Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome
Abstract
1. Introduction
2. Material and Methods
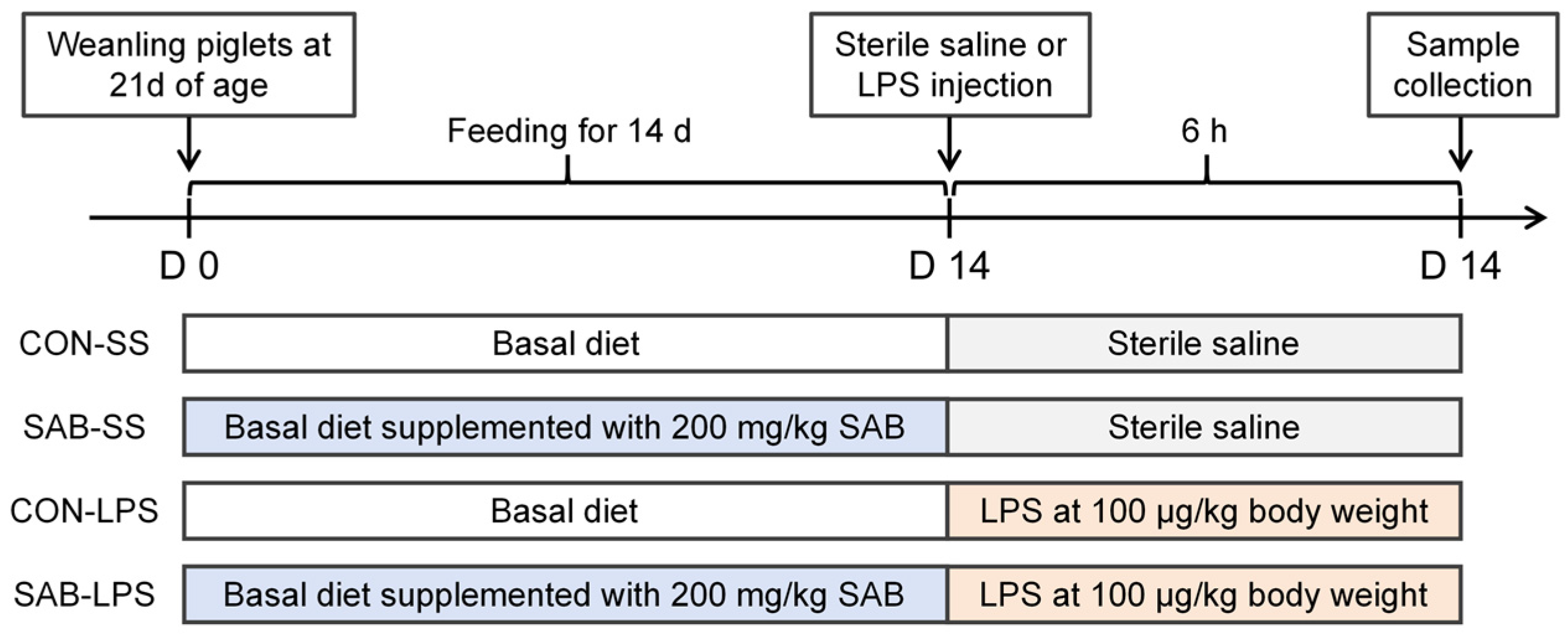
2.1. Experimental Design, Management, and Sample Collection
2.2. Routine Blood Indicators
2.3. Spleen Ultrastructure Observation
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Cell Apoptosis Analysis
2.6. Immunofluorescent Staining
2.7. Immune Status Analysis
2.8. Redox Status Analysis
2.9. Measurement of Adenosine Triphosphate (ATP) Levels
2.10. Determination of Mitochondrial Superoxide Radical Levels and Mitochondrial Complex Activities
2.11. Determination of Mitochondria Swelling
2.12. Evaluation of Mitochondrial Membrane Potential
2.13. RNA Isolation and Quantitative Real Time PCR (qRT-PCR) Analysis
2.14. Protein Extraction and Western Blot (WB) Analysis
2.15. Statistical Analysis
3. Results
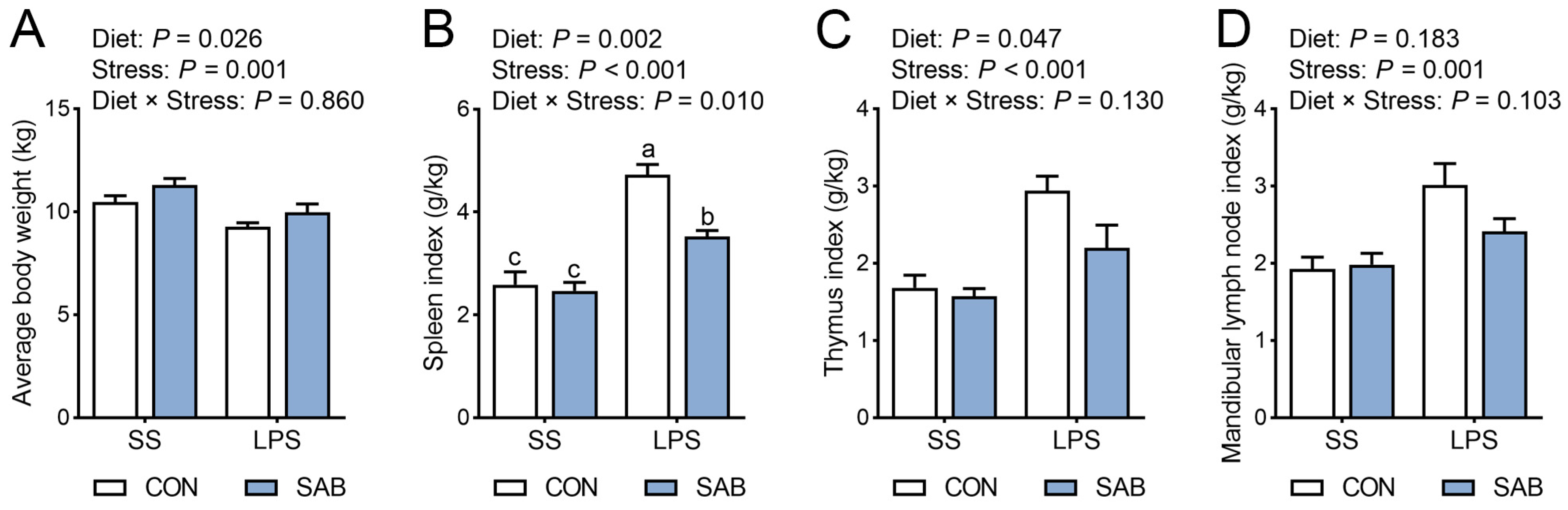
3.1. Average Body Weight and Immune Organ Indices
3.2. Blood Routine Examination
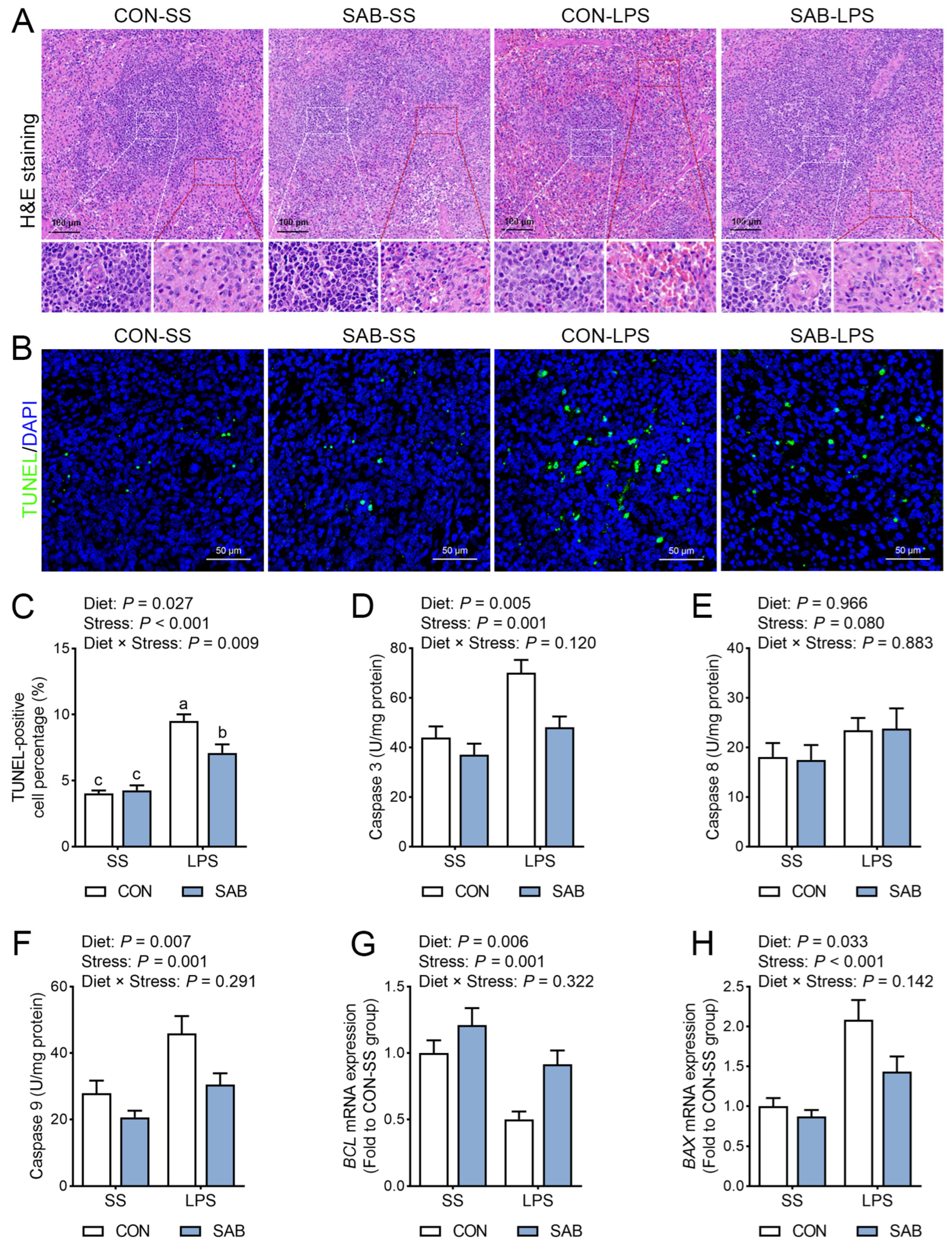
3.3. Histopathology and Cell Apoptosis of Spleen
3.4. Inflammation Response in the Spleen
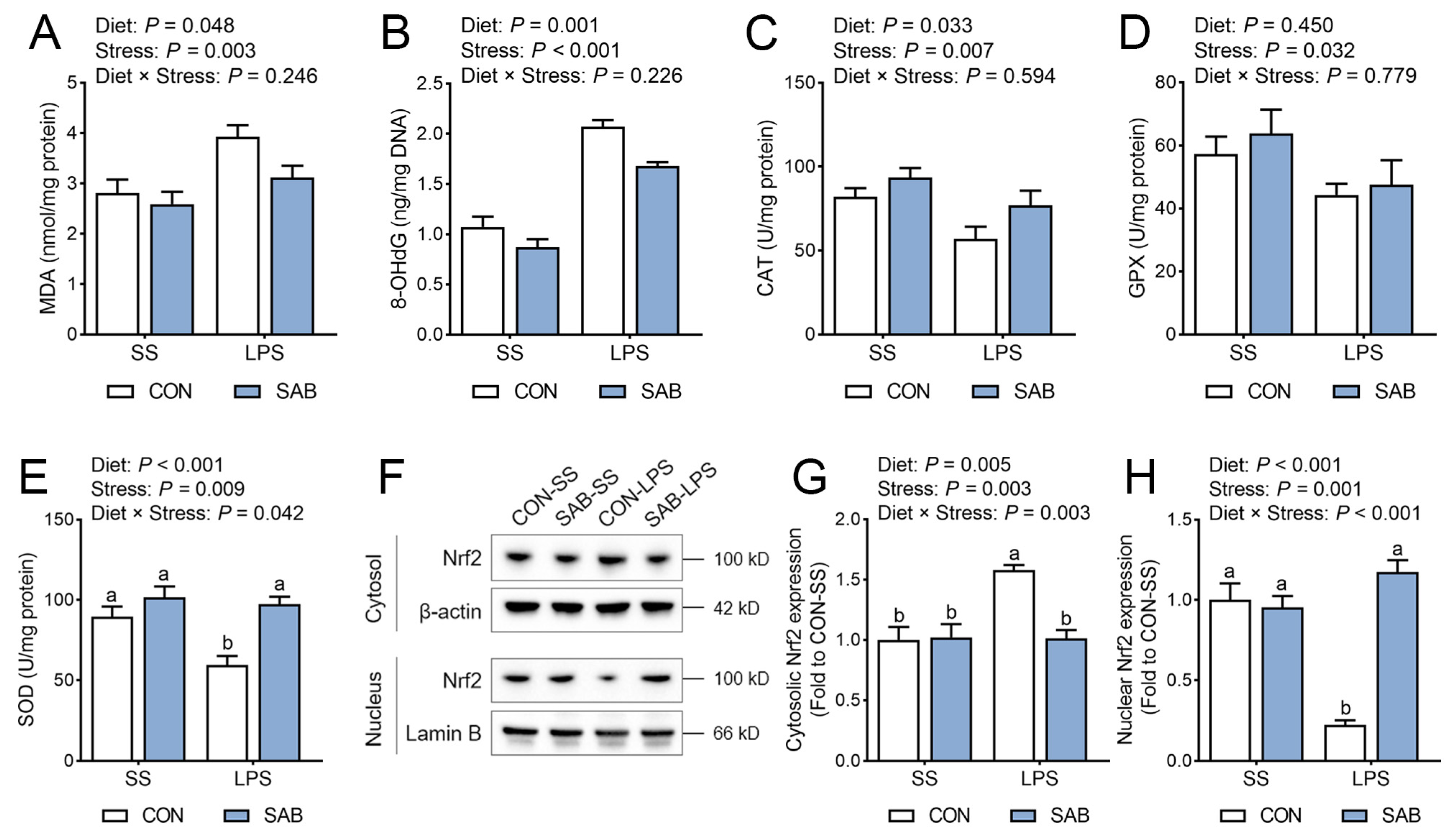
3.5. Redox Status of Spleen
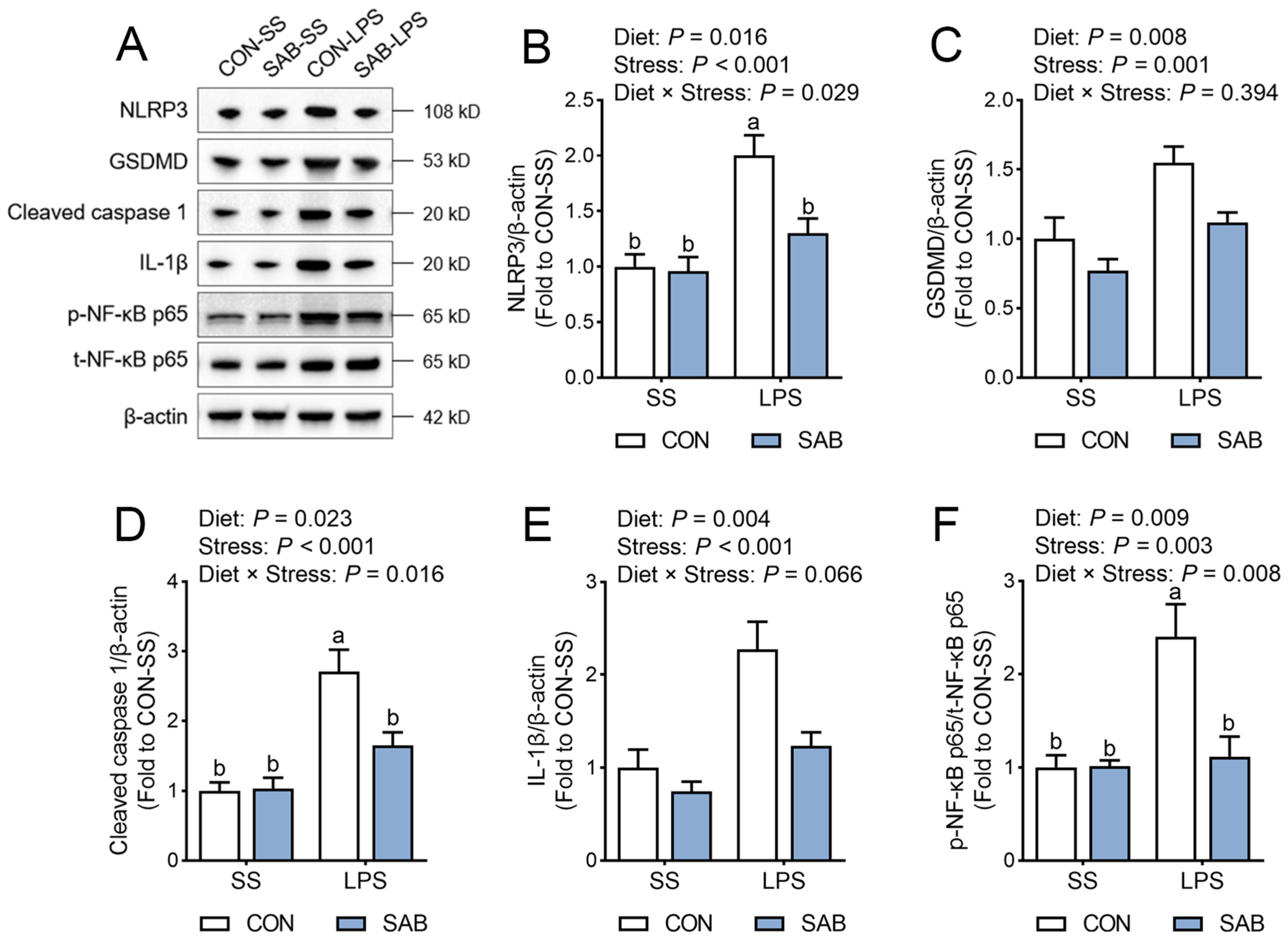
3.6. NLRP3 Inflammasome Activation of Spleen
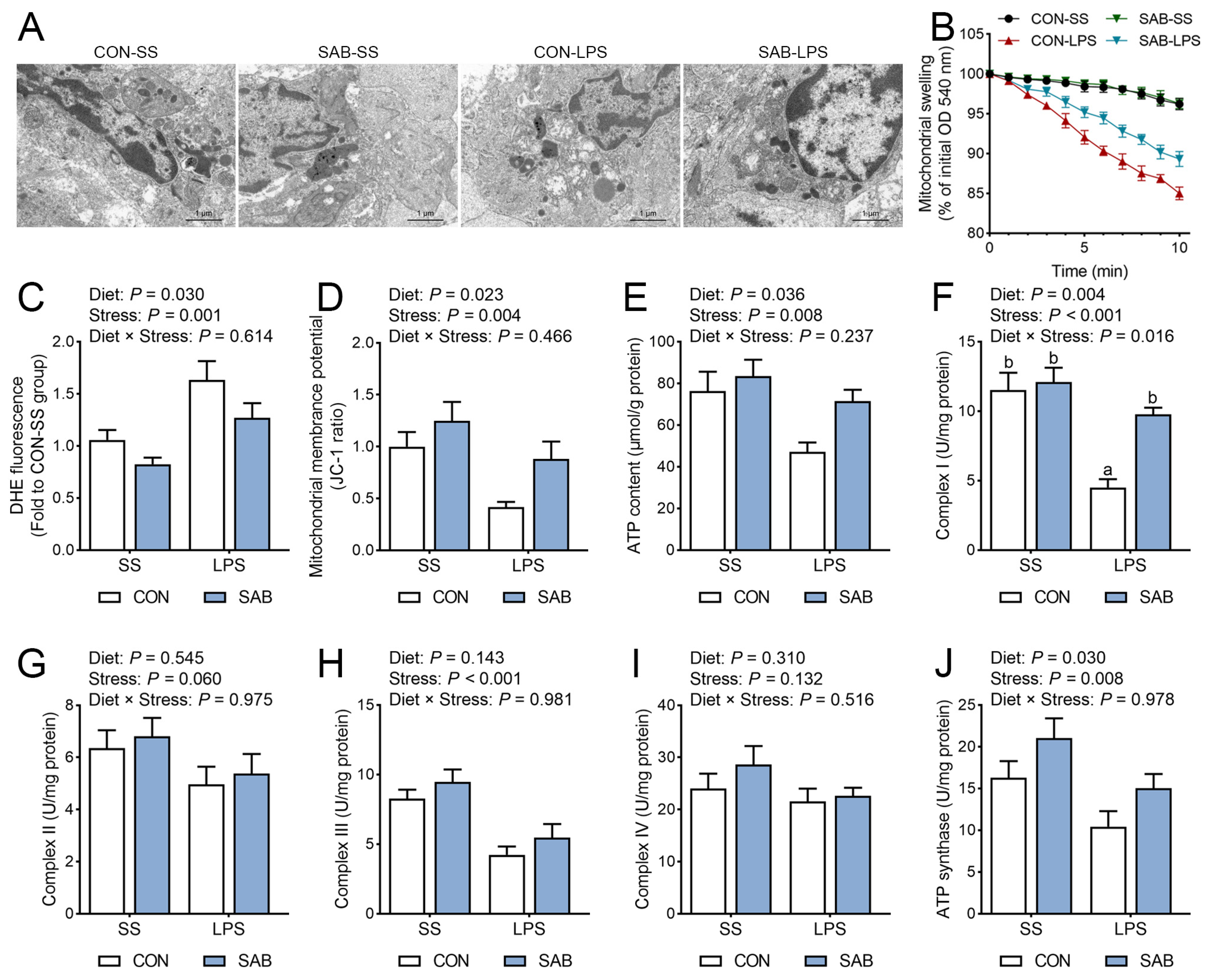
3.7. The Ultrastructure, Swelling, and Function of Mitochondria in the Spleen
4. Discussion
4.1. LPS Challenge Induces Severe Splenic Immune Injury
4.2. SAB Improves LPS-Induced Splenic Inflammation by Maintaining Redox Balance
4.3. SAB Alleviates LPS-Induced Splenic Inflammation by Inhibiting NLRP3 Inflammasome
4.4. SAB Preserves Mitochondrial Integrity to Attenuate Oxidative Stress and NLRP3 Inflammasome Activation
4.5. The Novelty and Limitation of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Wluka, A.; Olszewski, W.L. Innate and adaptive processes in the spleen. Ann. Transplant. 2006, 11, 22–29. [Google Scholar]
- Liu, Z.; Wang, Y.; Ning, Q.; Gong, C.; Zhang, Y.; Zhang, L.; Bu, X.; Jing, G. The role of spleen in the treatment of experimental lipopolysaccharide-induced sepsis with dexmedetomidine. SpringerPlus 2015, 4, 800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsokos, G.C. Autoimmunity and organ damage in systemic lupus erythematosus. Nat. Immunol. 2020, 21, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duan, M.; Chen, W.; Jiang, A.; Li, X.; Yang, J.; Li, Z. The spleen in liver cirrhosis: Revisiting an old enemy with novel targets. J. Transl. Med. 2017, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Guan, L.; Shi, H.; Tian, J.; Wang, X.; Liu, N.; Wang, C.; Zhang, Z. PM (2.5) induces the inflammatory response in rat spleen lymphocytes through autophagy activation of NLRP3 inflammasome. Mol. Immunol. 2023, 161, 74–81. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Li, J.; Dai, X.; Li, X.; Li, J. Lycopene ameliorates atrazine-induced pyroptosis in spleen by suppressing the Ox-mtDNA/Nlrp3 inflammasome pathway. Food Funct. 2022, 13, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; He, K.; Xu, M.; Gong, J. Cardiolipin inhibitor ameliorates the non-alcoholic steatohepatitis through suppressing NLRP3 inflammasome activation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8158–8167. [Google Scholar]
- Chen, C.; Li, H.; Yuan, Y.; Dai, H.; Yang, B. Antioxidant activity and components of a traditional Chinese medicine formula consisting of Crataegus pinnatifida and Salvia miltiorrhiza. BMC Complem. Altern. Med. 2013, 13, 99. [Google Scholar] [CrossRef]
- He, G.; Chen, G.; Liu, W.; Ye, D.; Liu, X.; Liang, X.; Song, J. Salvianolic acid B: A review of pharmacological effects, safety, combination therapy, new dosage forms, and novel drug delivery routes. Pharmaceutics 2023, 15, 2235. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Gu, T.; Yu, D.; Shi, Y.; Gao, Z.; Wang, Z.; Liu, W.; Fan, Z.; Hou, W.; et al. Astrocytic glycogen mobilization participates in salvianolic acid B-mediated neuroprotection against reperfusion injury after ischemic stroke. Exp. Neurol. 2022, 349, 113966. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Liu, X.; Zhang, J.; Zhang, Y.; Wen, Y.; Yang, G. Salvianolic acid B exerts protective effects against Aβ-induced neuroinflammation through the inhibition of NLRP3 inflammasome activation and switching of M1/M2 polarization. Tissue Cell 2023, 85, 102260. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, C.; Di, T.; Chen, L.; Zhao, W.; Wei, L.; Zhou, S.; Wu, X.; Wang, G.; Zhang, Y. Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells. Bioengineered 2022, 13, 3486–3502. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Wang, E.; Shi, L.; Ma, C.; Tan, X. Salvianolic acid B attenuates oxidative stress-induced injuries in enterocytes by activating Akt/GSK3β signaling and preserving mitochondrial function. Eur. J. Pharmacol. 2021, 909, 174408. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.; Du, Y. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Zhong, S.; Yu, W.; Wang, J.; Zhu, W.; Yang, T.; Zhao, G.; Jiang, Y.; Li, Y. Effects of continuous LPS induction on oxidative stress and liver injury in weaned piglets. Vet. Sci. 2022, 10, 22. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Tu, F.; Cao, J.; Hou, X.; Chen, Y.; Yan, J. Effects of resveratrol and its derivative pterostilbene on hepatic injury and immunological stress of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2022, 100, skac339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Lai, C.; Chen, Y. Salvianolic acid B ameliorates lipopolysaccharide-induced inflammatory liver injury via deacetylation of NF-κB RelA. Food Biosci. 2025, 68, 2212–4292. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Yan, S.; Sosunov, A.A.; McKhann, G.M.; Yan, S. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2010, 107, 18670–18675. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Al Amir Dache, Z.; Thierry, A.R. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023, 42, 112728. [Google Scholar] [CrossRef]
- Crane, G.M.; Liu, Y.C.; Chadburn, A. Spleen: Development, anatomy and reactive lymphoid proliferations. Semin. Diagn. Pathol. 2021, 38, 112–124. [Google Scholar] [CrossRef]
- Liu, G.; Sun, W.; Wang, F.; Jia, G.; Zhao, H.; Chen, X.; Tian, G.; Cai, J.; Wang, J. Dietary tryptophan supplementation enhances mitochondrial function and reduces pyroptosis in the spleen and thymus of piglets after lipopolysaccharide challenge. Animal 2023, 17, 100714. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-α-induced inflammation by regulating NF-κB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Sun, J.M.; Liu, Y.X.; Liu, Y.D.; Ho, C.K.; Tsai, Y.T.; Wen, D.S.; Huang, L.; Zheng, D.N.; Gao, Y.; Zhang, Y. Salvianolic acid B protects against UVB-induced skin aging via activation of NRF2. Phytomedicine 2024, 130, 155676. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Y.; Gu, F.; Zhuang, Y.; Lv, H.; He, X.; Yang, H.; Lu, J. Salvianolic acid B exerts cerebroprotective effects after traumatic brain injury via Nrf2-dependent antioxidant and anti-inflammatory cascades. Exp. Neurol. 2025, 391, 115305. [Google Scholar] [CrossRef]
- McManus, R.M.; Latz, E. NLRP3 inflammasome signalling in Alzheimer’s disease. Neuropharmacology 2024, 252, 109941. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Li, Q.; Pan, Y.; Xu, L. Salvianolic acid B alleviates myocardial ischemic injury by promoting mitophagy and inhibiting activation of the NLRP3 inflammasome. Mol. Med. Rep. 2020, 22, 5199–5208. [Google Scholar] [CrossRef]
- Li, P.; Li, S.; Wang, L.; Li, H.; Wang, Y.; Liu, H.; Wang, X.; Zhu, X.; Liu, Z.; Ye, F. Mitochondrial dysfunction in hearing loss: Oxidative stress, autophagy and NLRP3 inflammasome. Front. Cell Dev. Bio. 2023, 11, 1119773. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Wu, X.; Zhang, Q.; Xia, G.; Chu, X.; Xia, H.; Lin, S.; Shang, H. Salvianolic acid B alleviated myocardial ischemia-reperfusion injury via modulating SIRT3-mediated crosstalk between mitochondrial ROS and NLRP3. Phytomedicine 2025, 136, 156260. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, A.; Wang, Y.; Guo, L.; Dou, H.; Lu, L.; Rafiq, M.; Li, P.; Chen, X.; Xiao, Q. Salvianolic acid B improves mitochondrial dysfunction of septic cardiomyopathy via enhancing ATF5-mediated mitochondrial unfolded protein response. Toxicol. Appl. Pharmacol. 2024, 491, 117072. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Dou, X.; Wang, R.; Jiang, Y.; Zhang, J.; Qiao, X.; Liu, Y.; Zhang, H.; Lai, C.; Chen, Y.; et al. Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome. Antioxidants 2025, 14, 883. https://doi.org/10.3390/antiox14070883
Wang H, Dou X, Wang R, Jiang Y, Zhang J, Qiao X, Liu Y, Zhang H, Lai C, Chen Y, et al. Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome. Antioxidants. 2025; 14(7):883. https://doi.org/10.3390/antiox14070883
Chicago/Turabian StyleWang, Hao, Xiao Dou, Ruixue Wang, Yuxin Jiang, Jinsong Zhang, Xianjuan Qiao, Yingjun Liu, Hao Zhang, Chenhuan Lai, Yanan Chen, and et al. 2025. "Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome" Antioxidants 14, no. 7: 883. https://doi.org/10.3390/antiox14070883
APA StyleWang, H., Dou, X., Wang, R., Jiang, Y., Zhang, J., Qiao, X., Liu, Y., Zhang, H., Lai, C., Chen, Y., & Yong, Q. (2025). Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome. Antioxidants, 14(7), 883. https://doi.org/10.3390/antiox14070883





