3.1. Characterization of UAE Olive By-Products by HPLC-ESI-QTOF-MS
Representative base peak chromatograms (BPCs) of olive pomace and olive leaf extracts obtained by UAE are shown in
Figure 1. The detected compounds were characterized thanks to the chemical information provided by the HPLC-ESI-QTOF-MS instrument. In this sense, compounds were tentatively identified by interpretation of their mass spectra and the molecular formula provided by the DataAnalysis 6.0 software, together with the information previously reported in the databases and literature concerning olive pomace and leaf composition. Whenever possible, the putative identification was confirmed by a comparison of retention times and MS/MS spectra to commercial standards, while the remaining compounds remain tentatively identified, including several isomers that exhibited the same molecular formula and similar fragmentation patterns but different retention times.
A total of 85 compounds were detected in the analyzed olive pomace and olive leaf extracts, of which 63 of them could be tentatively identified, while the remaining 22 compounds were unknown, despite the efforts made for characterization. All detected compounds are shown in
Table 1, which summarizes the following information: peak number assigned, retention time (RT), experimental and theoretical
m/
z, error (ppm), molecular formula, milisigma value (mSigma), fragmentation pattern, and the proposed compound for each chromatographic peak. The mSigma value points out the statistical similarity between the theoretical and measured isotopic patterns, in such a way that a low mSigma value means that the experimental isotopic pattern of a peak is very similar to the theoretical isotopic pattern for the proposed molecular formula.
Most of the identified compounds are characteristic of olive-related products and by-products, or are derivative compounds, and as such, they have been extensively described in the vast literature concerning these matrices. As shown in
Table 1, a considerable number of compounds belong to or are derived from the secoiridoid family, with oleuropein, ligstroside, and their derivatives being the most widely represented. Relatively unaltered secoiridoids, such as oleuropein and ligstroside, along with their isomers (peaks 43, 62, 68, 74, and 45, 72, respectively), have been found in extracts from both studied matrices. In contrast, more complex derivatives, such as hydroxyoleuropein and its isomers (peaks 37, 38, 40), or dihydrooleuropein (peak 48), were identified only in olive leaves, which is consistent with the previously reported results [
25].
On the other hand, a high number of oleuropein aglycone isomers (peaks 57, 58, 60, 67, 69, 70, 73, 75, 78, 80, 83, 84) were exclusively found in olive pomace extracts, as the release of endogenous enzymes during the mechanical crushing of the fruit causes the hydrolysis of oleuropein [
26]. A similar number of isomers have been previously described in olive oil, with these species being attributed to the biotransformation of oleuropein aglycone due to the keto-enol tautomeric equilibrium on the exposed hemiacetal carbon after the removal of the glucose moiety, which involves ring opening [
27]. The extensive degradation of oleuropein by enzymatic action could explain the presence of elenolic acid and isomers (peaks 29, 34, 47, 76), and some derivatives (peaks 26, 27) exclusively in OP extracts.
Despite the absence of oleuropein aglycone in olive leaf extracts, oleacein (a decarboxymethylated derivative of oleuropein aglycone) was detected in this matrix, although its peak intensity was considerably lower compared to olive pomace extracts. Other related secoiridoids were also identified in both matrices, including oleoside isomers (peaks 20, 33) and its methyl ester derivative (peak 39), as well as glucosylated derivatives of elenolic acid (peaks 16, 22). Iridoids characteristic of olives, such as loganic acid (peak 17), 7-epiloganin (peak 28), and lamiol (peak 36), were also found in both by-products.
Belonging to the group of phenolic alcohols, hydroxytyrosol (peak 23) was detected in both matrices, as expected, along with its glucosylated derivatives (peaks 15 and 22), although only one isomer was found in OL extracts. The presence of these hydroxytyrosol glucoside isomers has been previously reported in olive pomace [
28]. In addition, an oxidized form of hydroxytyrosol was identified in OL extracts, which has been previously described in olive by-products, and ethylguaiacol (peak 25) was detected exclusively in olive pomace extracts.
On the other hand, the phenylpropanoid verbascoside (peak 41), as well as its isomer isoverbascoside (peak 53), were found in both matrices, both of which have also been previously described as olive phenolic compounds in other research studies [
29]. Several flavonoids were also present in both matrices, mainly luteolin (peak 83) and its different glycosylated derivatives (peaks 46, 50, 52, 55, 65). In contrast, apigenin (peak 85) was detected exclusively in olive pomace extracts, as described by Cecchi et al.
Apart from these phenolic families, a wide variety of organic acids have been identified, mainly in olive pomace extracts, including glucuronic acid (peak 1), citric acid (peak 9), xylonic acid (peak 2), treonic acid (peak 4), quinic acid (peak 5), and malic acid (peak 7), which is consistent with the previous scientific literature related to OP extracts [
30].
3.2. Optimization of Ultrasound-Assisted Extraction (UAE)
The UAE methodology was optimized following the procedure described in
Section 2.3, by applying a Box–Behnken design (BBD) to assess the following three independent variables: ethanol concentration in the extraction solvent, ultrasound amplitude, and energy applied to the sample by US, each at three levels, with three central points. This design resulted in the 15 randomized experiments shown in
Table 2. All experiments were conducted with a fixed solid–liquid ratio of 1:10, based on previously optimized conditions reported in other studies, both for olive pomace [
31] and for olive leaves [
32], where it was demonstrated that this solid-to-solvent ratio was appropriate and minimized both the amount of solvent used and the energy cost and duration of the subsequent solvent removal step. The type of solvent used for extraction was one of the most thoroughly investigated factors. Ethanol and water are the most suitable solvents for the food and nutraceutical industries because they are green and GRAS solvents that enhance the efficiency of phenolic compounds extraction [
33]. Furthermore, mixtures of water and ethanol prevent generation of radicals during cavitation, which may lead to the oxidation of bioactive compounds [
18]. Therefore, the extraction solvent composition was assessed across a range of ethanol concentrations, from 0 to 100%.
Regarding specific UAE parameters, there is no consensus on which variables are the most influential in the extraction process, and different studies describe the extraction method using different parameters such as power percentage or amplitude percentage, which are not reproducible across different ultrasonic probes. To overcome this limitation, in this study the US power was assessed as an ultrasound amplitude expressed in µm instead of a percentage to facilitate the reproducibility of the optimized conditions across different sonotrodes, making this parameter independent of the ultrasound device used [
34]. The range tested was from 30 to 60 μm to ensure the industrial scalability of the optimal conditions since industrial probes can operate at up to 63 μm.
Generally, the scientific literature agrees on the paramount importance of ultrasound intensity in UAE, since extraction yield usually increases with this parameter up to a certain threshold. Most reported studies evaluate US intensity as power density (W∙mL
−1) due to its independence from vessel geometry and extraction solvent volume, and extraction time as independent factors; however, these parameters are inherently interrelated. An increase in either variable translates into a greater energy delivery to the sample, enhancing cell disruption and promoting compound transfer from the matrix to the solvent. Nevertheless, excessive power density or prolonged extraction time intensifies cavitation, leading to elevated temperatures and potential compound degradation. The relationship between these two variables can be expressed as follows:
Therefore, in the present study, the third variable chosen for optimization was the specific energy input to the sample by US, measured in J∙mL
−1, as a single integrated parameter allowing simultaneous optimization of both power density and extraction time. This approach reduces experimental complexity and mitigates the risk of overfitting that may arise from attempting to independently optimize variables exerting overlapping effects on extraction efficiency. The studied range for this variable was 25 to 100 J∙mL
−1. In this regard, values exceeding 100 J∙mL
−1 could affect the phenolic composition, leading to some degradation of the bioactive compounds, whereas energies below 25 J∙mL
−1 are insufficient to rupture the vegetable cell wall, making the extraction incomplete [
35].
Table 2 summarizes the values of the independent variables, as well as the resulting total run time and temperature increase for the 15 experiments conducted according to the BBD. The temperature was monitored throughout the extraction procedure. The starting temperature was set at 15 °C. The different combinations of the extraction conditions showed the same variations in the temperature increase for both matrices, with a final value below 40 °C, ensuring that phenolic compounds were not exposed to high temperatures that could cause thermal degradation. Regarding extraction time, all experiments lasted between 1 and 4 min. In this regard, as many authors have noted, this technique does not require long extraction times to obtain elevated contents of phenolic compounds, highlighting the remarkable efficiency of this extraction technique [
36,
37]. Compared to using an ultrasonic bath for the extraction of phenolic compounds from olive by-products [
31], this method requires shorter extraction times and results in lower temperature increases, reducing the risk of degrading certain phenolic compounds and potentially offering additional advantages.
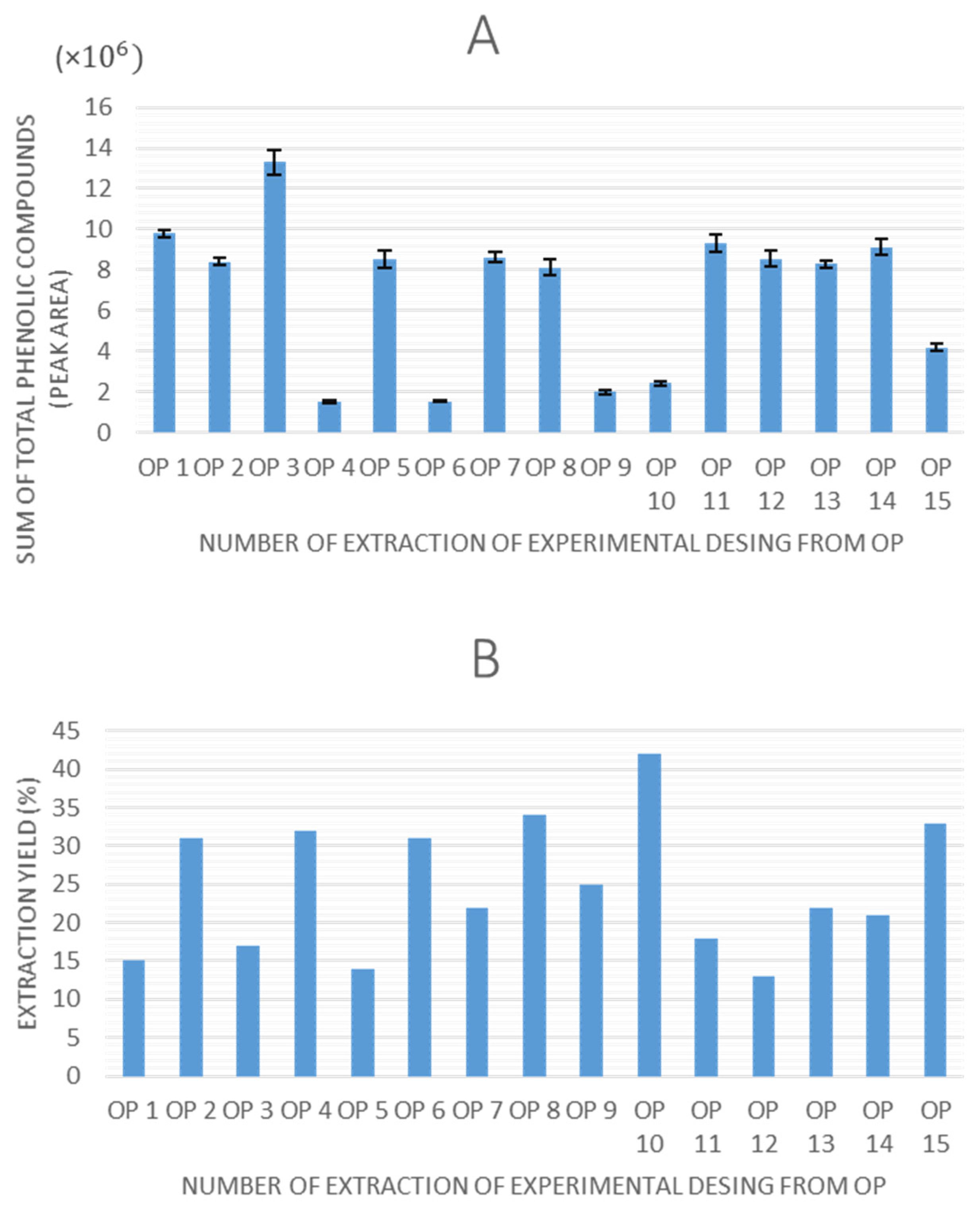
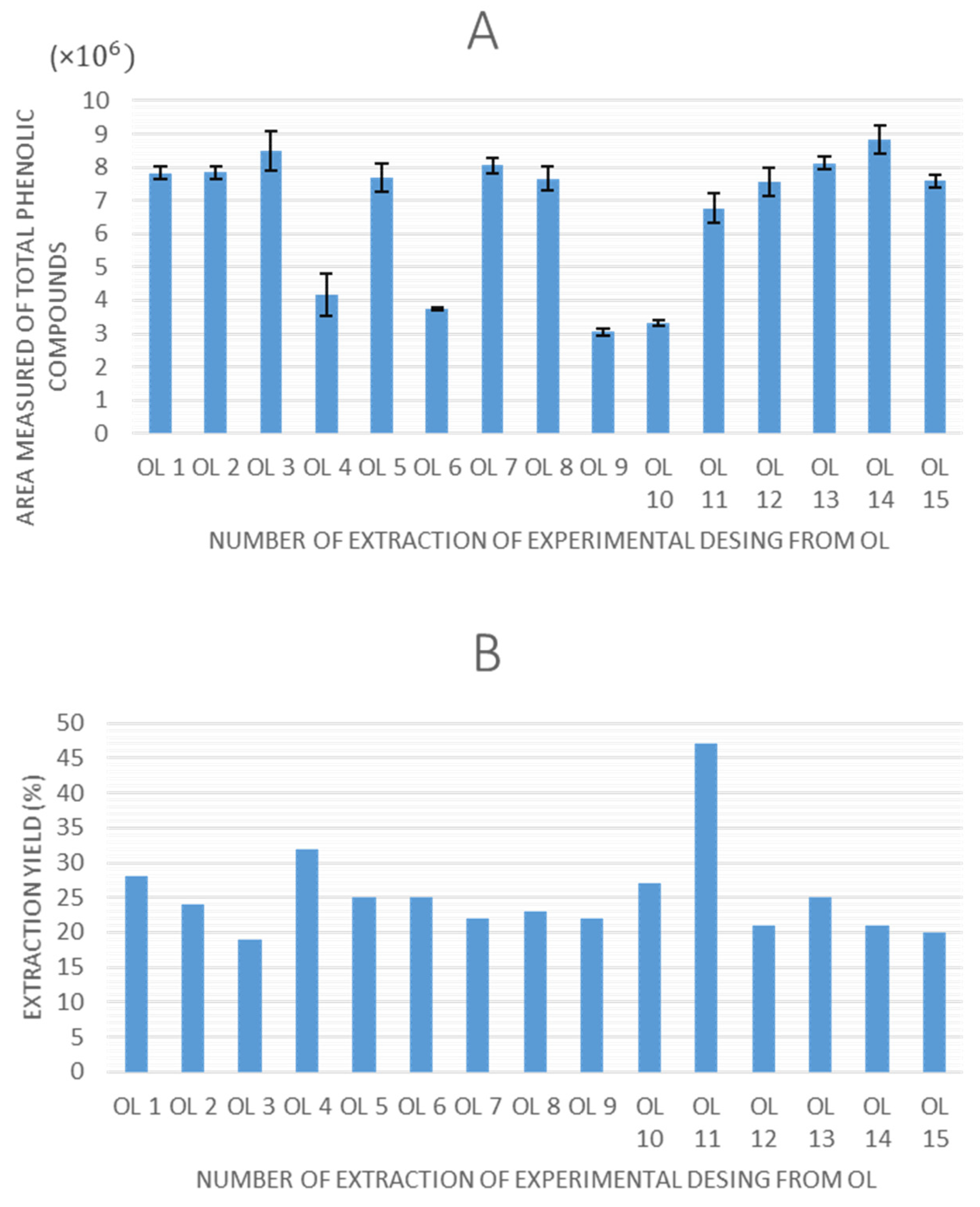
Once all of the experiments were completed, the extraction yield and total peak area of phenolic compounds obtained by HPLC-ESI-QTOF-MS were selected as response variables to evaluate the experimental designs. The values obtained for these response variables are shown in
Figure 2 and
Figure 3 for olive pomace and olive leaves, respectively.
Regarding the graphs illustrating the TPC for each experiment using olive pomace and olive leaves, a consistent trend is observed in both matrices, as follows: extractions performed without ethanol in the solvent system (0% ethanol) resulted in lower quantified areas of total phenolic compounds. This indicates that ethanol is a critical factor influencing extraction efficiency in UAE. Moreover, experimental points 7 (8.6 × 106 in OP, 8.2 × 106 in OL), 14 (9.1 × 106 in OP, 8.6 × 106 in OL), and 15 (8.8 × 106 in OP, 7.8 × 106 in OL), which correspond to the central points of the design, demonstrated high repeatability, confirming the precision and stability of the experimental setup.
In terms of extraction yield, in all experiments it ranges between 15–50%, which could be considered a relatively high value, and it is in agreement with the previously reported data for UAE in olive pomace [
31,
38]. However, no discernible trend is observed that accounts for the variation in yield values among the different experiments across any of the matrices.
The effect of the independent variables (US amplitude, ethanol content of extraction solvent, and specific energy input to the sample by US) on the different response variables (extraction yield and TPC) was evaluated. The experimental results were fitted to second-order polynomial models. To assess the adequacy of the proposed models and the fit of the obtained data, an analysis of variance (ANOVA) was performed for each experimental model corresponding to the different response variables. The analysis included an evaluation of model adequacy, the coefficient of determination (R
2), and the lack-of-fit test.
Table 3 presents the ANOVA results for each model for olive pomace and olive leaves.
Regarding olive pomace, the model built for extraction yield did not present any significant variable, showing that the independent variables within the studied ranges did not affect the extraction yield. Therefore, this model was discarded. On the other hand, ethanol content and its quadratic effect, as well as the quadratic effect of amplitude and the interaction between ethanol content and applied energy, had a significant effect on the total phenolic content. Additionally, the lack-of-fit test and R2 values confirmed the suitability of the model.
With respect to olive leaves, as with the case of olive pomace, only the model built for total phenolic content showed a significant influence of the studied independent variables, along with suitable fit and adequacy. In this case, the only variable that demonstrated a significant effect on TPC was the extraction solvent composition, as well as its quadratic effect.
As can be observed, among the assessed variables, the concentration of ethanol in extraction solvent was the most significant factor with the highest impact in both models. The concentration of ethanol is a key factor in ultrasound-assisted extraction (UAE) of phenolic compounds, as it significantly affects solvent polarity, solute–solvent interactions, and the permeability of plant cell membranes. The effectiveness of the extraction process relies heavily on aligning the solvent’s polarity with the wide polarity spectrum of phenolic compounds naturally found in plant materials. Using ethanol–water mixtures enables precise adjustment of polarity, improving the extraction of both water-soluble and moderately non-polar phenolics [
39]. In addition, ethanol can disrupt plant tissue structure by increasing membrane permeability and causing protein denaturation, which in turn promotes mass transfer and the release of intracellular compounds. These effects are enhanced by acoustic cavitation generated during UAE, which contributes to physical cell disruption and deeper solvent diffusion [
40]. The ethanol-to-water ratio also directly impacts cavitation by altering the solvent’s physical properties, such as vapor pressure, viscosity, and surface tension. Although the ideal ethanol concentration depends on the specific matrix and target compounds, it consistently proves to be the most critical parameter for improving selectivity and maximizing extraction efficiency in UAE of phenolic-rich plant materials [
41]. This effect has been previously described in the recovery of phenolic compounds from olive wastes by UAE [
42,
43], as well as by other extraction techniques [
44].
The interaction between ethanol concentration and the energy applied has been shown to have a negative effect on the extraction of total polyphenols in the case of olive pomace. This effect is linked to the enhancement of the cavitation phenomenon produced by a higher amount of applied energy. In this context, this phenomenon causes an increase in free radicals, which accelerates the degradation of the phenolic compounds extracted. However, this effect is partially counteracted by the ability of ethanol to scavenge the generated radicals; hence, their interaction significantly influences TPC levels [
45].
Regarding US amplitude, its quadratic effect has a lesser influence on the extraction of total phenolics from olive pomace than the other variables. In this sense, this slight influence on the extraction could be attributed to the previous breakup of the cellular matrix in this sample, as it had already been subjected to an extraction process during beating and crushing to obtain the olive oil [
46].
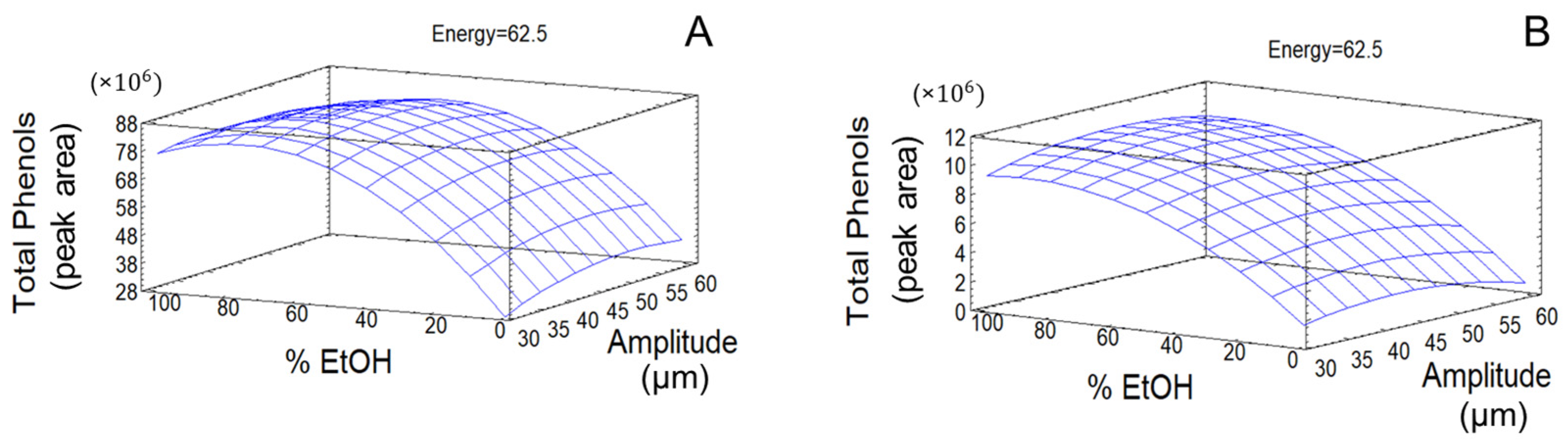
The response surface plots corresponding to the models built for TPC showed significant effects of studied variables, as well as a suitable fit, and the predictive capacities are shown in
Figure 4 for olive pomace and olive leaves.
The fitted simplified equations that explain the behavior of these response variables for olive pomace are displayed below, where A represents US amplitude, B corresponds to ethanol content in extraction solvent, and C denotes applied energy, as follows:
In the case of olive leaves, the simplified equations were as follows:
In this regard,
Table 4 presents the optimal experimental conditions for each model of both by-products, including the predicted values for the response variables.
As can be seen, the optimal parameters to maximize TPC in olive by-products were quite similar, unless EtOH content was present.
Once the optimal conditions for UAE were defined as described above, they were used to obtain both optimal extracts in triplicate, which were then analyzed by HPLC-ESI-QTOF-MS. To validate the designs, the experimentally obtained sum of the total phenolic peak areas were compared to the predicted values, and the results of this validation are presented in
Table 5, including the relative error between both values. The results show that the experimental peak areas were very close to the predicted ones, with relative errors below 25%, which may be considered a validation of these models.
It is worth noting that the extraction yields obtained from both by-products are comparable to those previously reported in the literature, despite the short extraction time and mild conditions applied [
37,
47,
48,
49]. This is of particular interest, since optimizing TPC in both by-products enables acceptable yields in the development of phenolic-enriched extracts.
The optimized conditions by the experimental design ensure that the resulting extracts are not only produced efficiently, but also genuinely enriched with target compounds. Quantitation of phenolic compounds by HPLC-QTOF-MS determines the meaningful relationships between the total extract yield (which includes the target compounds alongside other components present in the extract) and the relative concentrations of phenolic compounds.
These compounds were quantified using the standard calibration curves described in
Section 2.
Table S1 (Table attached in Supplementary Materials) presents the calibration equations used to quantify phenolic compounds from olive pomace and olive leaf extracts and the LOD and LOQ determined for each compound.
The chromatographic area of the detected peak for each compound was interpolated into the corresponding calibration curve of the selected standard based on structural similarity, obtaining the calculated concentration. In this way, hydroxytyrosol derivatives were quantified using the hydroxytyrosol standard curve; the luteolin-7-
O-glucoside curve was used to quantify glycosylated flavonoids; and secoiridoids and its derivatives were quantified using the oleuropein standard curve, while loganin was used as a surrogate standard for iridoids. In these cases, results are expressed as hydroxytyrosol equivalents (HyE), luteolin-7-
O-glucoside equivalents (LuGE), oleuropein equivalents (OE), or loganin equivalents (LgE), respectively. It should be noted that this is a commonly used approximation, and that the concentration results obtained using calibration curves of structurally similar compounds should only be used for comparative purposes, as the actual concentrations of these compounds may differ. The compound content, expressed in mg of compound/g of dry extract, was calculated for each extract in triplicate, and the resulting data, expressed as the mean value ± standard deviation, are shown in
Table 6 and
Table 7.
The TPC in the olive pomace extract, calculated as the sum of all of the individually quantified phenolic compounds, was approximately 43 mg TPC/g of dried extract, while the olive leaf extract presented a TPC of around 30 mg/g. These values are consistent with previous studies; for instance, Cioffi [
47] reported a similar TPC of approximately 30 mg/g from olive pomace extracts obtained by UAE. Other authors have documented lower concentrations, around 20 mg TPC/g dried extract, in both olive pomace and olive leaf UAE extracts [
48,
49,
50]. In the case of Chanioti and Tzia [
50] and Ghomari [
48], extractions were carried out over extended periods (4 h) using ultrasonic baths. Conversely, Mojerlou and Elhamirad [
49] employed an ultrasonic probe with an extraction time of 3 min, similarly to our optimized conditions, but at a higher temperature (60 °C), which would imply an increase in the energetic cost of the process.
Evaluation of the individual compound concentrations revealed that oleuropein and its isomers were present at approximately 10 mg/g in both samples, a level higher than previously reported in other studies. Those studies used solid–liquid extraction and PLE methods, which involve more severe conditions that may lead to the degradation of phenolic compounds [
23,
26,
28,
29].
Degradation products derived from oleuropein were also evaluated, including oleuropein aglycone. In the case of olive pomace, this compound is formed as a result of the mechanical processes involved in olive oil production. During this process, glycosylated forms are hydrolyzed by endogenous enzymes, leading to the formation of aglycone derivatives. A total of 12 isomers of oleuropein aglycone were identified in the optimal olive pomace extract, reaching concentrations exceeding 15 mg/g of dried extract, higher than those previously reported [
47,
51]. In contrast, oleuropein aglycone was not detected in olive leaf extract, likely due to the absence of mechanical processing, which limits the enzymatic hydrolysis of glycosidic bonds [
29]. Since this derivative could also be formed during the extraction process, when aggressive conditions such as high-temperature or long extraction time are applied, this absence suggests a lower level of degradation in the olive leaves matrix compared to what has been described in previous studies [
51,
52,
53].
Oleacein, another compound formed from oleuropein degradation, was also one of the main compounds quantified in olive pomace extract. Rosa reported an oleacein concentration of 7.85 mg/g in an extract obtained by UAE [
53], while in the present study, the olive pomace extract showed a concentration of 3.1 ± 0.1 mg/g. This occurred despite the higher oleuropein content in this matrix.
Hydroxytyrosol was also quantified in both extracts. In olive pomace, its concentration was 0.50 ± 0.05 mg/g of dried extract, whereas in olive leaves, it was 0.148 ± 0.004 mg/g. These values differ from those reported in other studies. For instance, conventional extraction and PLE of olive pomace have yielded extracts with hydroxytyrosol contents approximately 3 and nearly 20 times higher, respectively [
29]. Similarly, the UAE of olive leaves has been reported to produce hydroxytyrosol levels of around 15 mg/g, with oleuropein not being detected [
48]. The relatively low concentration observed in this study suggests a limited hydrolysis of oleuropein.
Oxidized hydroxytyrosol was detected exclusively in the olive leaf extract, only at trace levels, below the LOQ. This oxidized derivative typically arises from the hydrolysis and subsequent oxidation of oleuropein or oleuropein aglycone under harsh conditions, such as elevated temperatures or prolonged exposure [
23]. It is also frequently found in olive pomace, olive paste, and even in olive oil as a result of degradation occurring during olive oil production [
1]. However, its presence solely in olive leaf extracts, and not in any of the 15 olive pomace extracts prepared during optimization, despite identical extraction conditions, suggests that this derivative was formed through hydroxytyrosol oxidation prior to extraction. This is likely attributable to the extended ambient-temperature drying process used for olive leaves, as opposed to the rapid, low-temperature freeze-drying employed for olive pomace, which helps preserve phenolic compounds.
The relatively low hydroxytyrosol concentration, combined with only trace levels of its oxidized form in the olive leaf extract, together with the higher abundance of oleuropein and its derivatives, indicates that the optimized UAE conditions—characterized by low temperatures and short extraction times—effectively preserve the phenolic profile of the original matrix.
According to Xie et al., ultrasound intensity—controlled in this study as US amplitude and energy applied to the sample—plays a crucial role in the extraction of phenolic compounds [
54]. Low intensities may lead to insufficient cell disruption, resulting in reduced extraction efficiency, whereas higher intensities can enhance recovery, but may also induce compound degradation due to increased temperature. Therefore, this study highlights the importance of carefully balancing ultrasound parameters to maximize phenolic yield while minimizing degradation. The conditions optimized in the present study, significantly milder than those reported in prior studies [
37,
38,
42], appear to represent an effective compromise, achieving substantial extraction with minimal deterioration of bioactive compounds.
Finally, antioxidant activity of optimal olive pomace and olive leaf extracts was assessed by a DPPH assay in triplicate. The results, expressed in mg Trolox equivalents per gram of dry extract, are shown in
Table 8.
As can be observed, both optimal extracts exhibit comparable levels of antioxidant activity, with a slightly higher value for olive leaf extract. This contrasts with the previously discussed total phenolic content, which was somewhat higher in the olive pomace extract. This discrepancy may be explained by the greater antioxidant capacity of specific compounds, such as oleuropein and flavonoids, including luteolin and its derivatives, which were found in higher concentrations in olive leaves than in pomace and significantly contribute to the antioxidant activity of olive by-products [
55]. The potential synergistic effect of other types of compounds presents in olive leaves, which may have been co-extracted along with phenolic compounds, but whose presence has not been studied, should also not be ruled out. This could be the case for the pentacyclic triterpenic acids oleanolic and maslinic, whose antioxidant capacity against the DPPH radical has been previously demonstrated [
56].
Comparing these values to those reported in previous studies is challenging due to the wide variety of in vitro antioxidant activity protocols and the lack of standardized units for reporting results. However, some studies have expressed their findings in the same units employed in this work, facilitating direct comparison. For instance, conventional extraction of olive leaves has yielded extracts with substantially lower antioxidant activity, around 2 mg Trolox equivalents/g dry extract [
57]. In contrast, the use of more efficient techniques, such as microwave-assisted extraction, has resulted in extracts with antioxidant capacities of up to 90 mg Trolox equivalents/g dry extract [
58].
Regarding olive pomace, UAE using a 50:50 acetone–water mixture as its. extraction solvent has produced extracts with notably higher antioxidant activity, achieving DPPH values of approximately 400 mg Trolox equivalents/g dry extract [
59]. Nevertheless, in 2024 Vittorio Carlucci et al. reported antioxidant activities of 63.70 ± 6.03 mg Trolox equivalents per gram of dry extract when using an ultrasonic-assisted extraction (UAE) with water, and 65.92 ± 1.62 mg Trolox equivalents per gram of dry extract with a UAE water/ethanol (15%) extraction [
60]. In comparison, the antioxidant activity found in this study indicates that using optimal conditions can achieve higher antioxidant activity than what has been previously reported.