A Novel Glycosylated Ferulic Acid Conjugate: Synthesis, Antioxidative Neuroprotection Activities In Vitro, and Alleviation of Cerebral Ischemia–Reperfusion Injury (CIRI) In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Animals
2.3. Synthesis of FA–Glucose Conjugate
2.3.1. Synthesis of (E)-3-(3-Methoxy-4-(prop-2-yn-1-yloxy)phenyl)acrylic Acid (Compound 1)
2.3.2. Synthesis of FA-Glu
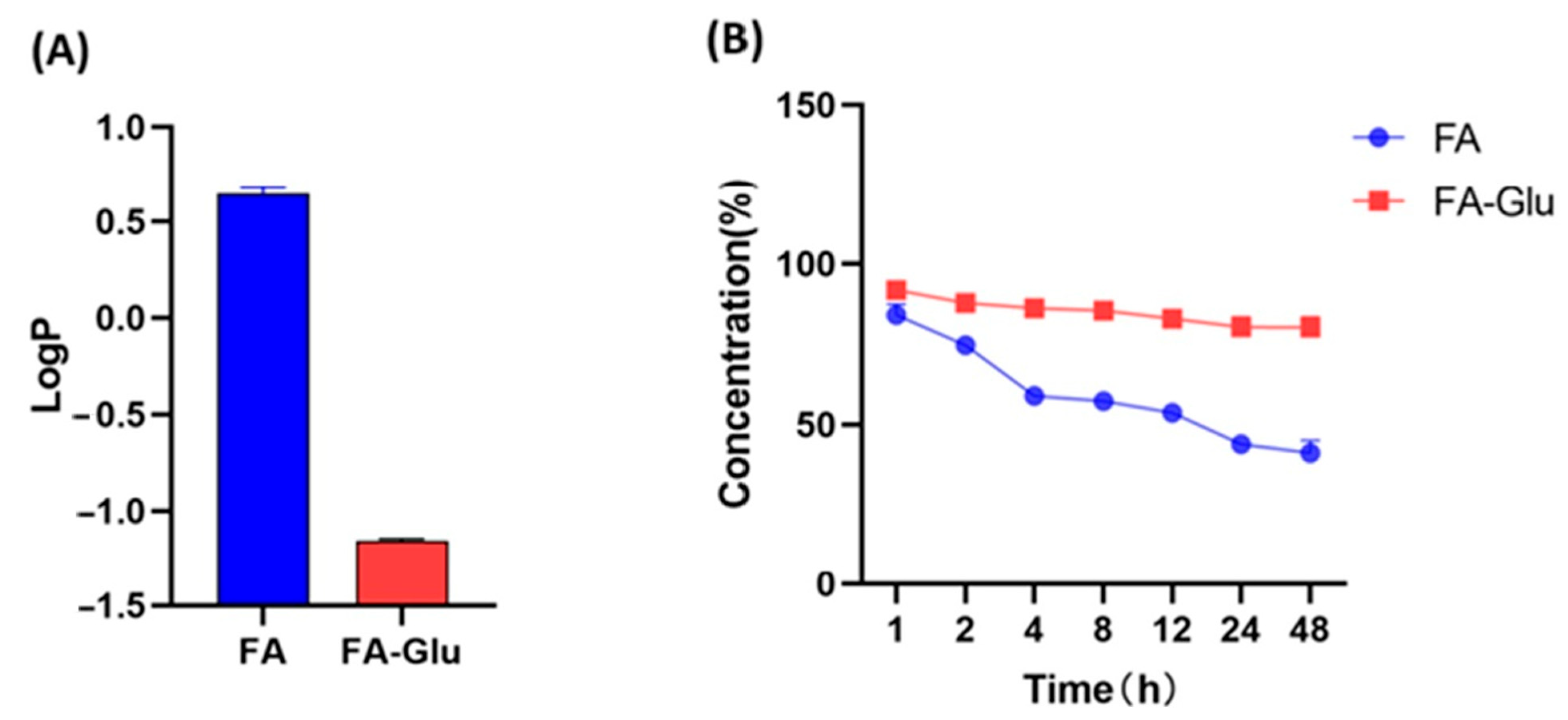
2.4. Test of LogP Value
2.5. Test of Stability
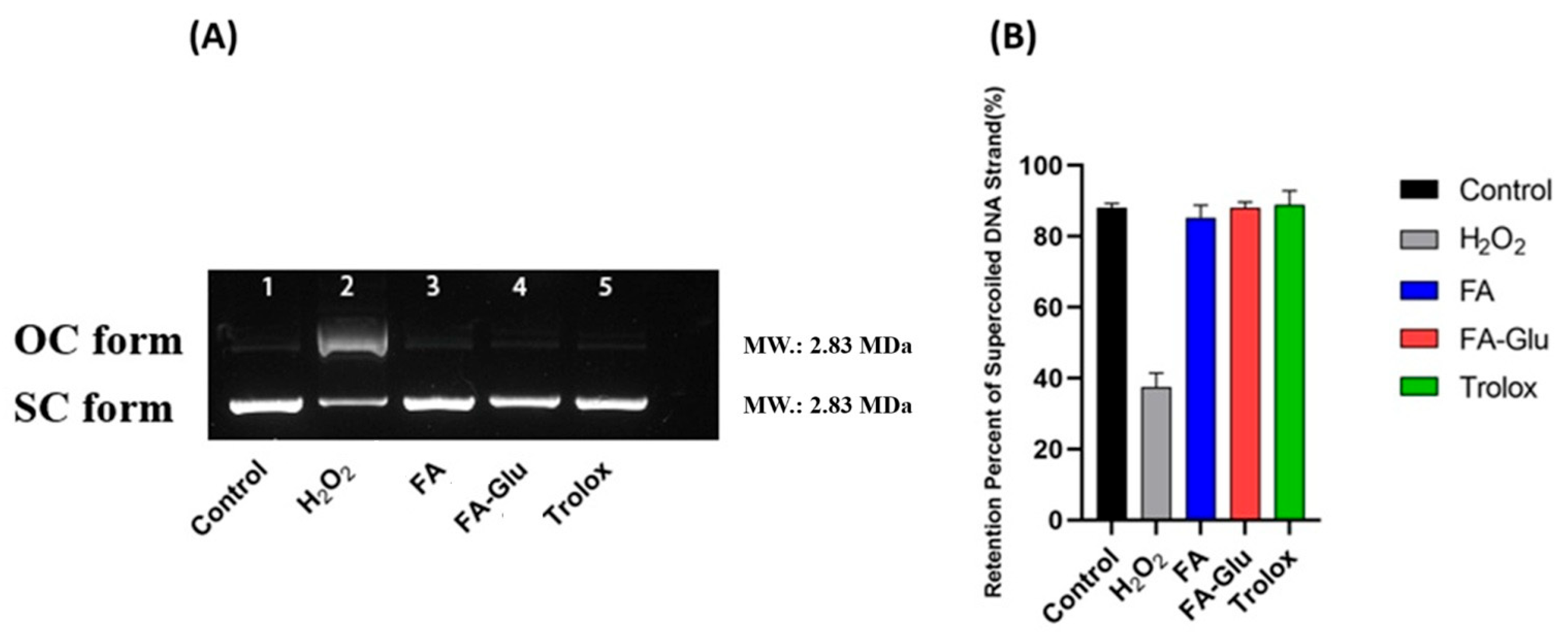
2.6. Determination of Preventive Activity Against Oxidative Damage in pBR322 Plasmid DNA
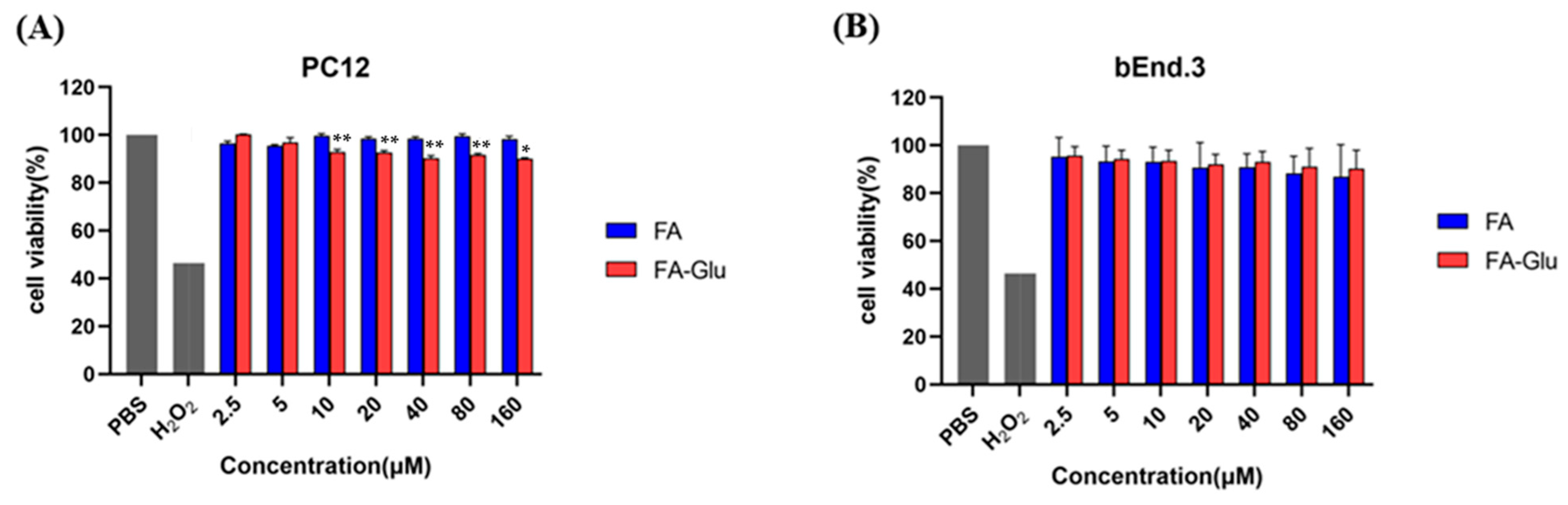
2.7. Cytotoxicity Test of FA-Glu
2.8. Evaluation of FA-Glu Neuroprotective Effect In Vitro
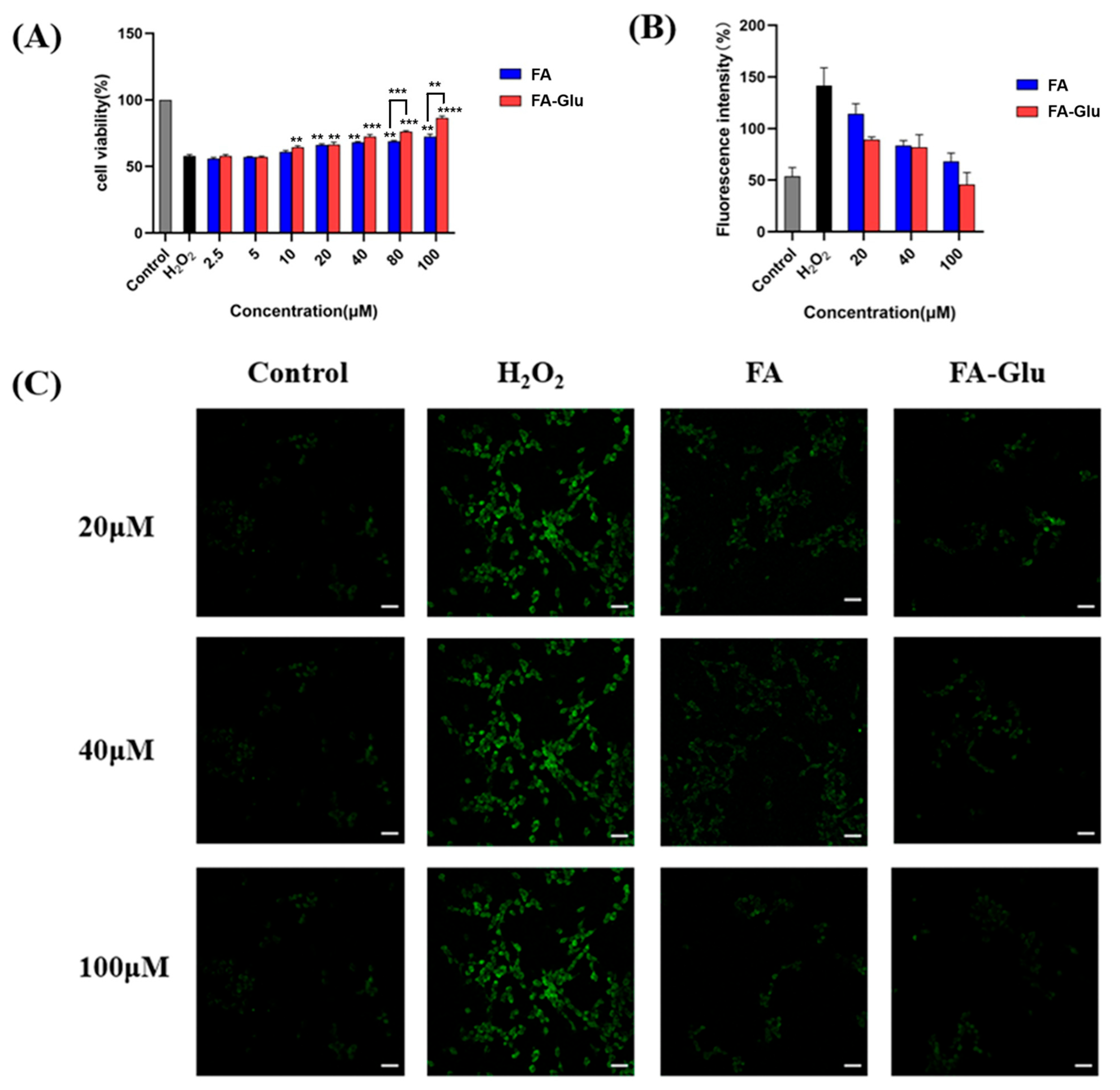
2.8.1. Antioxidative Protection to H2O2-Induced Injury on PC12 Cells
2.8.2. Intracellular ROS Evaluation
2.9. Neuroprotective Effect Evaluation In Vivo
2.9.1. Rat Model of Middle Cerebral Artery Occlusion (MCAO) and Treatments
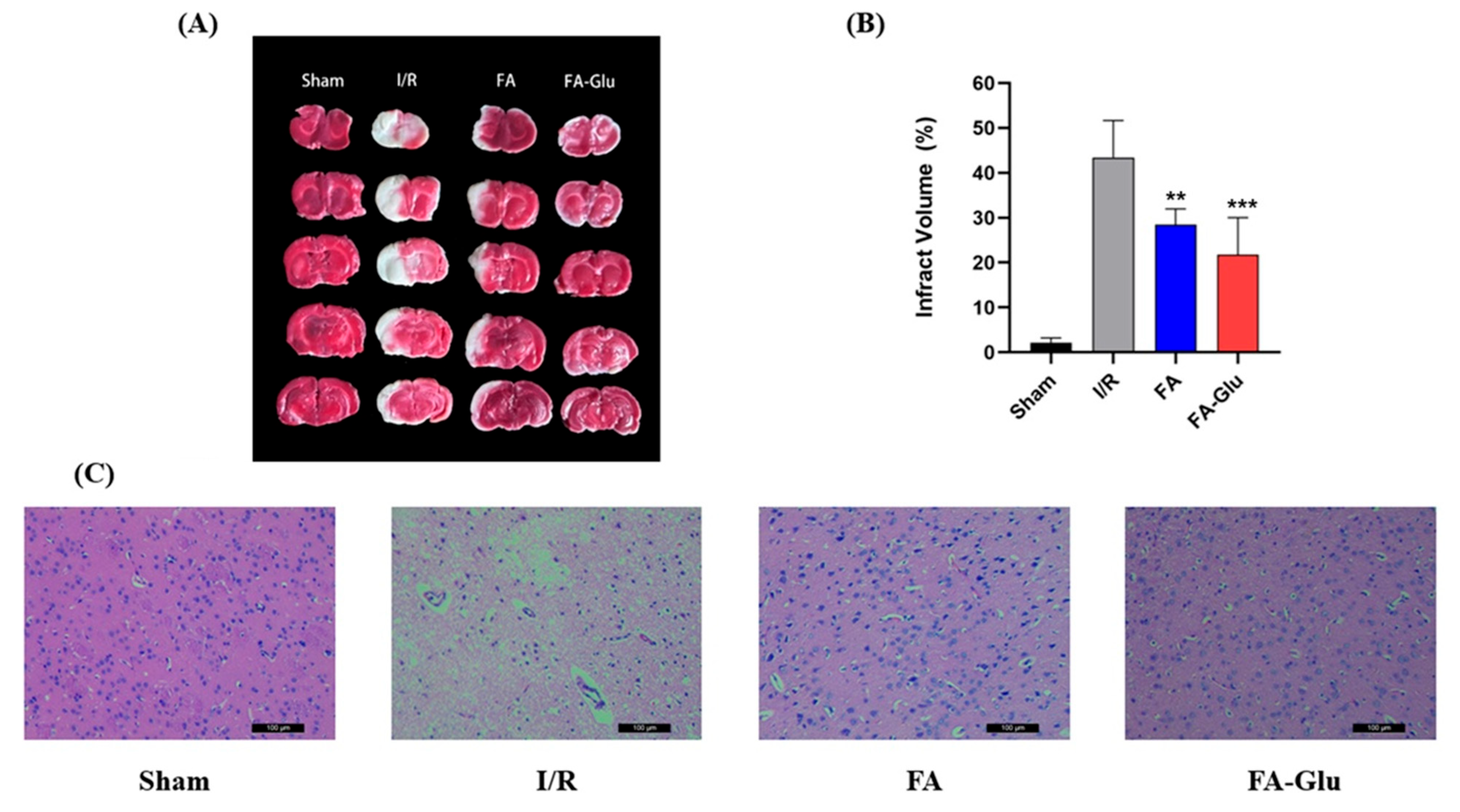
2.9.2. Cerebral Infarction Volume Measurement Assay
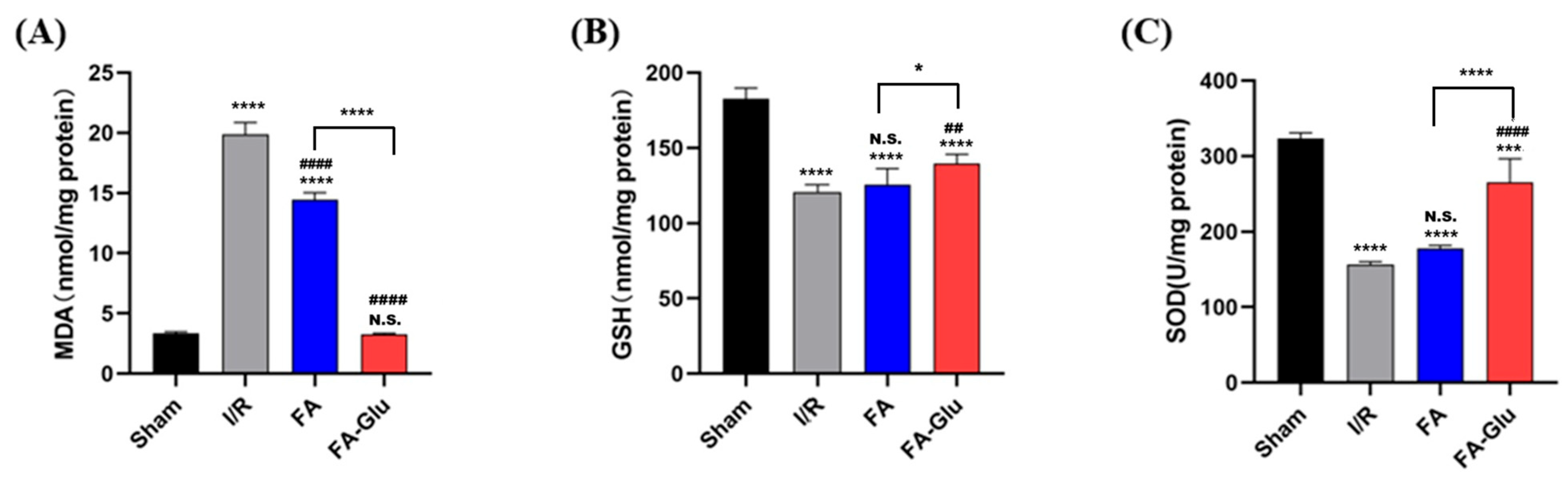
2.9.3. MDA, GSH, SOD Measurements
2.10. Statistical Analysis
3. Results and Discussion
3.1. Design and Synthesis of FA-Glu
3.2. Water Solubility and Stability
3.3. Preventive Activity Against Oxidative Damage of pBR322 Plasmid DNA
3.4. Cytotoxicity of FA-Glu
3.5. FA-Glu Attenuate H2O2-Induced Antioxidative Injury on PC12 Cells
3.6. FA-Glu Inhibition of ROS Production in the Oxidative Injury of PC12 Cells
3.7. Neuroprotective Effects of FA-Glu on Cerebral Ischemia–Reperfusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Du, Q.; Li, Z.; Xue, L.; Jia, Q.; Zheng, T.; Liu, J.; Ren, R.; Sun, Z. Multiomics profiling of the therapeutic effect of Dan-deng-tong-nao capsule on cerebral ischemia-reperfusion injury. Phytomedicine 2024, 128, 155335. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gong, P.; Zhang, M.; Li, C.; Xiao, P.; Yu, M.; Wang, X.; An, L.; Bi, F.; Song, X.; et al. Multi-parametric MRI assessment of melatonin regulating the polarization of microglia in rats after cerebral ischemia/reperfusion injury. Brain Res. Bull. 2023, 204, 110807. [Google Scholar] [CrossRef]
- Nakka, V.P.; Gusain, A.; Mehta, S.L.; Raghubir, R. Molecular Mechanisms of Apoptosis in Cerebral Ischemia: Multiple Neuroprotective Opportunities. Mol. Neurobiol. 2008, 37, 7–38. [Google Scholar] [CrossRef]
- Jin, C.; Huang, R.J.; Peterson, E.D.; Laskowitz, D.T.; Hernandez, A.F.; Federspiel, J.J.; Schwamm, L.H.; Bhatt, D.L.; Smith, E.E.; Fonarow, G.C.; et al. Intravenous tPA (Tissue-Type Plasminogen Activator) in Patients With Acute Ischemic Stroke Taking Non–Vitamin K Antagonist Oral Anticoagulants Preceding Stroke. Stroke 2018, 49, 2237–2240. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Yang, L.; Zhang, D.; Li, L.; Dong, X.; Li, Y.; Qun, S.; Li, W. Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J. Ginseng Res. 2022, 46, 515–525. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Savyuk, M.O.; Ponimaskin, E.; Vedunova, M.V. Hypoxia-Inducible Factor (HIF) in Ischemic Stroke and Neurodegenerative Disease. Front. Cell Dev. Biol. 2021, 9, 703084. [Google Scholar] [CrossRef]
- Halpani, C.G.; Mishra, S. Design, synthesis, characterization of ferulic acid and p-coumaric acid amide derivatives as an antibacterial/antioxidant agent. Pharm. Sci. Adv. 2024, 2, 100023. [Google Scholar] [CrossRef]
- Thapliyal, S.; Singh, T.; Handu, S.; Bisht, M.; Kumari, P.; Arya, P.; Srivastava, P.; Gandham, R. A Review on Potential Footprints of Ferulic Acid for Treatment of Neurological Disorders. Neurochem. Res. 2021, 46, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Shubham, S.; Kumar, A.; Kumar, V.; Oz, E.; Brennan, C.; Zeng, M.; Proestos, C.; Çadırcı, K.; et al. Ferulic acid: Extraction, estimation, bioactivity and applications for human health and food. J Sci Food Agr. 2025, 105, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Setzer, W.N.; Aldahish, A.A.; Sharifi-Rad, J.; Calina, D. Breaking free from free radicals: Harnessing the power of natural antioxidants for health and disease prevention. Chem. Pap. 2024, 78, 2061–2077. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals—Just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Enzymatic Glycosylation in plasticized glass phases: A novel and efficient route to O-glycosides. Angew. Chem. Int. Edit. 2000, 39, 3804–3808. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Zhang, Y.-M.; Qi, J.-P.; Liu, R.; Zhang, H.; He, L.-C. Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-κB pathway. Int. Immunopharmacol. 2015, 28, 1018–1025. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Characterisation of inclusion complex of trans-ferulic acid and hydroxypropyl-β-cyclodextrin. Food Chem. 2011, 124, 1069–1075. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Zhang, S.; Chang, S.; He, B. Efficient synthesis of 4-O-β-d-glucopyranosylferulic acid from ferulic acid by whole cells harboring glycosyltransferase GTBP1. Biochem. Eng. J. 2018, 130, 99–103. [Google Scholar] [CrossRef]
- Galland, S.; Mora, N.; Abert-Vian, M.; Rakotomanomana, N.; Dangles, O. Chemical Synthesis of Hydroxycinnamic Acid Glucosides and Evaluation of Their Ability To Stabilize Natural Colors via Anthocyanin Copigmentation. J. Agric. Food Chem. 2007, 55, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, H.; Shimoda, K.; Kubota, N.; Nakajima, N.; Hamada, H.; Hamada, H. Biotransformation of Cinnamic Acid, p-Coumaric Acid, Caffeic Acid, and Ferulic Acid by Plant Cell Cultures of Eucalyptus perriniana. Biosci. Biotechnol. Biochem. 2010, 74, 1920–1924. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, P.; Liang, J.; Chen, Y.; Yang, C.; Xia, C.; Deng, J.; Hai, L.; Chen, J.; Wu, Y. Synthesis and bioactivity evaluation of glycosylated resveratrol derivatives as antioxidative neuroprotection agents against cerebral Ischemia-Reperfusion injury. Bioorganic Chem. 2024, 153, 107791. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discovery. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Philip, L.; Yeagle. GLUT1: Facilitated Transport of glucose. In The Membranes of Cells, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128004869. [Google Scholar]
- Chen, Q.; Gong, T.; Liu, J.; Wang, X.; Fu, H.; Zhang, Z. Synthesis, in vitro and in vivo characterization of glycosyl derivatives of ibuprofen as novel prodrugs for brain drug delivery. J. Drug Target. 2009, 17, 318–328. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Luo, Y.; Ji, X.; Ling, F.; Li, W.; Zhang, F.; Cao, G.; Chen, J. Impaired DNA repair via the base-excision repair pathway after focal ischemic brain injury: A protein phosphorylation-dependent mechanism reversed by hypothermic neuroprotection. Front. Biosci. 2007, 12, 1852–1862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lan, J.; Li, W.; Zhang, F.; Sun, F.-Y.; Nagayama, T.; O’Horo, C.; Chen, J. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2003, 23, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, H.; Yang, S.; Chen, D. Ischemic preconditioning induces XRCC1, DNA polymerase-beta, and DNA ligase III and correlates with enhanced base excision repair. DNA Repair 2007, 6, 1297–1306. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, M.; Afzal, M.; Zaki, M.; Bharadwaj, P.K. Synthesis structure elucidation of a copper(II) Schiff-base complex: In vitro DNAbinding pBR322 plasmid cleavage HSAbinding studies. J. Photochem. Photobiol. B Biol. 2014, 140, 321–331. [Google Scholar] [CrossRef]
- Wang, Q.D.; Sun, M.M.; Liang, J.H. Reaction Mechanisms and Kinetics of the Hydrogen Abstraction Reactions of C4–C6 Alkenes with Hydroxyl Radical: A Theoretical Exploration. Int. J. Mol. Sci. 2019, 20, 1275. [Google Scholar] [CrossRef]
- Kim, K.-A.; Kim, D. Autophagy-mediated occluding degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers CNS 2020, 17, 21. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, J.; Liu, Y.; Zhao, M.; Qi, Z.; Shi, W.; Li, Y.; Lu, S.; Dong, J.; Wang, Q. Assessing the structural and foaming property changes in egg yolk proteins due to malondialdehyde: Experimental and molecular docking studies. Food Chem. 2024, 452, 139529. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Mi, L.; Wei, Y.; Zhang, Y.; Mao, W. Sequential activation strategy of triazinyl resorufin for high selectivity fluorescence GSH detection. Talanta 2024, 269, 125477. [Google Scholar] [CrossRef] [PubMed]
- Makhmalzadeh, B.S.; Dehkordi, S.K.H.; Rezaie, A.; Karami, M.A. Superoxide dismutase-contained solid lipid nanoparticles: Formulation development and In-vivo evaluation for second-degree burn wound healing in rats. Burns 2024, 50, 1823–1831. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorganic Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yuan, Y.; Tong, L.; Yu, M.; Zhu, Y.; Liu, Q.; Deng, J.; Wang, F.; Xiang, Z.; Xia, C. A Novel Glycosylated Ferulic Acid Conjugate: Synthesis, Antioxidative Neuroprotection Activities In Vitro, and Alleviation of Cerebral Ischemia–Reperfusion Injury (CIRI) In Vivo. Antioxidants 2025, 14, 953. https://doi.org/10.3390/antiox14080953
Chen J, Yuan Y, Tong L, Yu M, Zhu Y, Liu Q, Deng J, Wang F, Xiang Z, Xia C. A Novel Glycosylated Ferulic Acid Conjugate: Synthesis, Antioxidative Neuroprotection Activities In Vitro, and Alleviation of Cerebral Ischemia–Reperfusion Injury (CIRI) In Vivo. Antioxidants. 2025; 14(8):953. https://doi.org/10.3390/antiox14080953
Chicago/Turabian StyleChen, Jian, Yongjun Yuan, Litao Tong, Manyou Yu, Yongqing Zhu, Qingqing Liu, Junling Deng, Fengzhang Wang, Zhuoya Xiang, and Chen Xia. 2025. "A Novel Glycosylated Ferulic Acid Conjugate: Synthesis, Antioxidative Neuroprotection Activities In Vitro, and Alleviation of Cerebral Ischemia–Reperfusion Injury (CIRI) In Vivo" Antioxidants 14, no. 8: 953. https://doi.org/10.3390/antiox14080953
APA StyleChen, J., Yuan, Y., Tong, L., Yu, M., Zhu, Y., Liu, Q., Deng, J., Wang, F., Xiang, Z., & Xia, C. (2025). A Novel Glycosylated Ferulic Acid Conjugate: Synthesis, Antioxidative Neuroprotection Activities In Vitro, and Alleviation of Cerebral Ischemia–Reperfusion Injury (CIRI) In Vivo. Antioxidants, 14(8), 953. https://doi.org/10.3390/antiox14080953







