Alterations of the Intestinal Barrier and Inflammatory Response, Caused by Chronic Ozone Exposure in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure to O3
2.2. TBARS
2.3. Western Blot
2.4. Immunohistochemistry
2.5. Hematoxylin and Eosin (H-E) Histological Technique
2.6. Statistical Analysis
3. Results
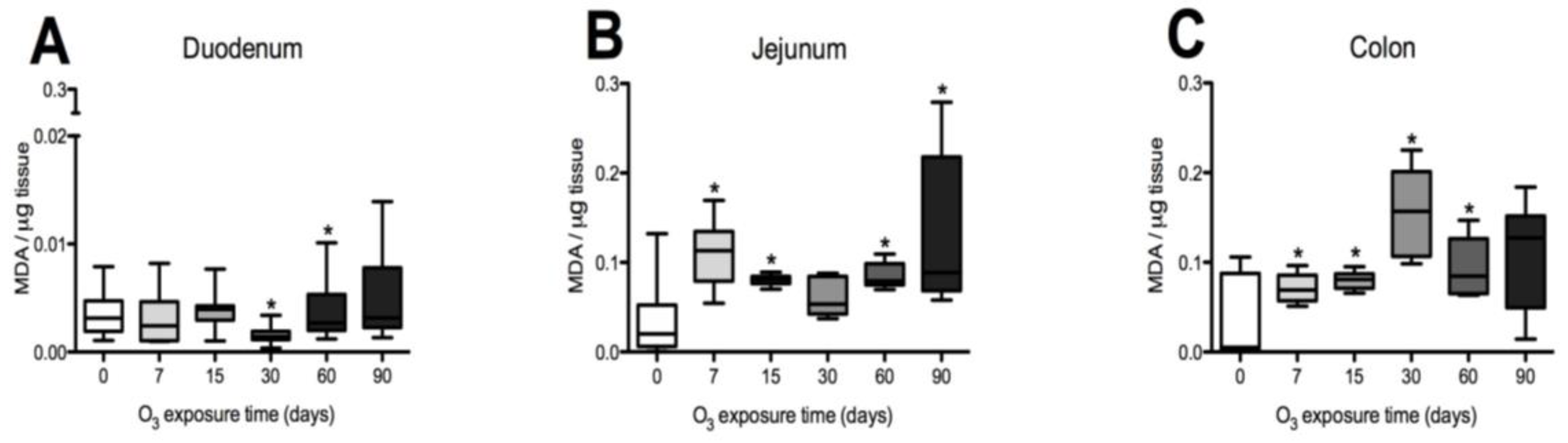
3.1. Oxidative Stress Status in the Duodenum, Jejunum, and Colon of the Rat
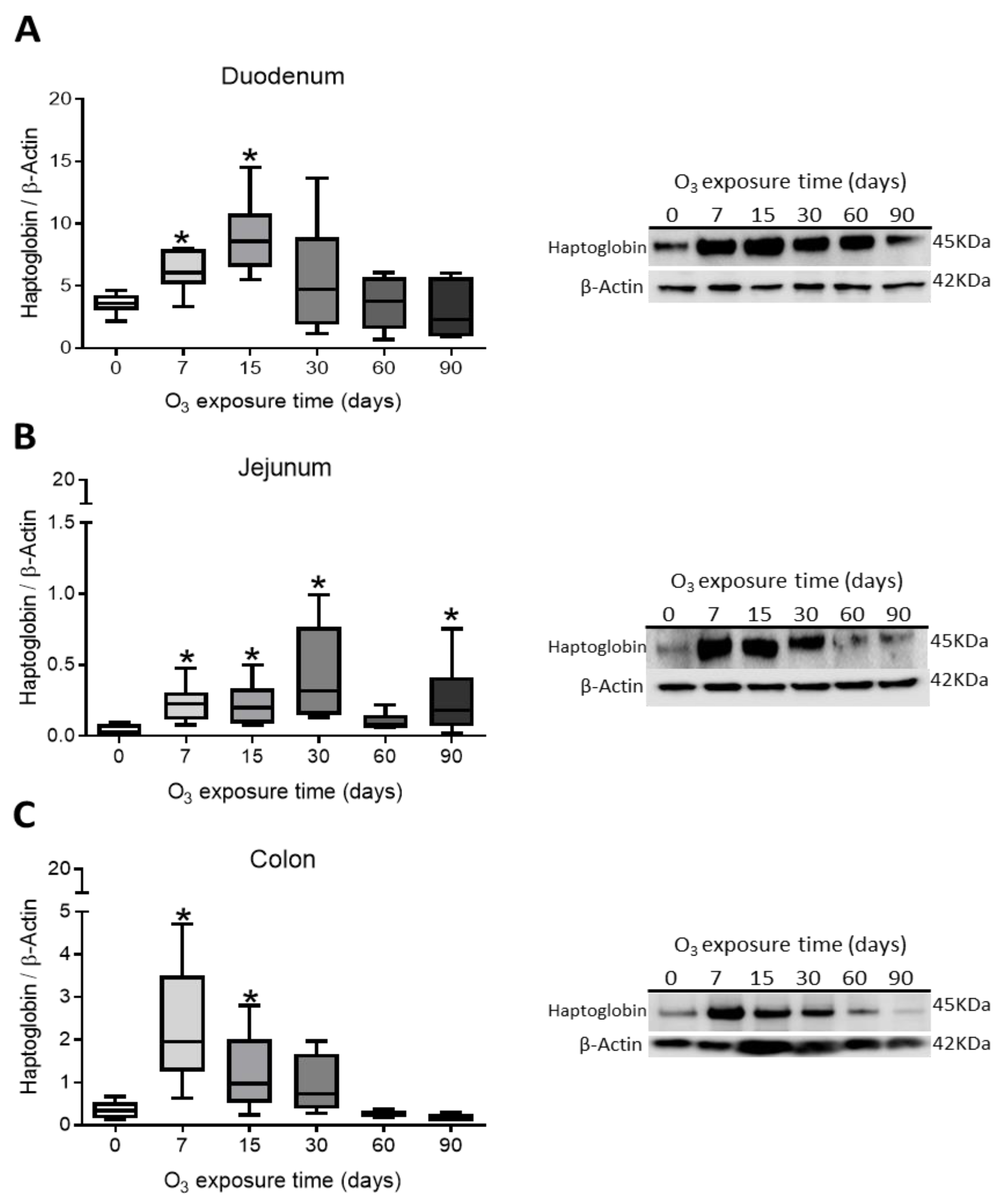
3.2. Western Blot Results in Duodenum, Jejunum, and Colon
Haptoglobin Content in Rat Duodenum, Jejunum, and Colon
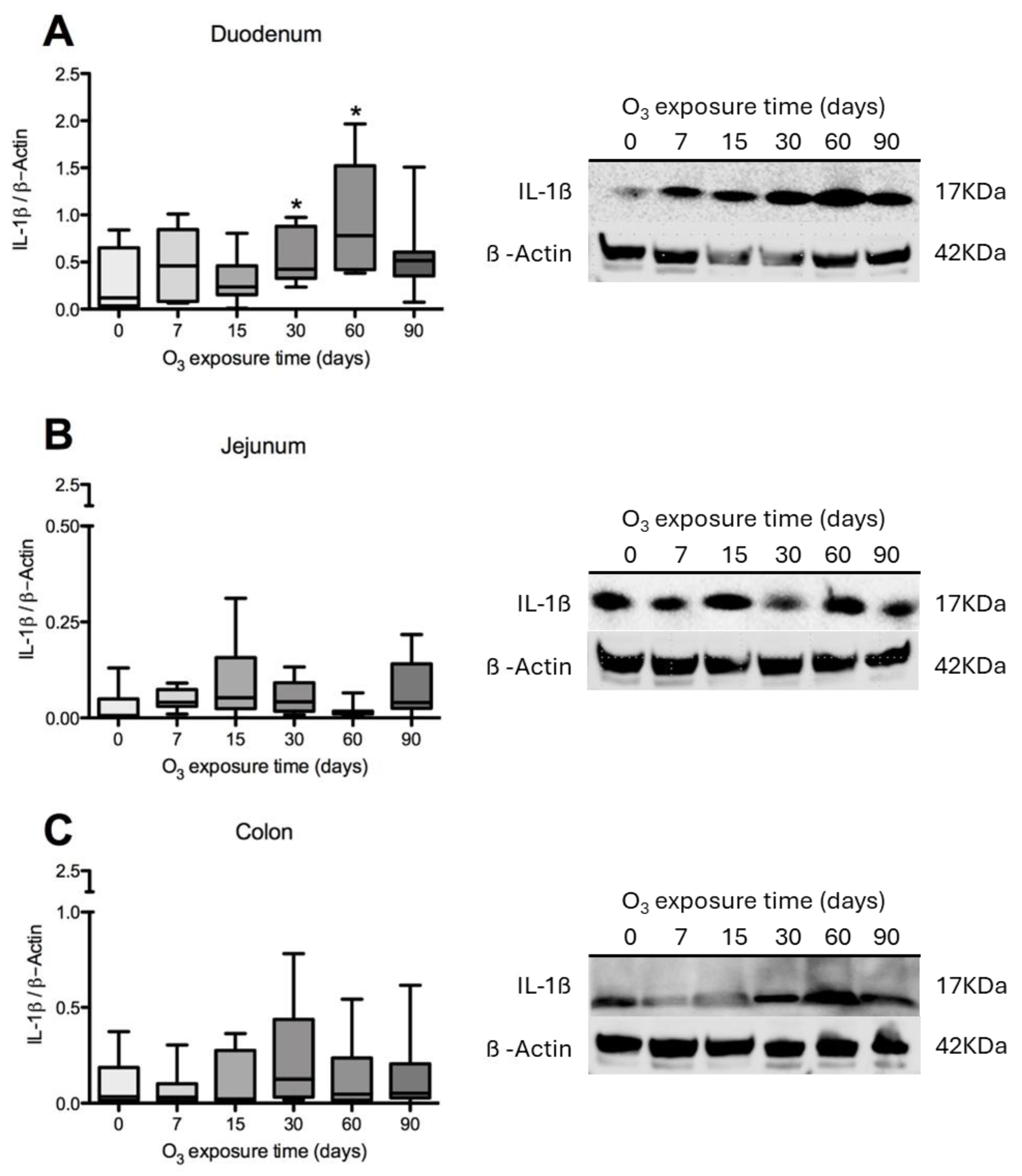
3.3. L-1β Content in the Duodenum, Jejunum, and Colon of Rats
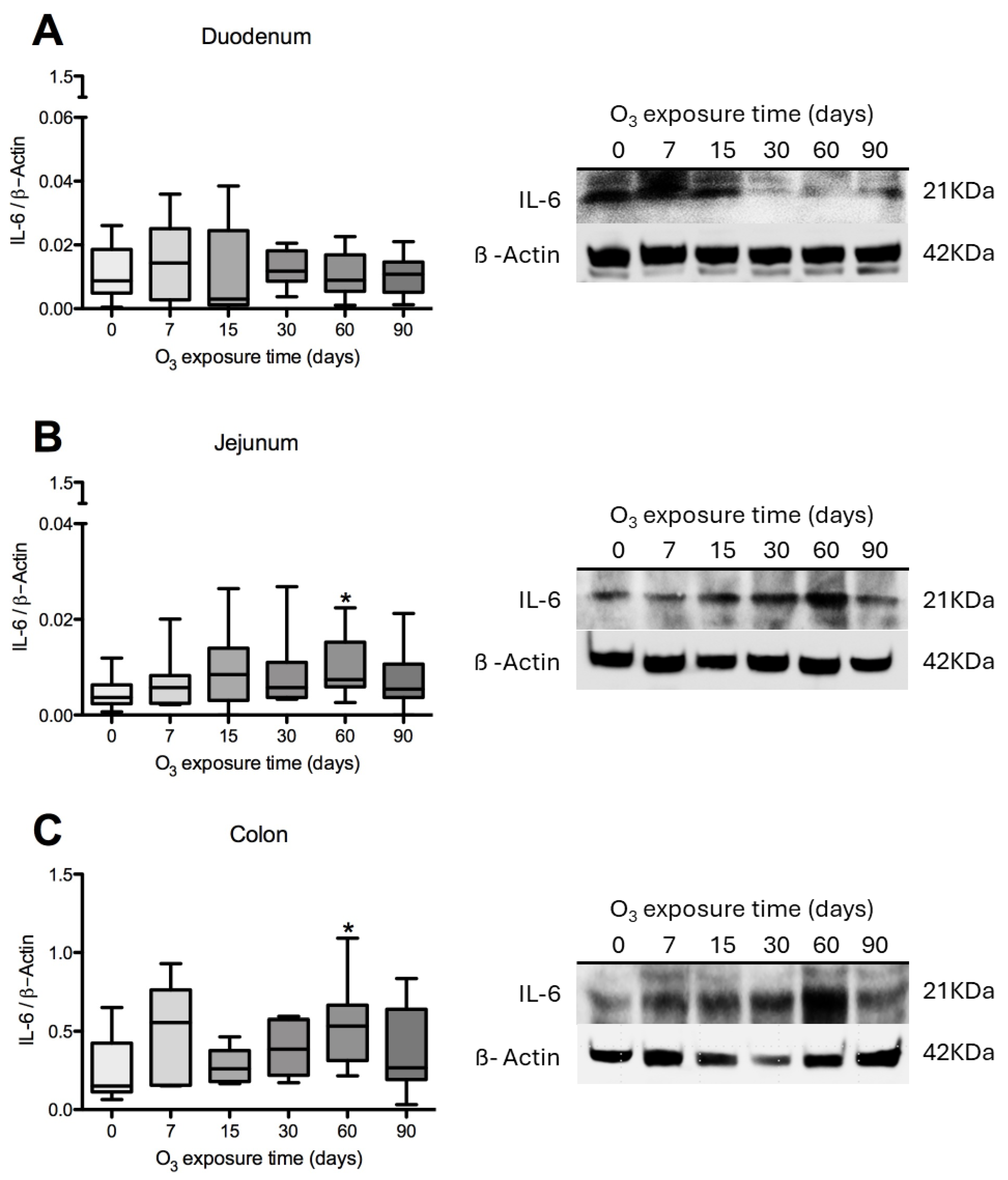
3.4. IL-6 Content in Rat Duodenum, Jejunum, and Colon
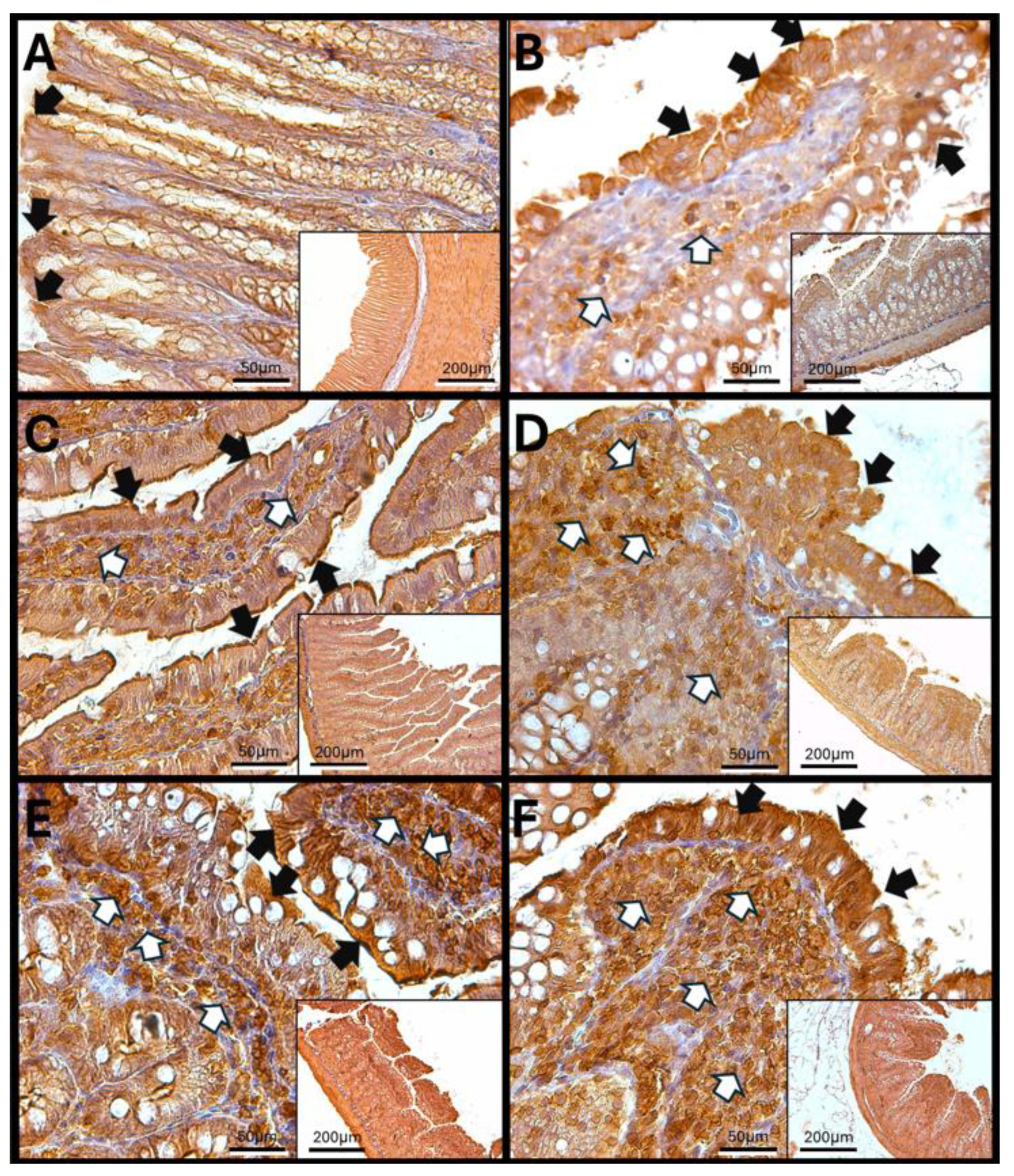
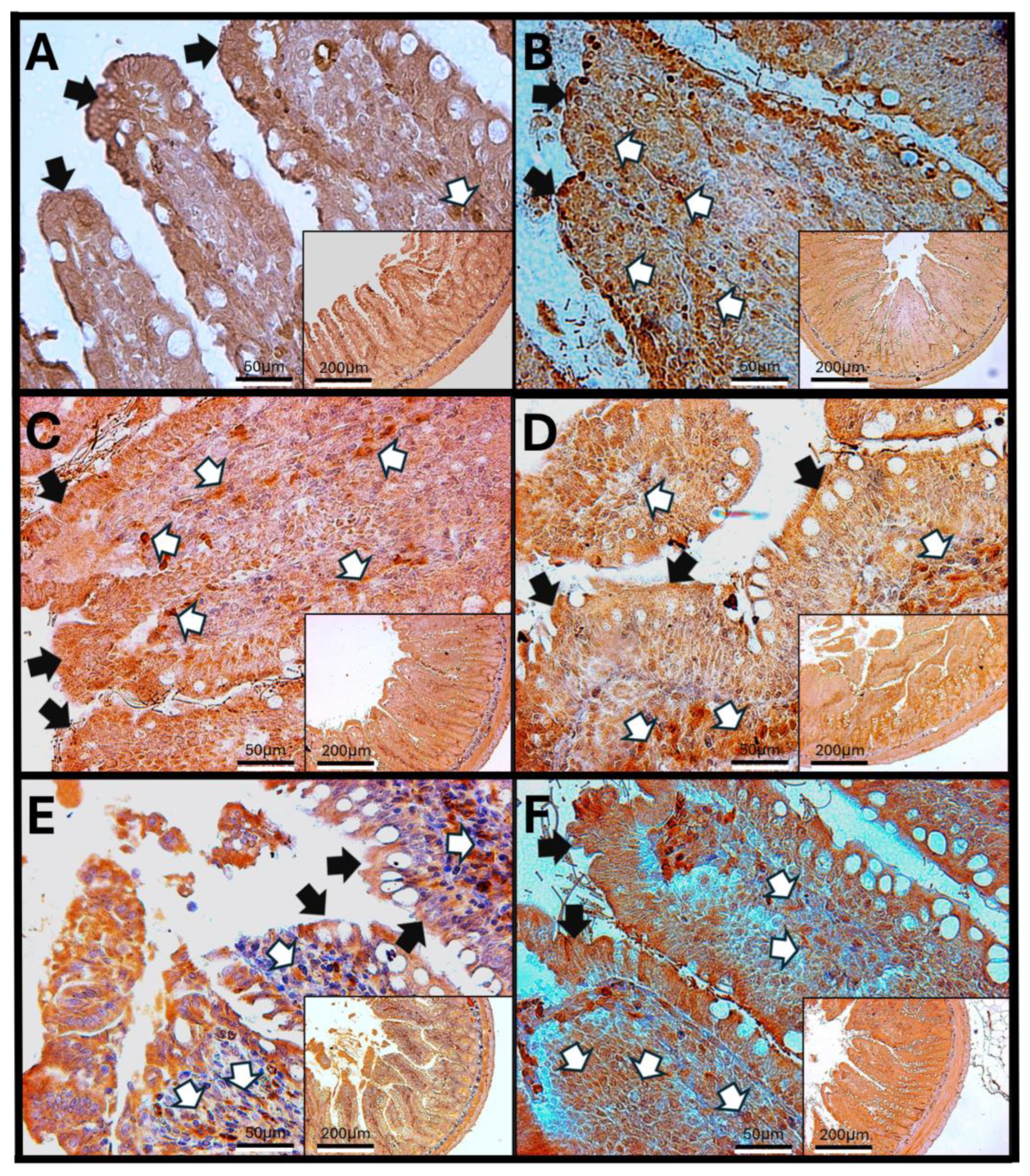
3.5. Immunohistochemical Test Results
Immunohistochemistry Against Haptoglobin
- Haptoglobin in the Duodenum
3.6. Haptoglobin in the Jejunum
3.7. Haptoglobin in the Colon
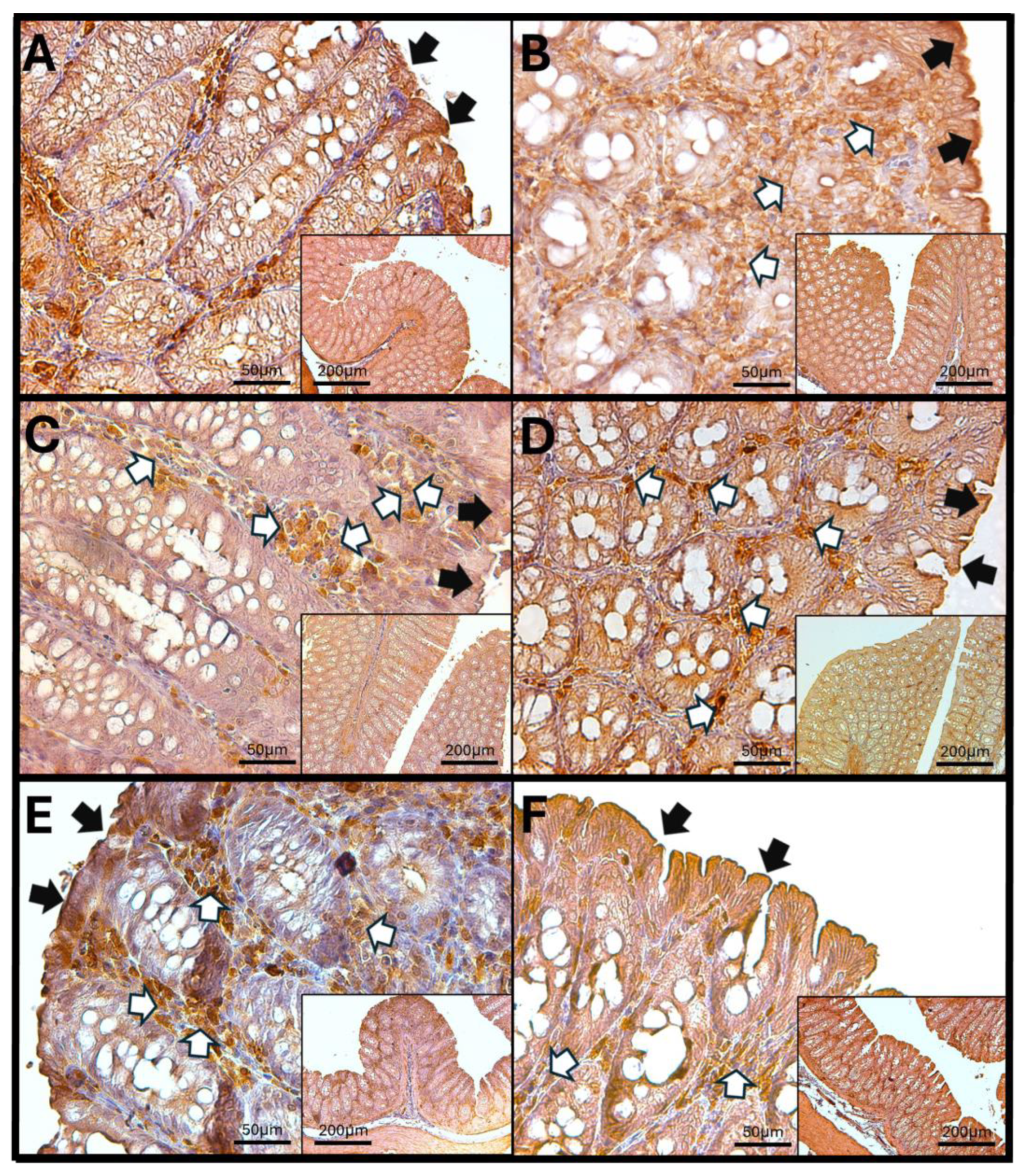
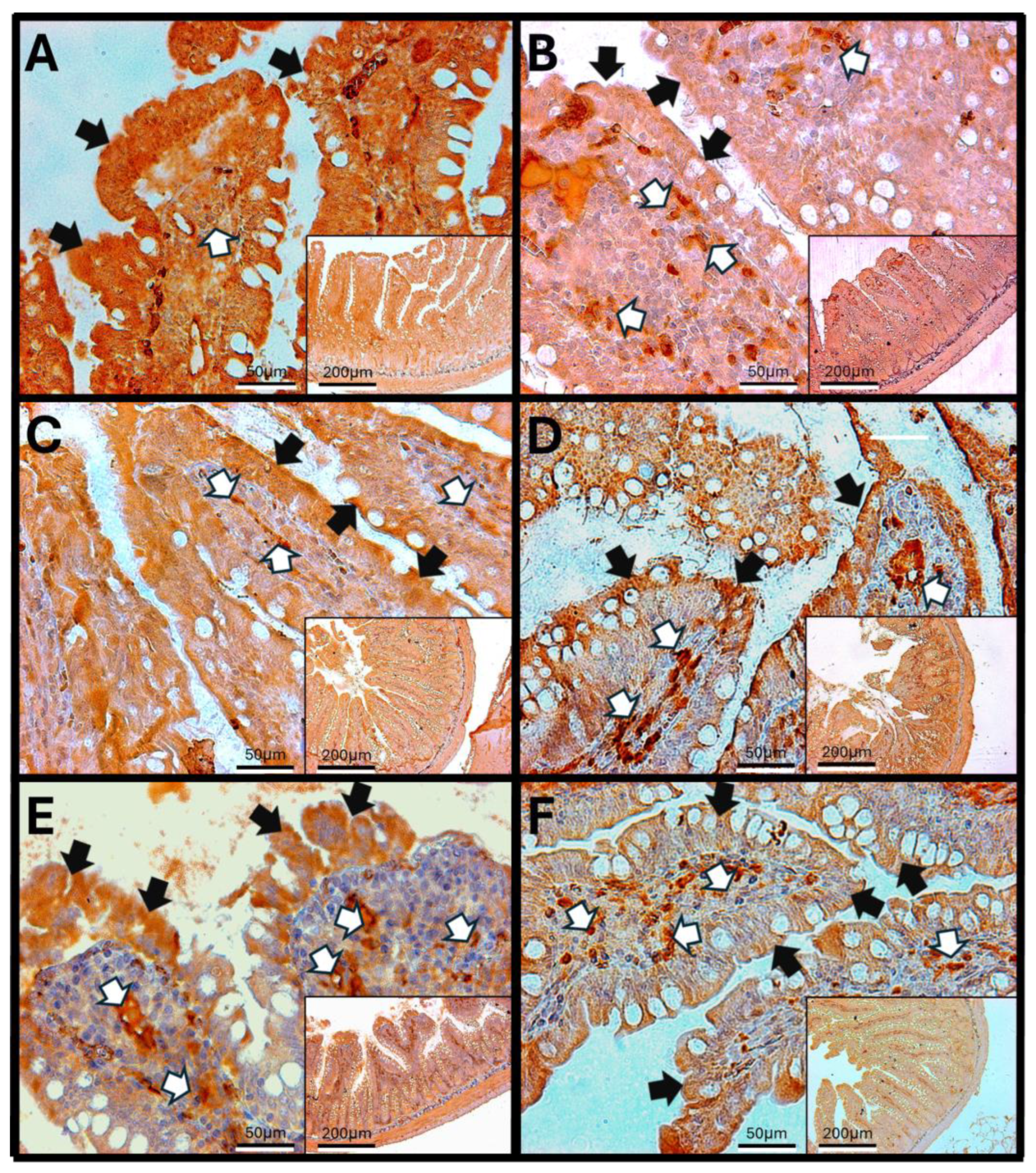
3.8. Immunohistochemistry Against IL-1β
3.8.1. IL-1β in Rat Duodenum
3.8.2. IL-1β in the Rat Jejunum
3.8.3. IL-1β in the Rat Colon
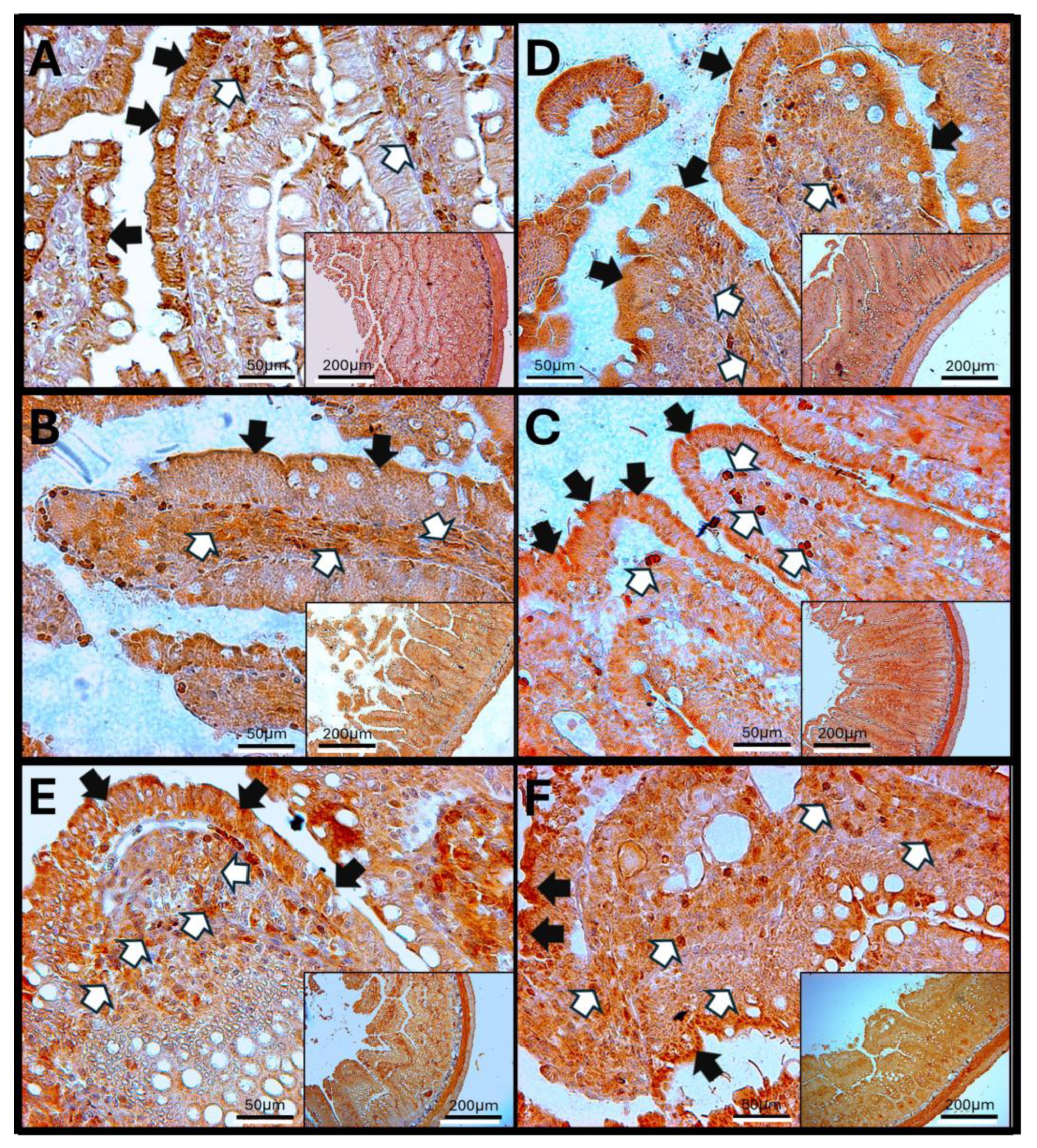
3.9. Immunohistochemistry Against IL-6
3.10. IL-6 in Rat Jejunum
3.11. IL-6 Immunoreactivity in the Rat Colon
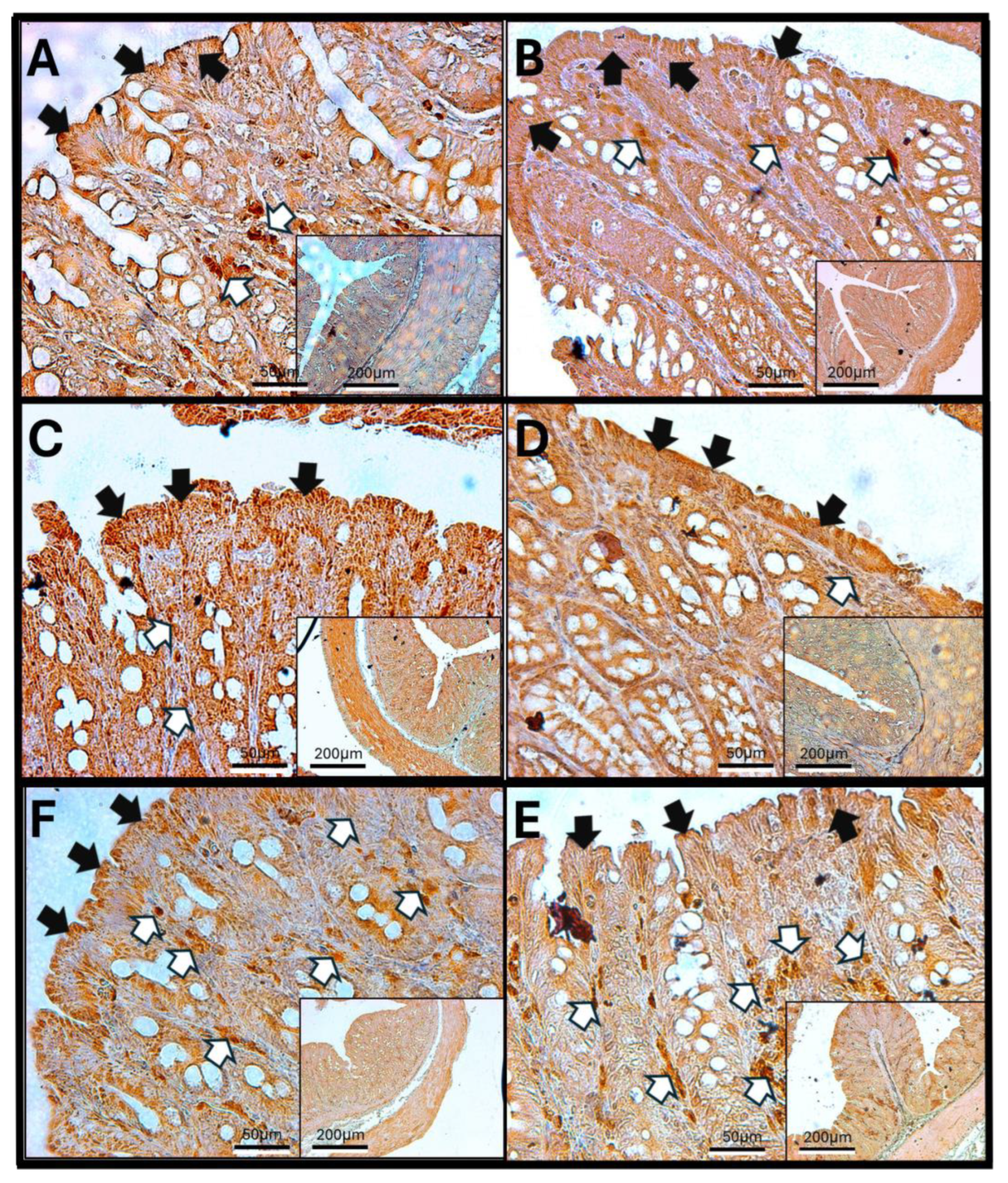
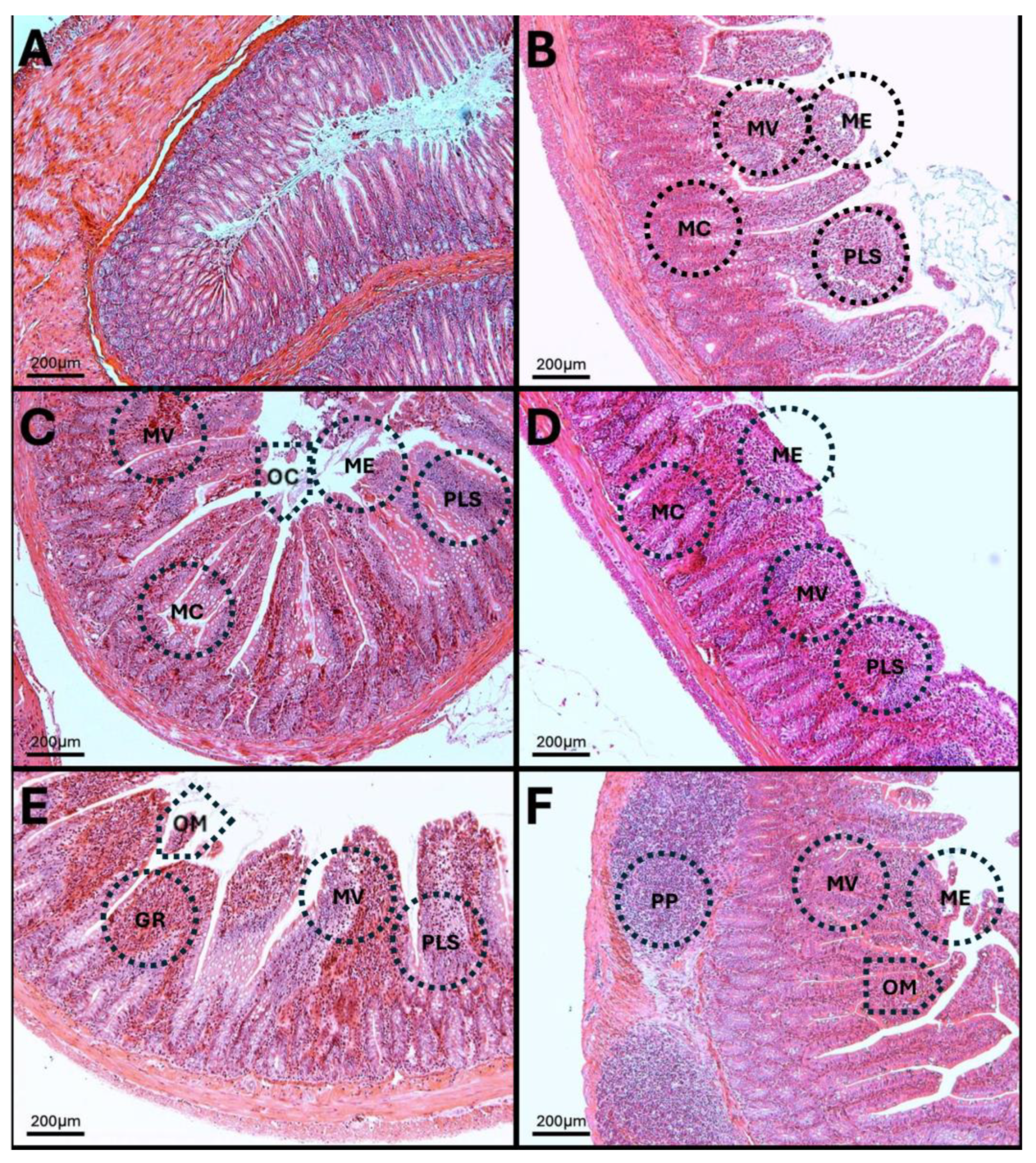
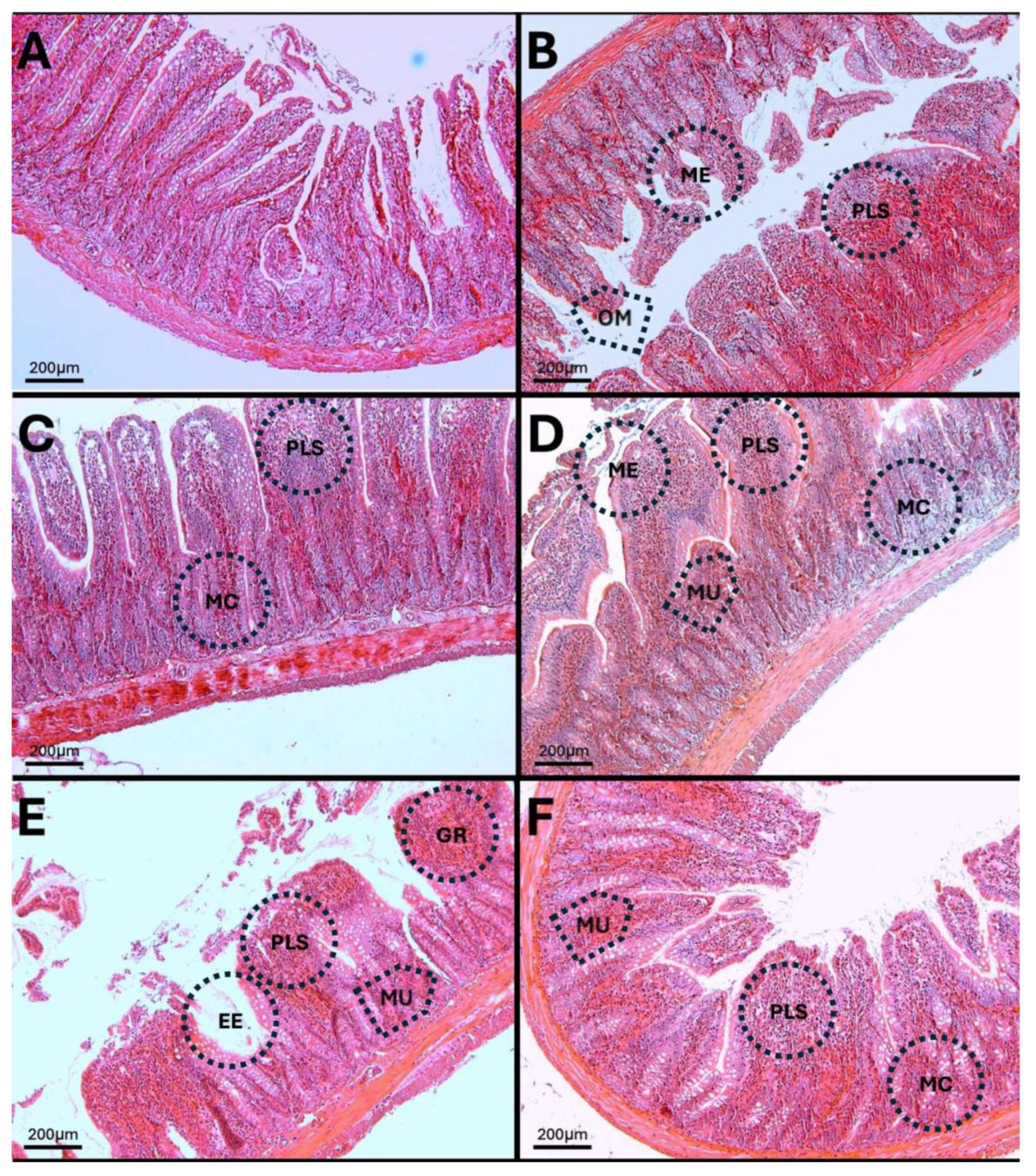
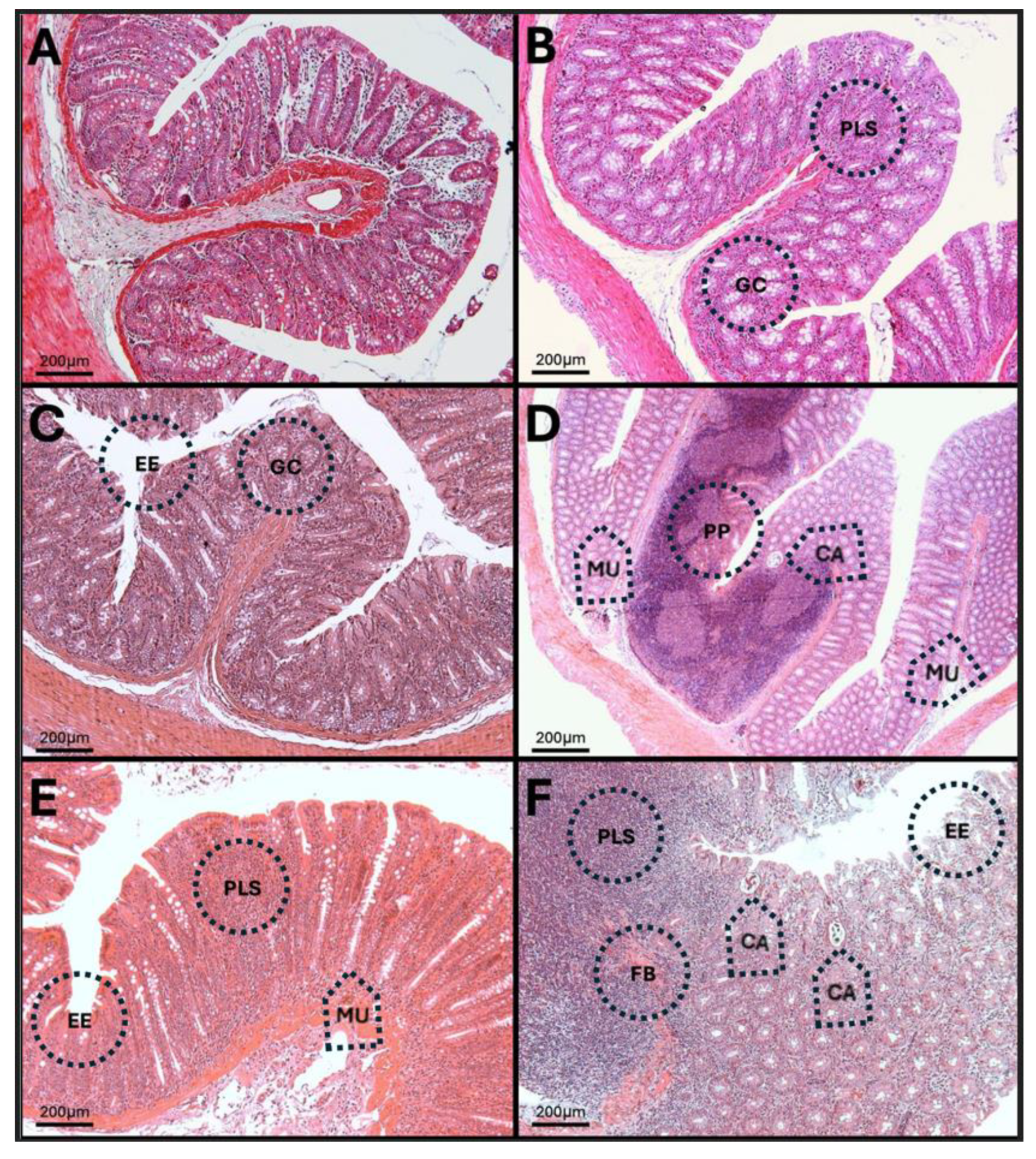
3.12. Results of H-E Staining in the Duodenum, Jejunum, and Colon of the Rat
3.13. H-E in Jejunum
3.14. H-E of the Colon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CCL4 | Chemokine (C-C motif) ligand 4 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| CCL11 | Chemokine (C-C motif) ligand 11 |
| CCL17 | Chemokine (C-C motif) ligand 17 |
| CA | Crypt abscess |
| ME | Modified epithelial |
| EE | Eroded epithelium |
| FB | Fibrosis |
| MC | Mucin cells |
| GR | Granulomas |
| H2O2 | Hydrogen peroxide |
| H-E | Hematoxylin and eosin |
| IBD | Inflammatory bowel disease |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| MU | Micro ulcers |
| MV | Modify villi |
| NK | Natural killer cells |
| OM | Opening crypt |
| O3 | Ozone |
| ppm | Parts per million |
| PP | Peyer’s patches |
| PLS | Plasmacytosis |
| PreHp-2 | Prehaptoglobin-2 |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| SCFAs | Short-chain fatty acids |
| TBARS | Thiobarbituric acid reactive substances |
| TBA | Thiobarbituric acid |
| TJs | Tight junctions |
| TLRs | Toll-like receptors |
| VCAM-1 | Vascular cell adhesion molecule 1 |
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Waxman, M.; Manczak, E.M. Air Pollution’s Hidden Toll: Links Between Ozone, Particulate Matter, and Adolescent Depression. Int. J. Environ. Res. Public Health 2024, 21, 1663. [Google Scholar] [CrossRef] [PubMed]
- Bista, S.; Chatzidiakou, L.; Jones, R.L.; Benmarhnia, T.; Postel-Vinay, N.; Chaix, B. Associations of air pollution mixtures with ambulatory blood pressure: The MobiliSense sensor-based study. Environ. Res. 2023, 227, 115720. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Lavigne, E.; Correa, P.M.; Ortega, N.V.; Achilleos, S.; et al. Interactive effects of ambient fine particulate matter and ozone on daily mortality in 372 cities: Two stage time series analysis. Bmj 2023, 383, e075203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Sadiq, I.Z. Free Radicals and Oxidative Stress: Signaling Mechanisms, Redox Basis for Human Diseases, and Cell Cycle Regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Hernández-Orozco, E.; Rivas-Arancibia, S. Effect of exposure to low doses of ozone on interleukin 17A expression during progressive neurodegeneration in the rat hippocampus. Neurol. (Engl. Ed.) 2021, 36, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Kulesza, R.J.; González-González, L.O.; Reynoso-Robles, R.; Mukherjee, P.S.; Torres-Jardón, R. Air Pollution, Combustion and Friction Derived Nanoparticles, and Alzheimer’s Disease in Urban Children and Young Adults. J. Alzheimers Dis. 2019, 70, 343–360. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Kulesza, R.J.; Mansour, Y.; González-González, L.O.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Mukherjee, P.S. Alzheimer disease starts in childhood in polluted Metropolitan Mexico City. A major health crisis in progress. Environ. Res. 2020, 183, 109137. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Ma, Y.; Yu, N.; Zheng, P.; Chen, Z.; Wang, T.; Jia, G. Association between Air Pollution and Lipid Profiles. Toxics 2023, 11, 894. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; García-Giménez, J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, E.; Martínez, F.; Espinosa-García, M.T.; Maldonado, P.; Rivas-Arancibia, S. Mitochondrial dysfunction in the hippocampus of rats caused by chronic oxidative stress. Neuroscience 2013, 252, 384–395. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, E.; Nava-Ruiz, C.; Escamilla-Chimal, E.; Borgonio-Perez, G.; Rivas-Arancibia, S. The Effect of Chronic Ozone Exposure on the Activation of Endoplasmic Reticulum Stress and Apoptosis in Rat Hippocampus. Front. Aging Neurosci. 2016, 8, 245. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Hernández-Orozco, E.; Rodríguez-Martínez, E.; Valdés-Fuentes, M.; Cornejo-Trejo, V.; Pérez-Pacheco, N.; Dorado-Martínez, C.; Zequeida-Carmona, D.; Espinosa-Caleti, I. Ozone Pollution, Oxidative Stress, Regulatory T Cells and Antioxidants. Antioxidants 2022, 11, 1553. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Junges, V.M.; Closs, V.E.; Nogueira, G.M.; Gottlieb, M.G.V. Crosstalk between Gut Microbiota and Central Nervous System: A Focus on Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 1179–1190. [Google Scholar] [CrossRef]
- Ojeda, J.; Ávila, A.; Vidal, P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Estados Unidos Mexicanos-Secretaría de Agricultura Ganadería, Desarrollo Rural, Pesca y Alimentación. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. In Norma Oficial Mexicana (NOM-062-ZOO-1999); Gobernación, S.d., Ed.; Diario Oficial de la Federación-SEGOB: Ciudad de México, Mexico, 2001; Volume 107, pp. 107–165. [Google Scholar]
- CICUAL (Comité Interno para el Cuidado y Uso de Animales de Laboratorio). In. División de Investigación, Facultad de Medicina: Ciudad de México, Mexico, 2018. Available online: https://di.facmed.unam.mx/comisiones/ManualCICUAL.pdf (accessed on 12 August 2025).
- Estados Unidos Mexicanos-Secretaría de Agricultura Ganadería; Desarrrollo Rural Pesca y Alimentación. Métodos Para Dar Muerte a Los Animales Domésticos y Silvestres. In NORMA Oficial Mexicana NOM-033-SAG/ZOO-2014; Gobernación, S.d., Ed.; Diario Oficial de la Federación-SEGOB: Ciudad de México, Mexico, 2015. [Google Scholar]
- Pereyra-Muñoz, N.; Rugerio-Vargas, C.; Angoa-Pérez, M.; Borgonio-Pérez, G.; Rivas-Arancibia, S. Oxidative damage in substantia nigra and striatum of rats chronically exposed to ozone. J. Chem. Neuroanat. 2006, 31, 114–123. [Google Scholar] [CrossRef]
- Fuentes-Venado, C.E.; Terán-Pérez, G.; Espinosa-Hernández, V.M.; Martínez-Herrera, E.; Segura-Uribe, J.J.; Mercadillo, R.E.; Pinto-Almazán, R.; Guerra-Araiza, C. Nutritional Status Influences Oxidative Stress and Insulin Resistance in Preschool Children. Metab. Syndr. Relat. Disord. 2021, 19, 513–523. [Google Scholar] [CrossRef]
- Miller, Y.I.; Shyy, J.Y. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends Endocrinol. Metab. 2017, 28, 143–152. [Google Scholar] [CrossRef]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106–2123. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy gut microbiota inflammatory responses, i.n.IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Dillon, S.M.; Rogers, L.M.; Howe, R.; Hostetler, L.A.; Buhrman, J.; McCarter, M.D.; Wilson, C.C. Human Intestinal Lamina Propria CD1c+ Dendritic Cells Display an Activated Phenotype at Steady State and Produce IL-23 in Response to TLR7/8 Stimulation. J. Immunol. 2010, 184, 6612–6621. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Kamada, N.; Kim, Y.-G.; Núñez, G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012, 209, 251–258. [Google Scholar] [CrossRef]
- Yonker, L.M.; Gilboa, T.; Ogata, A.F.; Senussi, Y.; Lazarovits, R.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; Rivas, M.N.; Porritt, R.A.; et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Investig. 2021, 131, e149633. [Google Scholar] [CrossRef] [PubMed]
- Rosser, F.; Balmes, J. Ozone and childhood respiratory health: A primer for US pediatric providers and a call for a more protective standard. Pediatr. Pulmonol. 2023, 58, 1355–1366. [Google Scholar] [CrossRef]
- Smith, J.K. IL-6 and the dysregulation of immune, bone, muscle, and metabolic homeostasis during spaceflight. NPJ Microgravity 2018, 4, 24. [Google Scholar] [CrossRef]
- Madaro, L.; Passafaro, M.; Sala, D.; Etxaniz, U.; Lugarini, F.; Proietti, D.; Alfonsi, M.V.; Nicoletti, C.; Gatto, S.; De Bardi, M.; et al. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat. Cell Biol. 2018, 20, 917–927. [Google Scholar] [CrossRef]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1BIncreases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p Which Degrades Occludin, m.RNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Ajamian, M.; Steer, D.; Rosella, G.; Gibson, P.R. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS ONE 2019, 14, e0210728. [Google Scholar] [CrossRef]
- Mariño-Crespo, Ó.; Cuevas-Álvarez, E.; Harding, A.L.; Murdoch, C.; Fernández-Briera, A.; Gil-Martín, E. Haptoglobin expression in human colorectal cancer. Histol. Histopathol. 2019, 34, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.L.; Lai, H.T.; Zhou, J.J.; Liu, W.Y.; Zhao, M.; Zhao, K. Nrf2 Signaling Pathway: Focus on Oxidative Stress in Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 2230–2249. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Kurti, Z.; Vegh, Z.; Gonczi, L.; Lakatos, P.L. Clinical Risk Factors: Lessons from Epidemiology. In Biomarkers in Inflammatory Bowel Diseases; Springer: Cham, Switzerland, 2019; pp. 9–22. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Martínez, A.; Rodríguez-Martínez, E.; Valdés-Fuentes, M.; Rivas-Arancibia, S. Alterations of the Intestinal Barrier and Inflammatory Response, Caused by Chronic Ozone Exposure in a Rat Model. Antioxidants 2025, 14, 1000. https://doi.org/10.3390/antiox14081000
Miranda-Martínez A, Rodríguez-Martínez E, Valdés-Fuentes M, Rivas-Arancibia S. Alterations of the Intestinal Barrier and Inflammatory Response, Caused by Chronic Ozone Exposure in a Rat Model. Antioxidants. 2025; 14(8):1000. https://doi.org/10.3390/antiox14081000
Chicago/Turabian StyleMiranda-Martínez, Alfredo, Erika Rodríguez-Martínez, Marlen Valdés-Fuentes, and Selva Rivas-Arancibia. 2025. "Alterations of the Intestinal Barrier and Inflammatory Response, Caused by Chronic Ozone Exposure in a Rat Model" Antioxidants 14, no. 8: 1000. https://doi.org/10.3390/antiox14081000
APA StyleMiranda-Martínez, A., Rodríguez-Martínez, E., Valdés-Fuentes, M., & Rivas-Arancibia, S. (2025). Alterations of the Intestinal Barrier and Inflammatory Response, Caused by Chronic Ozone Exposure in a Rat Model. Antioxidants, 14(8), 1000. https://doi.org/10.3390/antiox14081000




