1. Introduction
There is a growing demand for bioactive compounds from consumers interested in healthy foods of natural origin, and many fruits are important sources of such compounds with antioxidant ability. Moreover, the increasing focus on environmental sustainability and food waste reduction has encouraged research into innovative strategies for valorizing by-products from the fruit and vegetable industry [
1].
In this context, the Annurca apple from Campania (Malus pumila Mill.), a native cultivar recognized for its excellent nutritional and sensory properties, stands for a resource of particular interest. Specifically, peels and cores, often considered waste, are in fact rich in bioactive compounds such as polyphenols, flavonoids, and pectins, known for their antioxidant and health-promoting properties. The Annurca apple is called the “queen of apples” due to its remarkable organoleptic qualities, taste, flavor, and aroma, and it is a key part of the Mediterranean diet.
The
Annurca apple is a unique
cultivar in the world due to its traditional post-harvest reddening process. This distinctive trait significantly influences its biochemical composition and sets it apart from all other apple varieties studied to date. In fact, one of the most distinctive features of the
Annurca apple is its unique ripening process, known as “redding on the ground”. Unlike all apple varieties,
Annurca does not ripen on the tree: the fruits are harvested while still unripe. This is not simply due to the growers’ caution but rather to a specific botanical trait, the particularly short stem (typically 7 to 14 mm), which is not strong enough to support full ripening on the tree. Harvesting takes place in the last days of September (variable period because it depends on the climate), when the apples are still green with red streaks. After picking, the apples are laid out in the sun to ripen on beds made of wood shavings or pine needles, known as “melai”. To protect them from excessive solar radiation or adverse weather conditions, the “melai” are covered with special sheets or breathable cloths that allow light to pass through. The apples are regularly turned by hand and left to ripen for 20 to 40 days, until the skin turns 90–100% red. It is precisely this “melai” reddening process that elevates the quality of the
Annurca, endowing it with a unique identity that no other apple variety can match. Its potential health benefits are largely attributed to its high content of bioactive compounds, particularly polyphenols [
2].
Polyphenols are a diverse class of plant secondary metabolites renowned for their potent antioxidant activity, which enables them to effectively scavenge free radicals and mitigate oxidative stress in biological systems. Their health-promoting effects are largely attributed to their ability to modulate key cellular signaling pathways, exert anti-inflammatory actions, and confer protection against a range of chronic diseases, including cardiovascular disorders, diabetes, and cancer [
3,
4,
5,
6]. This broad group of compounds, including flavonoids, phenolic acids, and tannins, not only contributes to the organoleptic properties of fruits, such as color and flavor but also plays a crucial role in cellular defense mechanisms. The ability of polyphenols to prevent lipid peroxidation and DNA damage underpins their potential application in the development of functional foods and nutraceuticals. Importantly, the bioavailability and biological efficacy of polyphenols are influenced not only by their total concentration but also by their chemical structure, stability, and interactions within the food matrix, which can modulate their absorption and metabolic fate.
Among the main bioactive compounds in Annurca apples, polyphenols, especially quercetin, chlorogenic acid, and catechins, stand out for their high ability to neutralize free radicals, reduce oxidative stress, and modulate inflammatory processes at the cellular level. These properties make them highly interesting candidates for the development of functional ingredients and protective health products.
Various studies have proved the antioxidant and pro-oxidant effects of polyphenol extracts from
Annurca apple flesh on different cell models. The health benefits of
Annurca apple polyphenols include potential protective effects against obesity, liver disease, alopecia, diabetes, cancer, and cardiovascular diseases. Compared to other fruits,
Annurca apples generally show a higher content of bioactive compounds, making them ideal for nutraceutical applications. Extracts from
Annurca apple flesh show strong antioxidant activity in vitro, often surpassing that of other common apple cultivars. This activity is attributed to the synergistic effects of multiple polyphenols [
7,
8,
9]. Additionally,
Annurca biophenols reduce free radical formation in human erythrocytes [
10] by inhibiting the oxidation of low-density lipoproteins, a factor contributing to atherosclerosis development [
11].
Beyond antioxidant activity, polyphenolic molecules demonstrate antiproliferative effects, which may inhibit tumor formation and progression. For example,
Annurca apple flesh polyphenols can slow cell proliferation in human keratinocytes, a predictive model for dermo toxicity screening [
12]. This supports the hypothesis of
Annurca apple’s potential in phototherapy. These polyphenols significantly reduce cell viability in a dose-dependent manner, cause morphological changes, and induce apoptosis via an extrinsic, p53-independent pathway [
13]. One study highlighted the antitumor effects of
Annurca apple polyphenol flesh extract (AAPPE) on triple-negative breast cancer (TNBC) cell lines (MDA-MB-231 and MDA-MB-468). AAPPE selectively reduced TNBC cell viability by inducing G2/M cell cycle arrest and triggering reactive oxygen species (ROS) production in cancer cells while acting as an antioxidant in non-tumorigenic MCF10A cells. Moreover, AAPPE inhibited cell migration and metastasis, positioning it as a promising natural compound for TNBC prevention and treatment via ROS-mediated JNK activation and epithelial-to-mesenchymal transition inhibition [
14,
15].
Scientific interest in the
Annurca apple has also increased due to its excellent agronomic traits, long shelf life, and the potential for quality improvement through bio-stimulants and post-harvest innovations. A distinctive feature of the
Annurca apple is its post-harvest reddening process, which leads to significant changes in phytochemical composition between unripe (green) and ripe (red) fruits. Recent studies suggest that the content and bioavailability of phenolic compounds vary according to the maturation stage, thereby affecting the antioxidant potential of the extracts obtained [
16,
17]. These characteristics make the
Annurca apple an ideal model for multidisciplinary studies ranging from agronomy to nutraceuticals, with significant implications for public health and the valorization of local productions.
The phenolic composition of plants is influenced by multiple factors, including genotype, phenological stage, plant tissue, extraction method, environmental conditions, and geographic origin [
18,
19,
20]. These variables need site-specific and methodologically tailored analyses to assess phytochemical potential. However, during industrial processing and post-harvest handling, a significant portion of the fruit, particularly peel and core, is discarded. If properly valorized, these by-products could become valuable resources for the food, cosmetic, and nutraceutical industries. Recently, interest in agro-industrial by-products as alternative sources of bioactive compounds has increased.
This study aims to investigate and valorize by-products derived from the processing of Annurca apples, specifically peel and core, obtained from fruits at two ripening stages: unripe and fully ripe. The aims are to assess their polyphenol content, chemically characterize the extracts, and evaluate their antioxidant activity through various in vitro assays. The valorization of these by-products not only helps reduce environmental impacts related to organic waste disposal but also offers a concrete opportunity to recover high-value-added compounds usable in sectors such as functional foods, natural cosmetics, and pharmaceuticals. This approach aligns fully with the principles of the circular economy, promoting a more efficient and sustainable production model.
3. Results
3.1. Concentration of Phenolics, Flavonoids, and Ortho-Diphenols in Extracts of Annurca Apple
The concentration of the individual phenolic compounds in the apple is not constant. It depends on the cultivar, the maturity of the fruit, the growing conditions, the growth, the harvest, the storage, and the infections suffered. It can be changed by post-harvest factors, including conservation and processing [
28]. In general, the chemical profile and its variations are caused by the growing season, geographical location, and, above all, genetic variation [
29].
In our study, all apples analyzed belonged to the Annurca cultivar and were harvested in the same season from orchards under comparable agronomic and environmental conditions. This allowed us to minimize variability due to external factors and focus on changes related specifically to the ripening stage.
The concentrations of total polyphenols detected in the various extracts of the
Annurca apple are reported in
Table 1. In the unripe apple samples, an uneven distribution of polyphenols among the different fruit tissues was seen. Specifically, the peel showed a significantly higher concentration compared to both the flesh and the core (
p < 0.001). The flesh, in turn, showed approximately twice the number of polyphenols as the core (
p < 0.001).
In the ripe apple samples, a markedly different pattern in the distribution of polyphenols was seen. The peel had a polyphenol content approximately five times higher than that of the flesh and about seven times higher than that of the core (p < 0.001). Moreover, when comparing the peel of ripe apples to that of unripe apples, an approximately 4-fold increase in total polyphenol content was detected (p < 0.001), showing significant accumulation during the reddening and ripening process.
From a practical perspective, such a 4-fold increase may have relevant implications in terms of biological activity, considering that polyphenols are known for their antioxidant, anti-inflammatory, and potential health-promoting properties. Therefore, the more mature peel could be a more valuable source of functional compounds for nutraceutical or food applications.
This increase may be attributed to the action of ethylene, a key hormone in climacteric fruit ripening, known to stimulate the activity of the enzyme phenylalanine ammonia-lyase, which plays a central role in the biosynthesis of phenolic compounds. The characteristic red color of the apple peel is due to the accumulation in the vacuoles of cyanidin 3-galactoside, an o-diphenol belonging to the anthocyanin class.
About the other tissues, the flesh also showed an increase in polyphenol content during ripening, with an approximate 1.5-fold increase (p < 0.01), while, in the core, the increase was approximately 3-fold (p < 0.001). Overall, the data show that the ripening process leads to an increase in polyphenol content in all parts of the fruit, although this increase is more pronounced in the peel and core than in the flesh.
Given that the flesh represents about 85% of the edible part of the fruit, even a moderate increase in polyphenol content during ripening can contribute substantially to the overall intake of these compounds through diet.
Flash represents about 85% of the weight of the fruits (edible part); it has a high polyphenolic content, already present in the flesh obtained from the unripe fruit just harvested, therefore prior to the ripening process in the melai.
Regarding the total flavonoid content, the data are reported in
Table 1. As can be evaluated, during the ripening process, there is an increase of approximately 50% in the flavonoid content of the flesh. In contrast, the peel shows a more pronounced increase, about 2.5 times higher.
This significant increase in the peel may further enhance the antioxidant capacity of the fruit and suggests its potential value for the development of functional food ingredients or supplements.
Interestingly, no significant differences are detected in the core between ripe and unripe apple samples. It is also worth noting that, as previously evaluated for the total phenolic content, the peel is again the part with the highest concentration of flavonoids. Specifically, in unripe apple samples, the flavonoid concentration in the peel is about 10 times higher than in the flesh and core, while, in ripe apples, the increase reaches approximately 25 times.
As shown in
Table 1, the
ortho-diphenol content increases during ripening in all parts of the fruit. The most pronounced change is evaluated in the peel, where the concentration rises from 11.9 ± 1.9 in unripe apples to 36.1 ± 2.5 in ripe ones, which is an approximate 3-fold increase. In the flesh, the content increases modestly from 2.0 ± 1.2 to 2.8 ± 1.3, while the core shows a similar trend, with values rising from 2.4 ± 0.7 to 3.7 ± 1.1. These results confirm that the peel is the richest source of
ortho-diphenols, both in unripe and ripe stages, and suggest that the ripening process markedly enhances the accumulation of these compounds, especially in the external tissues of the fruit.
Such increases may be particularly relevant for applications in the nutraceutical sector, as ortho-diphenols have been associated with various beneficial biological effects, including antioxidant and cardioprotective properties.
3.2. UHPLC-MS/MS Analysis
A total of fourteen phenolic compounds (catechin, epicatechin, quercetin, quercetin hexoside, 4-hydroxybenzoic acid, protocatechuic acid, OH-tyrosol, vanillic acid, caffeic acid, ferulic acid, chlorogenic acid, rutin, synaptic acid, p-o-coumaric acid) were evaluated, primarily belonging to the classes of phenolic acids and flavonoids. UHPLC-MS/MS analysis revealed differences in phenolic compounds among the various parts of the Annurca apple (peel, flesh, and core) and between unripe and ripe stages.
The binary heat map analysis (
Figure 2) revealed distinct patterns in polyphenol presence across different tissue types and ripening stages. Notably, in unripe samples, key polyphenols such as epicatechin, catechin, chlorogenic acid, and quercetin hexoside were consistently detected, particularly in peel and flesh. This suggests their involvement in early-stage defense and developmental processes. In contrast, ripe samples showed the presence of compounds not detectable in immature tissues, such as 4-hydroxy-3-benzoic acid and quercetin, showing possible induction during ripening or senescence-related metabolic shifts. Vanillic acid and
p-coumaric acid were absent across all samples, suggesting either concentrations below detection thresholds or a lack of biosynthetic activity under the studied conditions. The ripe flesh displayed the highest diversity of polyphenols, including both flavonoids and phenolic acids, while ripe peel showed a comparatively reduced profile.
These observations highlight the maturity-dependent modulation of the polyphenolic composition, which appears to be tissue-specific and likely reflects underlying physiological and biochemical transitions associated with fruit development and ripening [
30,
31,
32].
3.3. Changes in Radical Scavenging Capacity During Annurca Apple Ripening
3.3.1. DPPH Assay
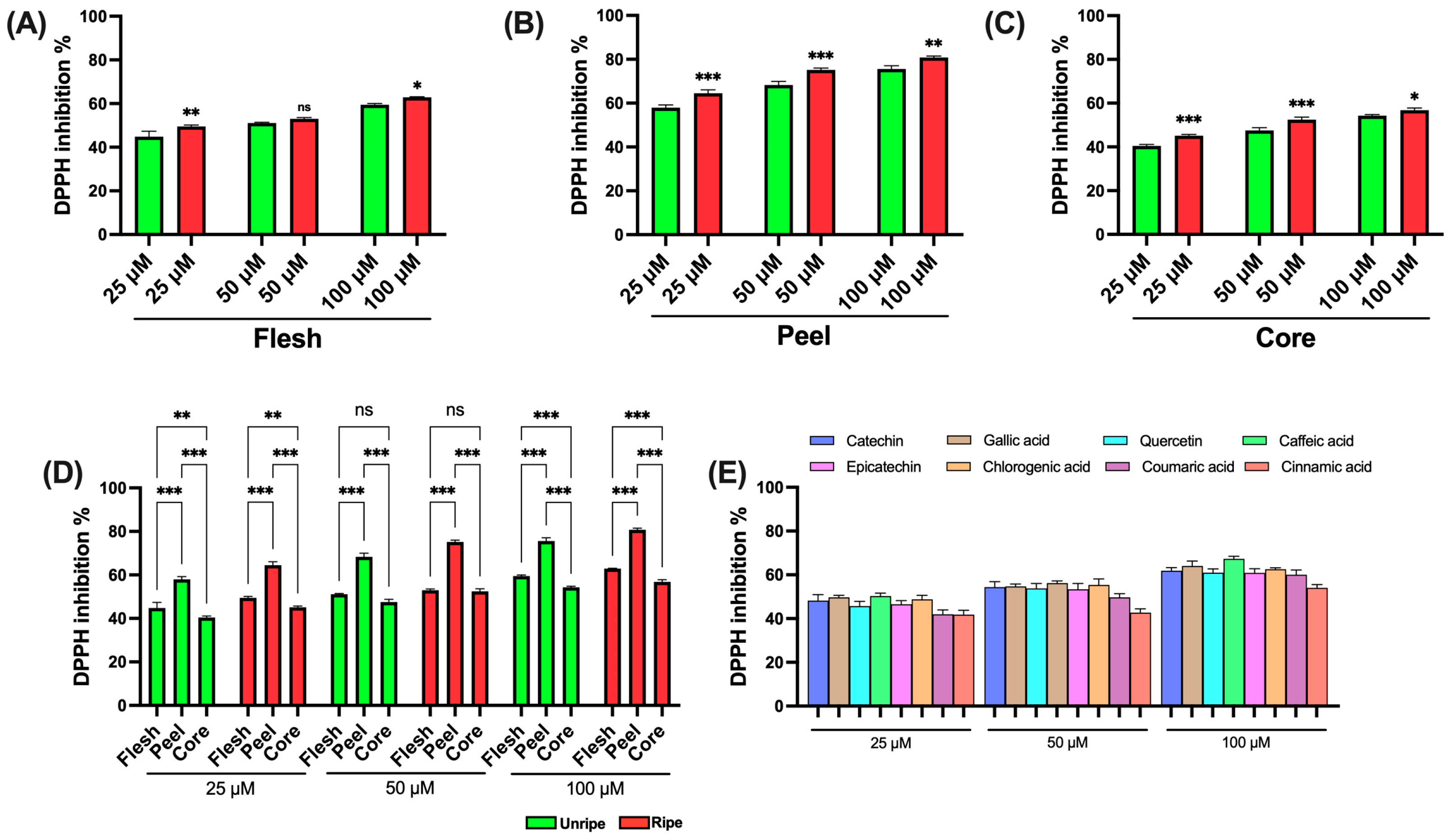
The antioxidant activity of various apple extracts was found using established spectrophotometric assays to ensure correct and comparable evaluation. The use of different methods is essential for a comprehensive characterization of the antioxidant potential of the samples analyzed. Initially, the DPPH assay was performed, based on the reduction in the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•), which results in a measurable decrease in absorbance. DPPH is commonly used to assess the antioxidant activity of natural compounds. Antioxidants interact with DPPH by donating an electron or a hydrogen atom, thus neutralizing the radical. The results shown in
Figure 2 show that all tested extracts were capable of significantly reducing the DPPH• radical. Specifically, flesh extracts displayed only minor differences between ripe and unripe apples across all tested concentrations (
Figure 3A). In contrast, peel extracts showed increased antioxidant activity in samples from ripe apples at all concentrations analyzed (
Figure 3B). A similar trend was evaluated in the core extracts, where samples from ripe apples showed higher antioxidant activity (
Figure 3C).
Figure 3D presents a comparison among the different parts of the apple. Peel extracts prove significantly higher antioxidant activity compared to both flesh and core, across all tested concentrations and for both ripeness stages. The flesh extracts showed higher activity than the core extracts only at low (25 μM) and high (100 μM) concentrations. Finally,
Figure 3E shows the data obtained from the standard compounds used as positive controls. All standards displayed a similar antioxidant profile across the tested concentrations. However, caffeic acid showed the highest antioxidant ability, while cinnamic acid showed the lowest.
Figure S1 shows the IC
50 values of the different components of the
Annurca apple. In particular, the data show that the components of the ripe apple exert a greater effect. In fact, the concentration needed to achieve 50% inhibition is lower in all three cases.
It is also interesting to note that the peel shows a significantly stronger effect compared to the other components. Specifically, the concentration needed to achieve 50% of the effect is about three times lower than that of the flesh and four times lower than that of the core.
Figure S1 also reports the IC
50 values of the standards used as positive controls. The greatest effect is seen as caffeic acid, while the weakest effect is seen as cinnamic acid.
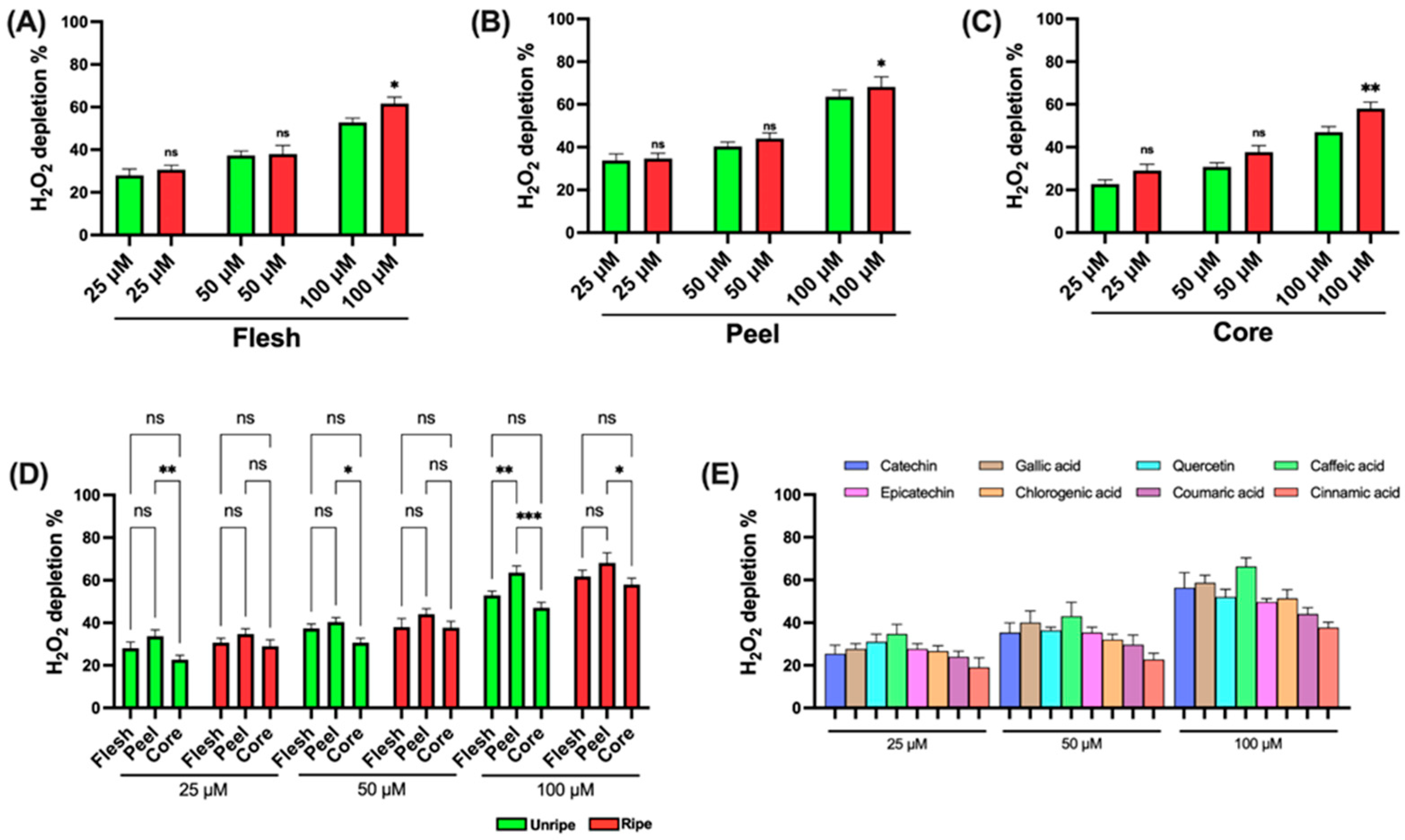
3.3.2. Hydrogen Peroxide Recovery Assay
Figure 4 shows the data on the H
2O
2 depletion capacity of the various components of the
Annurca apple. The assay used to evaluate antioxidant ability is based on the ability of the sample to neutralize H
2O
2: a lower amount of residual H
2O
2 indicates a higher antioxidant activity.
As shown in the figure, statistically significant differences between the components of ripe and unripe apples are detected only at higher concentrations (100 μM) across all three components analyzed (
Figure 4A–C). In
Figure 4D, statistically significant differences are noted between the flesh and core at 25, 50, and 100 μM in the unripe apple and at 100 μM in the ripe apple. Additionally, a difference is detected between peel and flesh in the unripe apple but only at the highest concentration (100 μM). Finally,
Figure 4E reports the data for the standard compounds used as positive controls. In this case as well, caffeic acid shows the strongest effect, while cinnamic acid shows the weakest.
Figure S2 presents the IC
50 values from the H
2O
2 depletion assay. The results show that the peel samples of unripe apples require a concentration approximately 1.5 times lower than the flesh samples and about 2 times lower than the core samples to achieve 50% inhibition.
Similarly, in the ripe apple samples, the peel also shows a higher inhibitory effect, although it is less pronounced than that detected in the unripe apple samples. Nevertheless, across all three components, the ripe apple samples display a more pronounced inhibitory activity compared to those derived from unripe apples.
Figure S2 reports the data obtained for the standard compounds employed as positive controls. The results are consistent with earlier observations: caffeic acid shows the most potent inhibitory effect among all standards tested, while cinnamic acid shows the least activity.
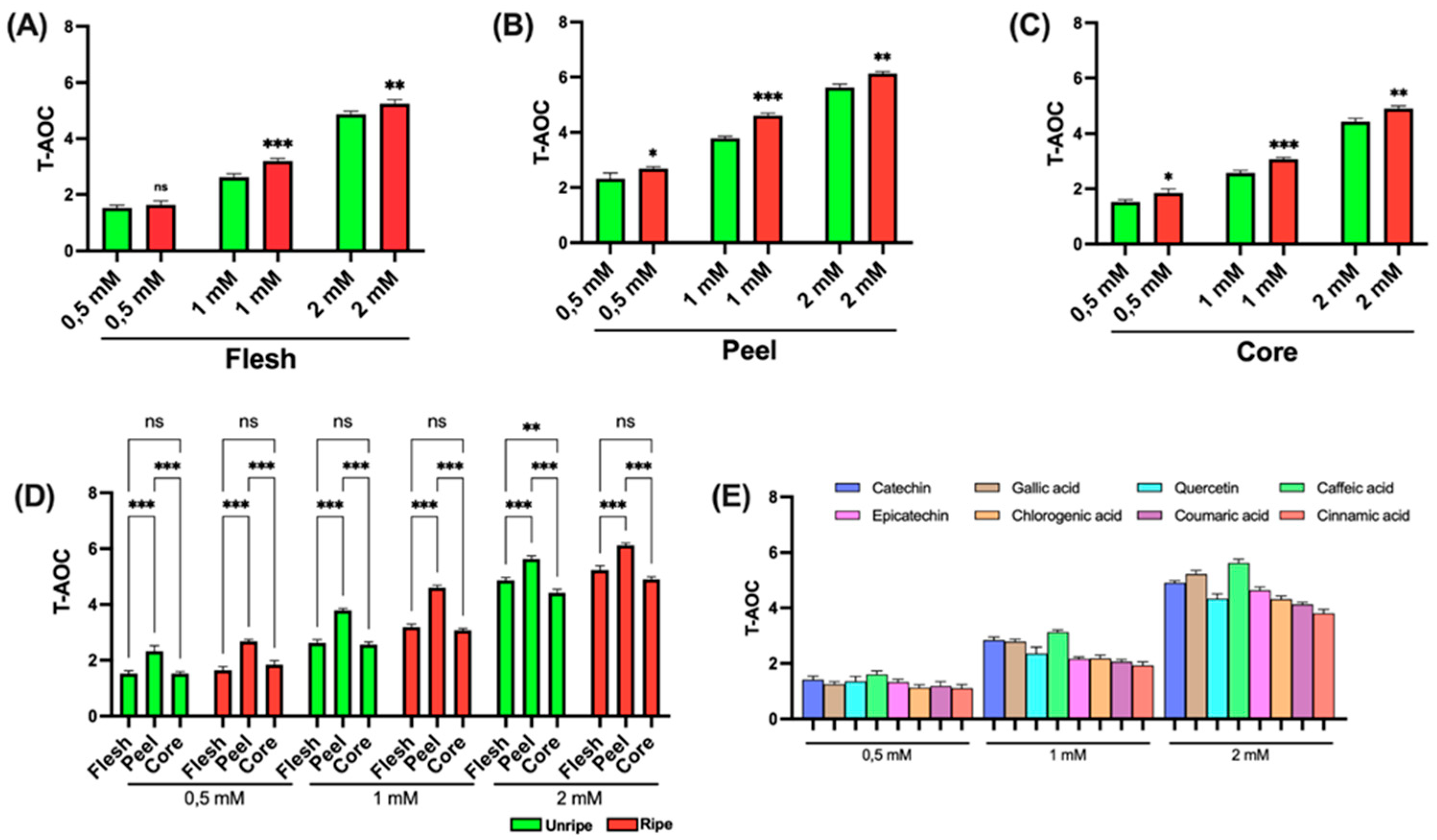
3.3.3. Total Antioxidant Capacity Assay
Figure 5 shows the data obtained from the TAC analysis of
Annurca apple components. As previously described, the assay is based on the reduction of Cu
2+ to Cu
+ by antioxidants present in the sample. The resulting Cu
+ forms a colored complex with a proprietary chromogenic reagent, allowing for a quantitative colorimetric determination of TAC.
As shown in
Figure 5A–C, a statistically significant difference between ripe and unripe apple samples is detected only in the peel at high concentrations (2 mM); no relevant differences are detected in the other components. In
Figure 5D, a statistically significant difference among peel, flesh, and core is noted across all tested concentrations. Furthermore, at the highest concentration (2 mM), a significant difference between flesh and core becomes clear in both ripe and unripe apple samples.
Figure 5E presents the results for the standard compounds used as positive controls. Notably, at the lowest concentration tested (0.5 mM), the various standards show relatively comparable antioxidant activities. In contrast to earlier data, at the intermediate concentration (1 mM), gallic acid proves to have the highest antioxidant effect. At the highest concentration (2 mM) and consistent with earlier findings, caffeic acid shows the most pronounced activity.
Across all concentrations, cinnamic acid consistently shows the lowest antioxidant potential.
Figure S3 shows the EC
50 values associated with the different components of the
Annurca apple. As illustrated in panels A, B, and C, no significant differences are evaluated between the mature and immature apple samples. Once again, the lowest concentrations needed to achieve 50% of the effect are associated with the peel samples.
Figure S3 also presents the data for the reference standards. Caffeic acid and gallic acid display lower and relatively similar EC
50 values, while the other standards show comparable but slightly higher values. Notably,
p-coumaric acid and cinnamic acid require substantially higher concentrations, approximately 2.5 and 3.5 times greater, respectively, to reach 50% of the effect.
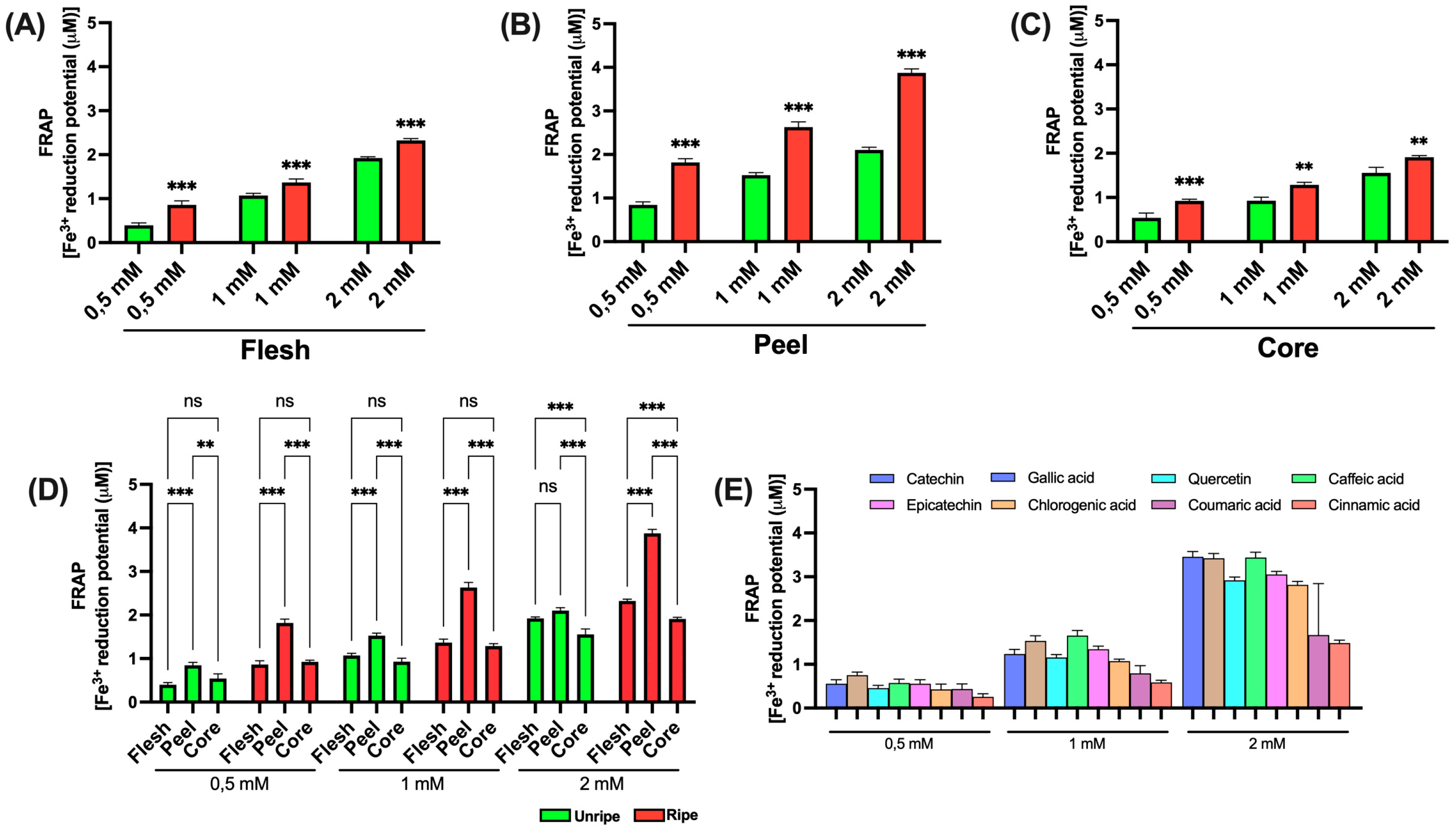
3.3.4. FRAP
The FRAP assay is a spectrophotometric technique commonly used to assess the total antioxidant ability of a sample. The method is based on the reduction of the ferric (Fe
3+)–TPTZ (2,4,6-tripyridyl-s-triazine) complex to its ferrous (Fe
2+)–TPTZ form by antioxidants present in the sample, under acidic conditions. This reaction results in the formation of a blue-colored complex, the intensity of which, measured at 593 nm, is directly proportional to the reducing power of the sample. As shown in
Figure 6 (panels A, B, and C), statistically significant differences were seen between mature and immature apple samples for all three components analyzed, at all tested concentrations. Data presented in panel D further reveal a significant difference between peel samples and those of flesh and core, both in mature and immature apples. An exception was noted in immature apples at a concentration of 2 mM, where no significant difference was seen between peel and flesh. Consistent with earlier results, a significant difference between flesh and core was also found in both mature and immature apples but only at the highest concentration tested (2 mM). Finally, the data related to reference standards, shown in
Figure 6E, show that caffeic acid shows the highest antioxidant activity, except at 2 mM, where its effect is comparable to that of catechin and gallic acid. In line with earlier findings, cinnamic acid showed the lowest antioxidant activity.
The EC
50 values obtained from the FRAP assay, presented in
Figure S4, clearly support the previously described findings. A marked difference is detected between the mature and immature apple samples. Peel samples show significantly lower EC
50 values compared to flesh and core, which are approximately 0.5-fold and 1-fold lower, respectively. The same figure shows the data for the reference standards. Once again, cinnamic acid displays the lowest antioxidant activity, while the remaining standards show relatively similar effects, with only minor variations among them.
3.3.5. ABTS Assay
The ABTS assay is a spectrophotometric method commonly used to evaluate antioxidant ability. The principle of the assay involves the generation of the ABTS
+ radical cation through the oxidation of ABTS by an oxidizing agent. The resulting green-blue chromophore is after reduced by antioxidants present in the sample, leading to a decrease in absorbance measured at 734 nm. The extent of absorbance reduction is directly proportional to the antioxidant ability of the sample.
Figure 7 presents the data obtained from
Annurca apple samples. As shown in panels A, B, and C, statistically significant differences are not between mature and immature apple samples for all three components analyzed. While significant differences are clear at all tested concentrations for peel and core, in the case of flesh, significance is only found at intermediate and high concentrations (1 mM and 2 mM). Panel D displays data comparing the different apple components. Peel consistently shows greater antioxidant ability compared to both flesh and core, across all tested concentrations and in both pre- and post-ripening samples. Notably, a statistically significant difference between flesh and core is seen at the highest concentration (2 mM), but only in immature apple samples. Finally, the results related to the reference standards, shown in Panel E, show that, consistent with earlier findings, caffeic acid shows the highest antioxidant activity, while cinnamic acid shows the lowest. However, in this assay, the difference between compounds appears less pronounced than in the other tests.
The EC
50 values obtained from the ABTS assay confirm marked differences between mature and immature apple samples across all three components analyzed. Peel samples show higher antioxidant ability compared to flesh and core, which, in contrast, show relatively similar values. Finally, the standard data, presented in
Figure S5, further support earlier findings, showing that caffeic acid shows the highest antioxidant activity, while the lowest activity is associated with cinnamic acid.
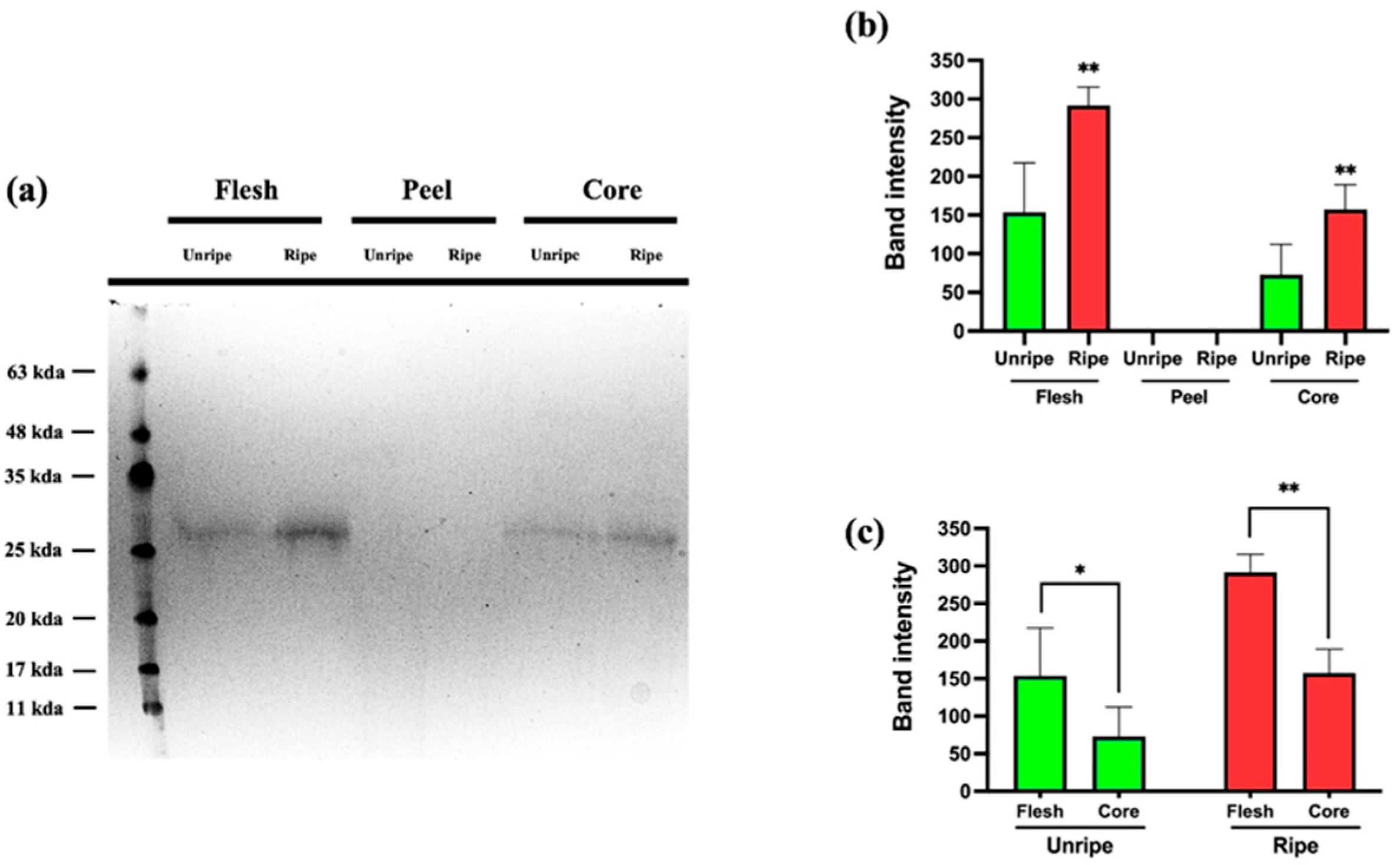
3.4. SDS-PAGE
The SDS-PAGE analysis shown in
Figure 8 reveals a band corresponding to a molecular weight of approximately 27–28 kDa. In an earlier study [
33], this band was identified as Thaumatin-like Protein 1a (TLP1a). We investigated whether the concentration of this protein varied among different components of the
Annurca apple at both mature and immature stages. As shown in
Figure 8A, the band was detected exclusively in the flesh and core samples, while it was completely absent in the peel, regardless of the ripening stage. Panel B presents the quantification of band intensity. The graph clearly proves a marked increase in Thaumatin-like Protein 1a levels during ripening in both the flesh and core. Finally, the data shown in Panel C indicate that the highest concentration of the protein is found in the flesh, in both mature and immature apple samples.
4. Discussion
4.1. Polyphenolic Changes During Ripening
In this study, samples were analyzed in their fresh form without prior drying, and the results are expressed as mg GAE per gram of Fresh Weight (FW). This approach reflects a realistic industrial scenario, where polyphenol extraction may occur directly from fresh apple processing by-products. While expressing results on a dry weight basis is common in the literature, we believe that using Fresh Weight offers a more practical and application-oriented perspective.
The results obtained in this study confirm that the ripening of the Annurca apple leads to significant changes in the composition and antioxidant ability of the various fruit’s tissues. In particular, the peel proved to be the richest part in polyphenols, with content that increases approximately 4-fold from the unripe to the ripe stage. This increase is consistent with the known role of ethylene in the ripening of climacteric fruits, which stimulates the biosynthesis of phenolic compounds through the activation of the enzyme phenylalanine ammonia-lyase. Moreover, the characteristic red coloration of the ripe peel is attributable to the accumulation of anthocyanins, particularly cyanidin 3-galactoside, which also contributes to antioxidant activity.
The ripening process of the
Annurca apple is accompanied by a substantial reshaping of the phenolic profile, with increasing total polyphenols, as also reported in other traditional apple cultivars where maturation and post-harvest reddening trigger secondary metabolite biosynthesis [
34]. The shift in composition may reflect enzyme-mediated transformations or redistribution of phenolics aligned with physiological needs for protection or storage. Interestingly, similar data were also seen for the profile of flavonoids and ortho-diphenols. These increases may be particularly relevant for nutraceutical applications, as flavonoids and ortho-diphenols have been associated with several beneficial biological effects, including antioxidant and cardioprotective properties. Overall, the findings reinforce that
Annurca apple by-products, particularly peel and core, are rich sources of antioxidant polyphenols, supporting their potential use as nutraceutical ingredients, a conclusion aligned with in vitro bioactivity assays showing significant antioxidant and enzyme-inhibitory effects [
35].
4.2. Antioxidant Capacity of Apple Tissues
Spectrophotometric analyses conducted using DPPH, H2O2 depletion, TAC, FRAP, and ABTS assays provided a comprehensive overview of the antioxidant potential of the different extracts. In all tests, the peel showed significantly higher antioxidant ability compared to the flesh and core, in both unripe and ripe samples. This result was also confirmed by the IC50 and EC50 values, which were lower for the peel, showing greater effectiveness in neutralizing free radicals and reducing oxidative compounds.
The flesh, although holding fewer phenolics than the peel, showed a significant increase during ripening (approximately 1.5 times), accompanied by an improvement in antioxidant activity. This suggests that the flesh also actively contributes to the fruit’s antioxidant defense mechanisms, albeit with lower efficacy. In the DPPH and ABTS tests, the flesh showed an intermediate response between peel and core, with significant differences especially at the lowest and highest concentrations. This behavior may reflect a more homogeneous but less concentrated distribution of phenolic compounds within the flesh matrix, which is the main edible part of the fruit and is therefore of greater nutritional interest.
The core, often considered a waste product, proved to have notable antioxidant potential, especially in ripe samples. Data showed an approximately 3-fold increase in phenolic content from unripe to ripe fruit, suggesting that this part also takes part in the biochemical processes associated with ripening. Although the total polyphenol content in the core is lower than in the peel and flesh, its antioxidant activity was still significant, as shown by the assays performed.
From the perspective of agri-food waste valorization, these findings are particularly relevant. Peel and core represent potential sources of bioactive compounds that could be exploited to produce functional ingredients, supplements, or natural extracts. These applications, however, should be further confirmed by added research, particularly concerning bioavailability, safety, and efficacy in vivo.
4.3. Expression and Potential Role of TLP1a
The SDS-PAGE analysis confirmed the presence of a protein band at ~27–28 kDa, previously identified as Thaumatin-like Protein 1a (TLP1a) in
Annurca apple tissues [
33]. Interestingly, this protein was detected exclusively in the flesh and core, with no expression seen in the peel at either ripening stage. This suggests a tissue-specific distribution of TLP1a, potentially reflecting distinct physiological roles, such as internal defense or stress response mechanisms localized to the fruit’s inner compartments. The progressive increase in band intensity during maturation shows a ripening-associated upregulation of TLP1a. This trend is consistent with earlier findings reporting enhanced expression of TLPs during fruit development and senescence, likely due to their role in pathogen resistance, osmotic regulation, and cellular stabilization. Moreover, the highest levels of TLP1a were evaluated in the flesh, both in unripe and ripe fruits, which may have implications for the nutritional or functional properties of apple-derived products. Given the known bioactivity of thaumatin-like proteins, including antifungal, sweet-tasting, and immunomodulatory effects, these findings could support future applications of
Annurca apple by-products in the development of functional foods or nutraceuticals.
Although the role of TLP1a in antioxidant defense is not yet fully elucidated, earlier studies have reported its upregulation under abiotic stress conditions, such as drought or salinity, where ROS accumulate [
36]. This could suggest a potential involvement in oxidative stress responses, either through direct ROS interaction or through indirect modulation of antioxidant systems.
The presence of TLP1a specifically in tissues that also showed high antioxidant potential further supports the hypothesis that this protein might contribute, directly or indirectly, to the functional performance of polyphenol-rich extracts. TLP1a could potentially interact with phenolic compounds through protein–polyphenol binding mechanisms, influencing their solubility, stability, or bioavailability in formulated products. Such interactions have been documented in other food systems, where TLPs bind polyphenols forming soluble or insoluble complexes [
37]. In addition, its possible role as a carrier or structural stabilizer might enhance the shelf life or activity of antioxidant extracts under stress conditions (e.g., temperature, pH, oxidation).
From an applied perspective, understanding the behavior of TLP1a in fruit matrices is crucial for improving extraction processes, especially when targeting both antioxidant and protein fractions for synergistic effects in nutraceuticals or food formulations. Furthermore, since TLPs are also associated with allergenic responses in sensitive individuals, their quantification and tissue distribution are essential for the safe use of apple by-products in health-oriented applications.
Further studies involving proteomic and transcriptomic profiling would be beneficial to elucidate the regulatory pathways underlying TLP1a expression and its functional significance during ripening. Investigating whether TLP1a modulates antioxidant mechanisms or contributes to the stability and efficacy of polyphenol-rich extracts may offer new perspectives for the valorization of Annurca apple by-products in sustainable and functional product development.
4.4. Tissue-Specific Impact of Ripening
Overall, the data supports the idea that all components of the Annurca apple, including by-products such as peel and core, can be valorized within a circular economy framework, contributing to waste reduction and the development of new high-value products. The comparison between ripe and unripe samples revealed significant differences in both phenolic composition and antioxidant ability. In all analyzed tissues, ripening led to an increase in total polyphenol content, with more pronounced effects in the peel and core. This increase was accompanied by improved antioxidant activity, as proven by the reduction in IC50 and EC50 values across the various assays.
However, the extent of the increase varied among tissues: approximately 4-fold in the peel, 1.5-fold in the flesh, and 3-fold in the core. These differences suggest that the biochemical response to ripening is not uniform but depends on the physiological function and structure of each tissue. From a functional standpoint, ripe samples show greater antioxidant efficacy, making them particularly interesting for nutraceutical and industrial applications. Nevertheless, unripe samples, despite having lower activity, still support a relevant antioxidant profile, which could be useful in contexts where a more moderate phenolic content or a milder sensory profile is desired.
4.5. Environmental and Industrial Implications
While the results suggest promising potential for the use of apple by-products, these conclusions must be interpreted with caution. In terms of environmental sustainability, the results of this study highlight the importance of valorizing by-products such as peel and core, generally considered waste, as valuable sources of bioactive compounds. Their use for the extraction of functional ingredients allows for the optimization of natural resource use and the creation of added value from low-cost materials that would otherwise have a high environmental impact if conventionally disposed of. In this context, the Annurca apple proves to be a strategic raw material, not only for its organoleptic and nutritional qualities but also for its potential for full use. Unlike other more extensively studied apple cultivars, the Annurca is unique in the world due to its traditional post-harvest reddening process, which significantly influences its polyphenolic profile. To date, limited information is available on the valorization of its by-products, especially in relation to ripening stages. The present study contributes novel data on this underexplored cultivar, offering new insights relevant for both scientific understanding and industrial application. The use of natural extracts obtained from by-products such as peel and core may also contribute to the replacement of synthetic antioxidants in food, cosmetic, and pharmaceutical products, responding to the growing demand for natural, safe, and sustainable ingredients.
However, the use of these extracts as direct replacements for synthetic antioxidants in food, cosmetic, or pharmaceutical applications cannot be fully justified based solely on in vitro results. Further toxicological, application, and formulation studies are necessary to confirm their safety and effectiveness under real-world conditions. In addition, some limitations of the present study should be acknowledged. The analyses were conducted exclusively in vitro and therefore do not provide information on the bioavailability, metabolism, or actual physiological effects of the compounds in vivo. Moreover, the number of biological replicates was limited, and the potential influence of seasonal or inter-annual variability was not considered. Furthermore, no toxicological or sensory evaluations were performed to confirm the safety and acceptability of using apple by-products in food applications. These aspects should be addressed in future studies to improve the translational relevance and applicability of the findings.