, M.A.; Naya, L.; James, E.K.; Rouhier, N.; Sato, S.; Tabata, S.; Becana, M. The glutathione peroxidase gene family of Lotus japonicus: Characterization of genomic clones, expression analyses and immunolocalization in legumes. New Phytol. 2009, 181, 103–114. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, L.; Li, B.; Xiao, J.; Wang, H.; Dong, C.; Li, X.; Xu, P. Genome-wide mining of gpx gene family provides new insights into cadmium stress responses in common carp (Cyprinus carpio). Gene 2022, 821, 146291. [Google Scholar] [CrossRef]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Vannozzi, A.; Palumbo, F.; Barcaccia, G. Exogenous EBR ameliorates endogenous hormone contents in tomato species under low-temperature stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
- Sreelakshmy, V.; Anbarasi, G.; Vishnupriya, B. Salicylic acid pre-treatment induced physiological and biochemical changes in Solanum lycopersicum L. under salinity stress. Not. Sci. Biol. 2021, 13, 10917. [Google Scholar] [CrossRef]
- Zelinová, V.; Mistrík, I.; Pavlovkin, J.; Tamás, L. Glutathione peroxidase expression and activity in barley root tip after short-term treatment with cadmium, hydrogen peroxide and t-butyl hydroperoxide. Protoplasma 2013, 250, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Wang, C.; Chang, Y.; Han, K.; Gao, Y.; Xie, J. Characterization of GPX gene family in pepper (Capsicum annuum L.) under abiotic stress and ABA treatment. Int. J. Mol. Sci. 2024, 25, 8343. [Google Scholar] [CrossRef]
- Islam, T.; Manna, M.; Kaul, T.; Pandey, S.; Reddy, C.S.; Reddy, M.K. Genome-wide dissection of Arabidopsis and rice for the indentification and expression analysis of glutathione peroxidases reveals their stress-specific and overlapping response patterns. Plant Mol. Biol. Report. 2015, 33, 1413–1427. [Google Scholar] [CrossRef]
- Song, W.; Xin, S.; He, M.; Pfeiffer, S.; Cao, A.; Li, H.; Schick, J.; Jin, X. Evolutionary and functional analyses demonstrate conserved ferroptosis protection by Arabidopsis GPXs in mammalian cells. FASEB J. 2021, 35, e21550. [Google Scholar] [CrossRef]






| Gene Name | Gene ID | Chromosome Location | Number of Amino Acid Residues | Molecular Weight (KDa) | Isoelectric Point | Instability Coefficient | Hydrophilicity Coefficient | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| CqGPX1 | AUR62024797 | Chr01: 46337198–46340575 | 241 | 26.49 | 8.79 | 32.42 | −0.196 | Chloroplasts, mitochondria |
| CqGPX2 | AUR62032425 | Chr04: 52843865–52847436 | 235 | 25.85 | 8.81 | 30.2 | −0.137 | Chloroplasts, mitochondria |
| CqGPX3 | AUR62002981 | Chr06: 14361837–14364180 | 171 | 19.55 | 9.33 | 25.62 | −0.269 | Chloroplasts, mitochondria |
| CqGPX4 | AUR62036788 | Chr07: 52295212–52298236 | 171 | 19.08 | 9.3 | 26.59 | −0.33 | mitochondrion |
| CqGPX5 | AUR62002269 | Chr07: 65062016–65067011 | 199 | 22.37 | 6.6 | 42.34 | −0.063 | Chloroplasts, mitochondria |
| CqGPX6 | AUR62015425 | Chr11: 20062320–20067149 | 200 | 22.56 | 7.63 | 41.98 | −0.137 | Chloroplasts, mitochondria |
| CqGPX7 | AUR62010667 | Chr13: 9382496–9384086 | 141 | 16.05 | 4.54 | 34.93 | −0.156 | Chloroplasts, mitochondria |
| CqGPX8 | AUR62010585 | Chr13: 10828050–10834294 | 455 | 50.87 | 5.22 | 42.6 | −0.225 | Chloroplasts, mitochondria |
| CqGPX9 | AUR62010584 | Chr13: 10861209–10865017 | 234 | 26.19 | 8.61 | 37.64 | −0.317 | Chloroplasts, mitochondria |
| CqGPX10 | AUR62035236 | Chr14: 7766701–7773388 | 323 | 36.17 | 9.43 | 34.67 | −0.416 | nucleus |
| CqGPX11 | AUR62005465 | Chr14: 52044958–52047589 | 141 | 15.98 | 9.06 | 42.97 | −0.415 | mitochondrion |
| CqGPX12 | AUR62017227 | Chr16: 68266257–68269618 | 170 | 18.91 | 6.73 | 30.86 | −0.221 | Chloroplasts, mitochondria |
| CqGPX13 | AUR62017225 | Chr16: 68316179–68319386 | 235 | 26.13 | 8.82 | 38.37 | −0.311 | Chloroplasts, mitochondria |
| CqGPX14 | AUR62033706 | Chr17: 53972176–53976971 | 171 | 19.04 | 9.29 | 28.3 | −0.333 | mitochondrion |
| CqGPX15 | AUR62033705 | Chr17: 53998828–54003613 | 171 | 19.04 | 9.29 | 28.3 | −0.333 | mitochondrion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xu, A.; An, K.; Wang, L.; Luo, T.; Yu, X.; Yin, H.; Guo, S.; Zhang, X. The Glutathione Peroxidase Gene Family in Chenopodium quinoa: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis. Antioxidants 2025, 14, 940. https://doi.org/10.3390/antiox14080940
Yang J, Xu A, An K, Wang L, Luo T, Yu X, Yin H, Guo S, Zhang X. The Glutathione Peroxidase Gene Family in Chenopodium quinoa: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis. Antioxidants. 2025; 14(8):940. https://doi.org/10.3390/antiox14080940
Chicago/Turabian StyleYang, Jing, Anna Xu, Kexin An, Lilong Wang, Taiping Luo, Xinyue Yu, Haibo Yin, Shanli Guo, and Xia Zhang. 2025. "The Glutathione Peroxidase Gene Family in Chenopodium quinoa: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis" Antioxidants 14, no. 8: 940. https://doi.org/10.3390/antiox14080940
APA StyleYang, J., Xu, A., An, K., Wang, L., Luo, T., Yu, X., Yin, H., Guo, S., & Zhang, X. (2025). The Glutathione Peroxidase Gene Family in Chenopodium quinoa: Genome-Wide Identification, Classification, Gene Expression and Functional Analysis. Antioxidants, 14(8), 940. https://doi.org/10.3390/antiox14080940







