1. Introduction
Skeletal muscle atrophy is defined as the reduction in muscle mass and cross-sectional area as a result of decreased muscle protein content, fiber shrinkage, and imbalances in the processes of protein synthesis and degradation [
1]. This condition diminishes muscle strength and functional capacity. Skeletal muscle atrophy can be classified according to its primary cause. Disuse atrophy arises from prolonged immobilization, which may occur due to accidents or extended bed rest, as well as exposure to microgravity conditions such as those experienced during spaceflight [
2]. In contrast, denervation atrophy is initiated by the loss of neural stimulation, which can result from spinal cord injury, amyotrophic lateral sclerosis, and other conditions [
1]. Various illnesses, including cancer, chronic obstructive pulmonary disease, and heart failure, can lead to a specific form of skeletal muscle wasting known as cachexia. Sarcopenia, which is characterized by age-related muscle loss, is primarily attributed to sedentary lifestyles and hormonal changes associated with aging [
3]. Furthermore, the chronic use of certain medications, particularly corticosteroids, can induce glucocorticoid-induced atrophy [
4].
The World Health Organization assigned sarcopenia an independent disease code (ICD-10 M62.84) in 2016, and the revised Korean Standard Classification of Diseases-8 (KCD-8) also included sarcopenia as a clinical condition in 2021, with diagnostic code M62.5 [
5,
6]. According to a 2024 study based on the Korean National Health and Nutrition Examination Survey, the prevalence of sarcopenia among Koreans aged 60 years and older is reported to be 6.8%, with rates of 5.5% in men and 7.9% in women. Among those aged 80 years and above, the prevalence increases sharply to 21.5% in men and 25.9% in women [
7]. Despite increasing recognition of sarcopenia as a significant health issue, there are currently no approved pharmacological therapies for its treatment either in Korea or globally. Although several investigational agents, including myostatin inhibitors and anabolic compounds, are undergoing clinical trials, none have yet been approved for clinical use [
8].
Skeletal muscle atrophy has profound physiological, metabolic, and functional implications that significantly impact overall health and quality of life. The reduction in muscle strength and endurance leads to a decreased ability to produce force, thereby complicating physical activities, such as walking, standing, and lifting. Given that the skeletal muscle serves as the primary site for glucose uptake, skeletal muscle atrophy can lead to decreased insulin sensitivity, thereby increasing the risk of developing type 2 diabetes, hyperglycemia, and metabolic syndrome due to compromised glucose metabolism [
9,
10]. Furthermore, the skeletal muscle functions as a source of myokines (e.g., interleukin-6 and irisin), which play critical roles in regulating inflammation and immune responses [
11]. Consequently, muscle loss diminishes anti-inflammatory signaling and thus exacerbates chronic inflammation and immune suppression, particularly in the context of aging or disease. Importantly, skeletal muscle atrophy is not merely localized; it exerts systemic effects on metabolism, cardiovascular health, immune function, and psychological well-being [
2]. Therefore, preventive strategies such as regular exercise, adequate protein intake, and early rehabilitation are essential to mitigate the effects of muscle atrophy and enhance outcomes in aging populations, individuals with chronic diseases, and those experiencing immobilization.
Glycine Semen Preparata (GSP), commonly referred to as fermented black soybeans or “Douchi” in traditional Chinese medicine, has been utilized for centuries [
12,
13]. In traditional Chinese medicine, GSP is recognized for its cooling properties, which are believed to be effective in dispelling “heat” and alleviating exterior pathogenic factors. This characteristic aligns with its application in the treatment of various conditions, including fever, irritability, and restlessness [
14,
15]. Soybeans are widely acknowledged as a significant source of plant-based proteins and are processed to obtain diverse products. The preparation of GSP involves the fermentation of black soybeans, a process that may enhance its medicinal properties [
13]. GSP is rich in bioactive compounds, including phenolic acids, flavonoids, and isoflavones, and has demonstrated substantial antioxidant effects [
14,
16]. These compounds reduce oxidative stress by scavenging free radicals, thereby protecting cells from damage and potentially lowering the risk of chronic diseases, such as cardiovascular disease and cancer [
17,
18]. Research indicates that GSP mitigates inflammation by modulating inflammatory mediators, including cytokines and enzymes such as cyclooxygenase-2, rendering it beneficial in the treatment of arthritis and other inflammatory disorders [
19]. Furthermore, studies have suggested that GSP possesses anticancer properties. Fermentation enhances the production of bioactive peptides and isoflavones, such as genistein, which inhibit cancer cell growth, induce apoptosis (programmed cell death), and prevent metastasis [
20,
21,
22]. GSP also exhibits antimicrobial properties by inhibiting the growth of various pathogenic bacteria and fungi. This antimicrobial effect may be attributed to the production of organic acids such as lactic acid during fermentation, which lowers the pH and creates an unfavorable environment for microbial proliferation [
23,
24]. Some studies have suggested that GSP reduces blood cholesterol levels by decreasing cholesterol absorption in the intestines and increasing bile acid excretion [
25]. Additionally, GSP exerts immunomodulatory effects by enhancing macrophagic activity and increasing the production of immune-related cytokines [
13,
14,
19]. Emerging evidence indicates that GSP may have neuroprotective effects, potentially aiding the prevention of neurodegenerative diseases such as Alzheimer’s disease [
13,
26]. The antioxidants and isoflavones present in GSP may help mitigate oxidative damage to the brain, thereby contributing to improved cognitive function. In summary, GSP exhibits a broad range of biological activities, including antioxidant, anti-inflammatory, anticancer, antimicrobial, and neuroprotective activities. These properties are attributed to its rich biochemical composition, which is enriched during fermentation.
Soy-based products have been extensively researched to determine their potential to mitigate muscle atrophy. Both in vitro and in vivo studies have indicated that soy protein and isoflavones may play significant roles in preserving muscle mass and function [
27,
28]. Research into various fermented soybean products has provided valuable insights into their effectiveness in promoting muscle health. For instance, “Miso”, a traditional Japanese fermented soybean paste, suppresses muscle atrophy induced by high-fat/high-sucrose diets in murine models by enhancing the gut microbiota and increasing the production of short-chain fatty acids [
29]. However, studies on the effects of GSP on muscle health are scarce. This study aimed to determine whether the ethanolic extract of GSP can alleviate skeletal muscle atrophy under oxidative stress and elevated corticosteroid levels. Furthermore, we sought to validate the effectiveness of GSP in an in vivo dexamethasone (Dexa)-induced muscle atrophy model and identify the specific compounds responsible for some of its observed activities.
2. Materials and Methods
2.1. Preparation of GSP
Freeze-dried GSP extract was prepared and supplied by KOC Biotech (Daejeon, Republic of Korea) following a standardized protocol. Raw GSP plant materials, processed in accordance with the Korean Pharmacopeia, were purchased from Omniherb (Daegu, Republic of Korea). They were mechanically ground and extracted at 85 °C with 70% ethanol at a volume ratio of 10:1 for 3 h using a reflux extraction system (MS-DM609; MTOPS, Seoul, Republic of Korea). The extract was clarified using a cartridge filter with a 5 µm removal rating (SYP05-25, Synopex, Hwaseong, Republic of Korea), concentrated using a rotary evaporator (Ev-1020, SciLab, Seoul, Republic of Korea), and lyophilized in an LP20 freeze dryer (Ilshin-Bio-Base, Dongducheon, Republic of Korea). The yield of the freeze-dried GSP extract was 5.98%. A voucher specimen (KOC-70E-085) was deposited at the KM Convergence Research Division of the Korea Institute of Oriental Medicine (Daejeon, Republic of Korea).
2.2. Phytochemical Analysis of GSP via High-Performance Liquid Chromatography (HPLC)
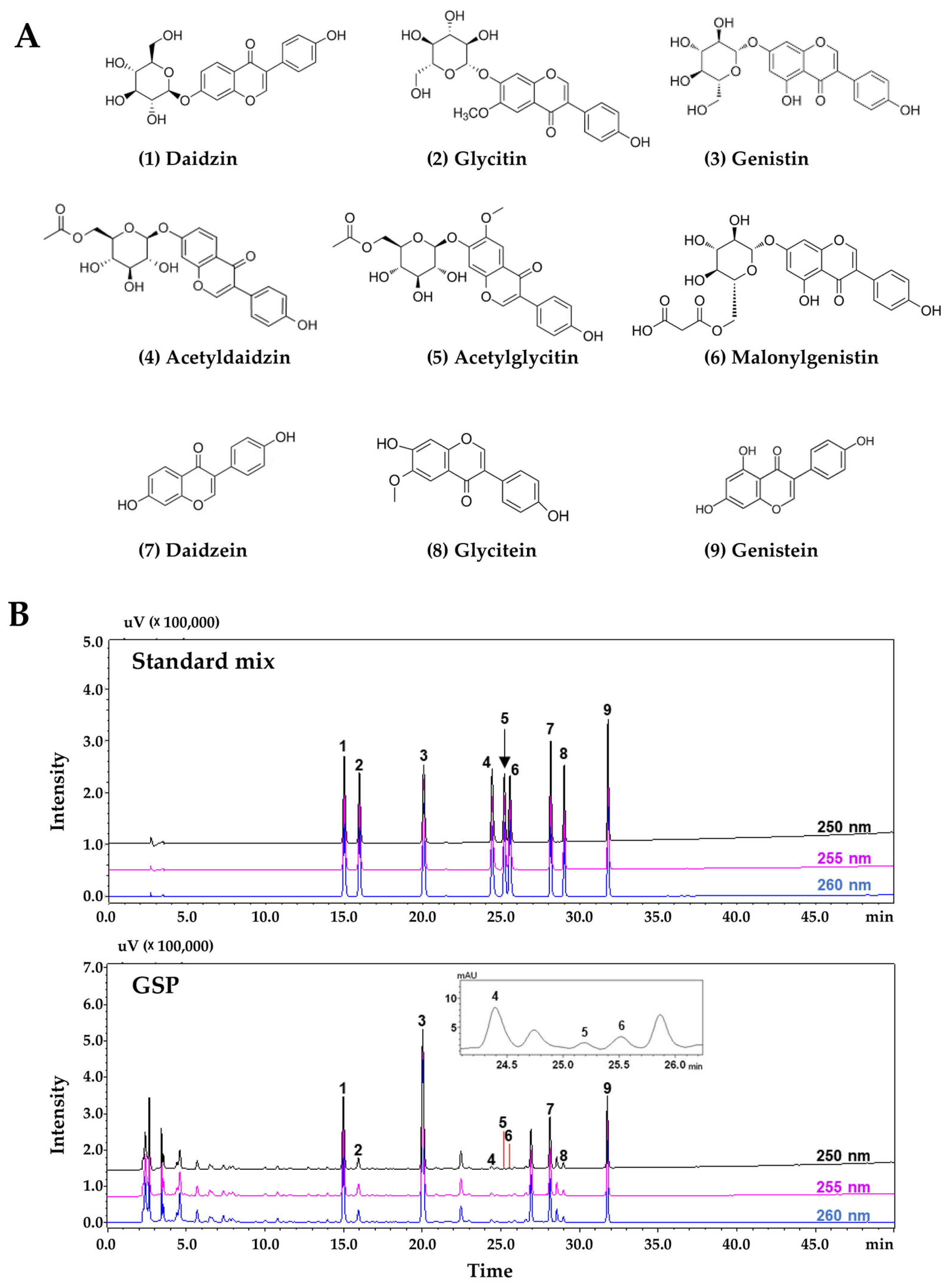
Phytochemical analysis of nine flavonoids (daidzin, glycitin, genistin, acetyldaidzin, acetylglycitin, malonylgenistin, daidzein, glycitein, and genistein) from the GSP extract was performed using a Prominence LC-20A series HPLC system (Shimadzu, Kyoto, Japan) consisting of a solvent delivery unit, online degasser, column oven, autosampler unit, photodiode array detector (PDA), and system controller (LCsolution software, Version 1.24, Shimadzu). These flavonoid compounds were subjected to chromatographic separation using a Capcell Pak UG80 reversed-phase column (250 mm × 4.6 mm, 5 µm, Shiseido, Tokyo, Japan) maintained at 40 °C and gradient elution with a distilled water (solvent A)-acetonitrile (solvent B) mobile phase, with both solvents containing 0.1% (v/v) formic acid. The gradient elution conditions for the mobile phase were as follows: 10% B (initial time), 25% B (40 min), 90% B (45 min, followed by holding for 10 min), and 10% B (60 min, followed by holding for 10 min). The flow rate and injection volume were 1.0 mL/min and 10 µL, respectively. The target compounds were simultaneously scanned at 190–400 nm using a PDA.
2.3. Chemicals
Ascorbic acid (A4544), avertin (T48402), carboxymethylcellulose sodium salt (CMC-Na, C5678), crystal violet solution (V5265), Dexa for in vitro studies (D4902), water-soluble Dexa for animal studies (D2915), dimethyl sulfoxide (DMSO, D8418), hydrogen peroxide (H2O2) solution (#216763), resveratrol (RsV, R5010), sodium dodecyl sulfate (SDS, L3771), 10% formalin (HT5012), 4′,6-diamidino-2-phenylindole (DAPI, D8417), and tert-amyl alcohol (#152463) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Formic acid (#695076) and acetonitrile (#100030) were obtained from Merck KGaA (Darmstadt, Germany). 1,1-Diphenyl-2-picrylhydrazyl (DPPH, #14805) and 2,2′-azino-bis[3-ethylbenzothiazoline-6-sulfonic acid] (ABTS, A2166) were sourced from Cayman Chemical (Ann Arbor, MI, USA) and Tokyo Chemical Industry (Tokyo, Japan), respectively. Tissue-Tek O.C.T. compound was purchased from Sakura Finetek (Torrance, CA, USA). Ethanol (ER1009-500-00), xylene (XR1014-100-01), and Triton X-100 (TR1020) were purchased from Biosesang (Yongin, Republic of Korea). Harris hematoxylin (#3801562) and alcohol-based eosin (#3801602) solutions were obtained from Leica Biosystems (Wetzlar, Germany). A xylene-based mounting solution (#3801730; Leica Biosystems) was used. Nine flavonoid standard compounds used to assess the quality of GSP extracts using the HPLC system were purchased from high-purity compound manufacturers: daidzin (C21H20O9, CAS No. 552-66-9, purity 99.3%, DR10952) and acetylglycitin (C24H24O11, CAS No. 134859-96-4, purity 99.3%, DR12228) from Shanghai Sunny Biotech (Shanghai, China); glycitin (C22H22O10, CAS No. 40246-10-4, purity 99.6%, CFN99105), malonylgenistin (C24H22O13, CAS No. 51011-05-3, purity 98.1%, CFN90631), and glycitein (C16H12O5, CAS No. 40957-83-3, purity 98.9%, CFN99106) from Wuhan ChemFaces Biochemical (Wuhan, China); genistin (C21H20O10, CAS No. 529-59-9, purity 98.3%, BP0635) from Biopurify Phytochemicals (Chengdu, China); acetyldaidzin (C23H22O10, CAS No. 71385-83-6, purity 95.3%, TBW03633) from ChemNorm Biotech (Wuhan, China); and daidzein (C15H10O4, CAS No. 486-66-8, purity 98.0%, D7802) and genistein from (C15H10O5, CAS No. 446-72-0, purity 98.0%, G6649) from Merck KGaA (Darmstadt, Germany). HPLC-grade solvents (methanol, acetonitrile, and water) were purchased from J. T. Baker (Phillipsburg, NJ, USA).
2.4. Cultivation of C2C12 Myoblasts and Their Differentiation into Myotubes
The C2C12 murine myoblast cell line (CRL1772) was obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in growth medium, specifically Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/L glucose, 10% heat-inactivated fetal bovine serum, and 100 IU penicillin/100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. To induce myotube formation, C2C12 myoblasts were placed in growth medium (GM) until confluence and then switched to differentiation medium (DM) consisting of DMEM with 2% heat-inactivated horse serum and 100 IU penicillin/100 μg/mL streptomycin. DM was refreshed every alternate day for 5–7 days. All reagents for cell culture were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
2.5. Cytotoxicity Assay for C2C12 Myoblasts
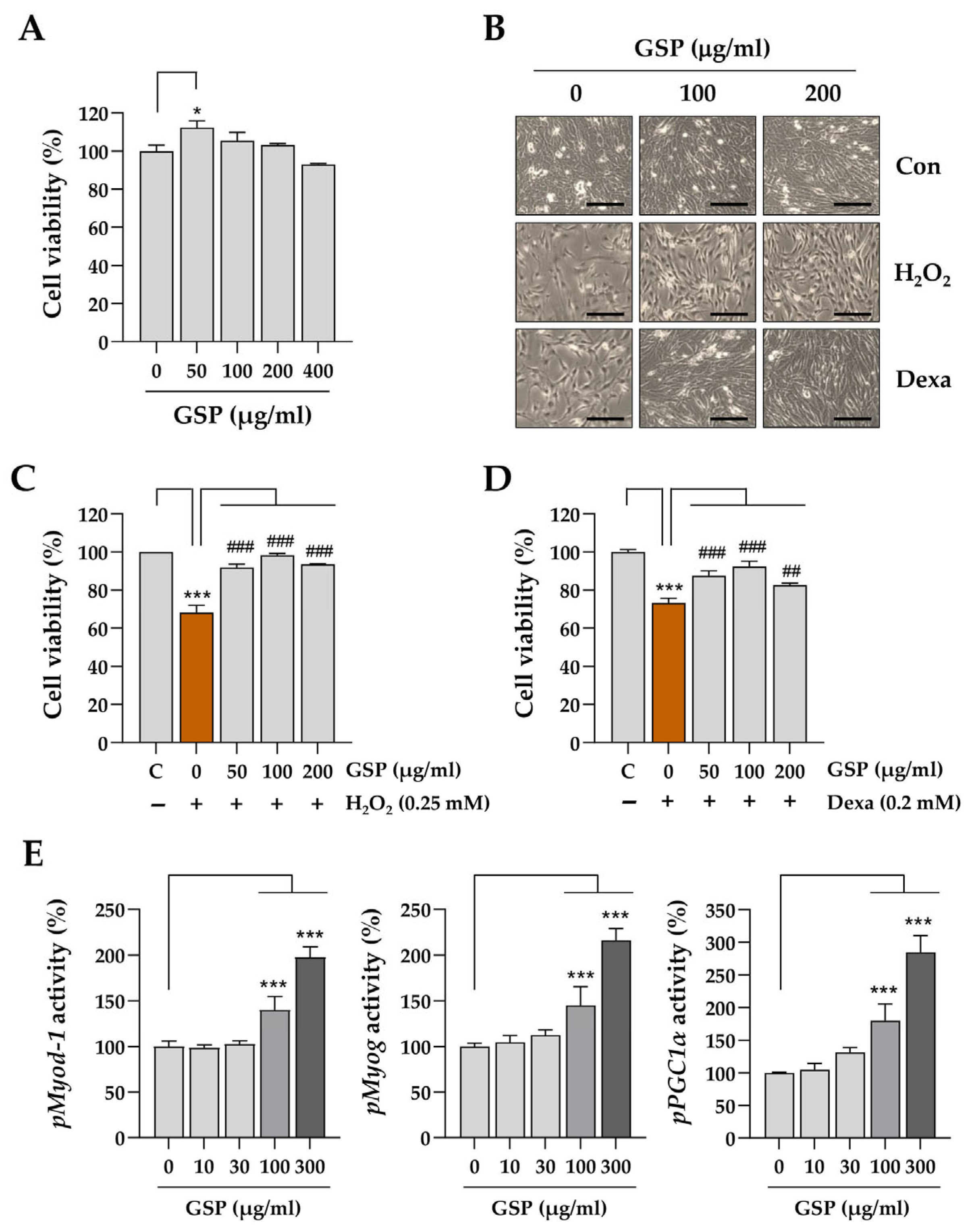
To evaluate the cytotoxic effects of GSP on C2C12 myoblasts, 5000 cells were seeded in each well of a 96-well plate. The cells were treated with GSP or vehicle (0.1% DMSO) after overnight incubation. To investigate the protective effects of GSP under stressful conditions inducing cellular damage, the cells were pretreated with GSP for 12 h and subsequently exposed to 0.25 mM H2O2 or 0.2 mM Dexa. After 24 h, the supernatants were discarded, and the cells were washed twice with phosphate-buffered saline (PBS; Thermo Fisher Scientific). Adherent cells were analyzed with the EZ-Cytox Enhanced Cell Viability Assay Kit (Daeil Lab Service Co., Ltd., Seoul, Republic of Korea) and a SpectraMax i3 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). The viability of these cells was relatively higher than that of vehicle-treated cells.
2.6. Crystal Violet Staining for Assessing Myotube Density
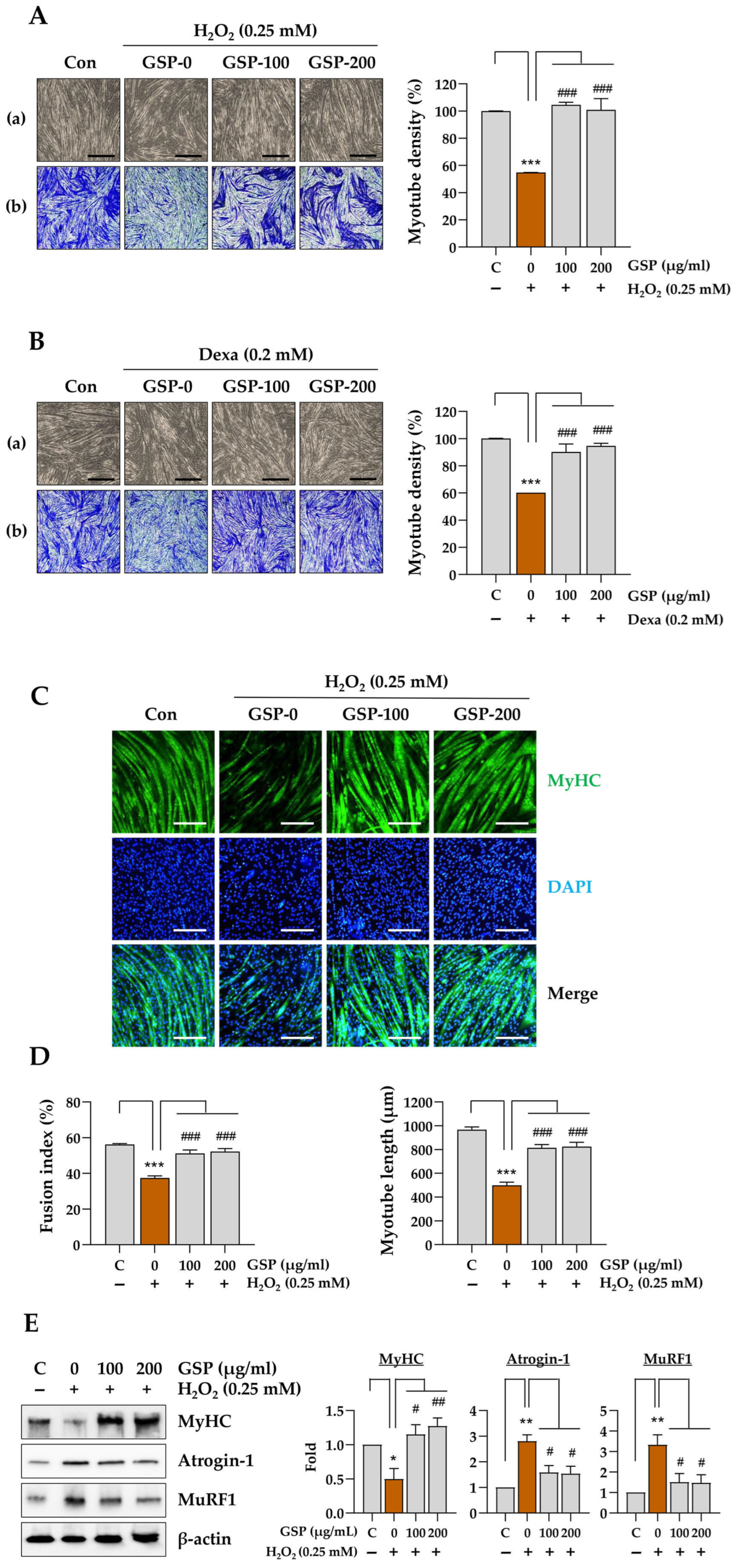
To evaluate the protective effects of GSP and flavonoids against myotube degradation, myotubes differentiated for 5 days were pretreated with GSP, flavonoids, or a vehicle for 12 h, then subsequently treated with 0.25 mM H2O2 or 0.2 mM Dexa for 24–48 h. After washing with PBS, the cells were stained with a crystal violet solution (0.2% crystal violet containing 20% methanol) for 30 min at 25 °C, washed with tap water, dried, and photographed. To quantify myotube density, the stained cells were dissolved in 1% SDS solution for 30 min at 37 °C, and the absorbance was measured at 590 nm using a SpectraMax i3 microplate reader.
2.7. Immunofluorescence Staining
C2C12 myotubes that had been differentiated for 5 days on glass-bottom dishes (SPL Life Sciences, Pocheon, Republic of Korea) were pretreated with GSP for 12 h and then exposed to 0.25 mM H2O2 for 24 h. After rinsing with PBS, the cells were fixed in 10% neutralized formalin, permeabilized with 0.1% Triton X-100, and blocked with 3% bovine serum albumin (Thermo Fisher Scientific) in PBS for 30 min at 25 °C. After washing, they were incubated overnight at 4 °C with a mouse anti-myosin heavy chain (MyHC) antibody (1:1000 dilution, B4470, R&D Systems, Minneapolis, MN, USA) and then treated with an Alexa Fluor 488-conjugated goat anti-mouse IgG antibody (1:1000 dilution, #A11001, Thermo Fisher Scientific) for 3 h at 25 °C. The cells were counterstained with DAPI, then fluorescent images were captured using a fluorescence microscope (IX71; Olympus Co., Tokyo, Japan). The fusion index and myotube length were assessed in five representative images per group using the ImageJ software, version 1.54f (National Institute of Health, Bethesda, MD, USA). The fusion index was calculated using the following formula: Fusion index (%) = (number of nuclei in multinucleated cells/total number of nuclei) × 100.
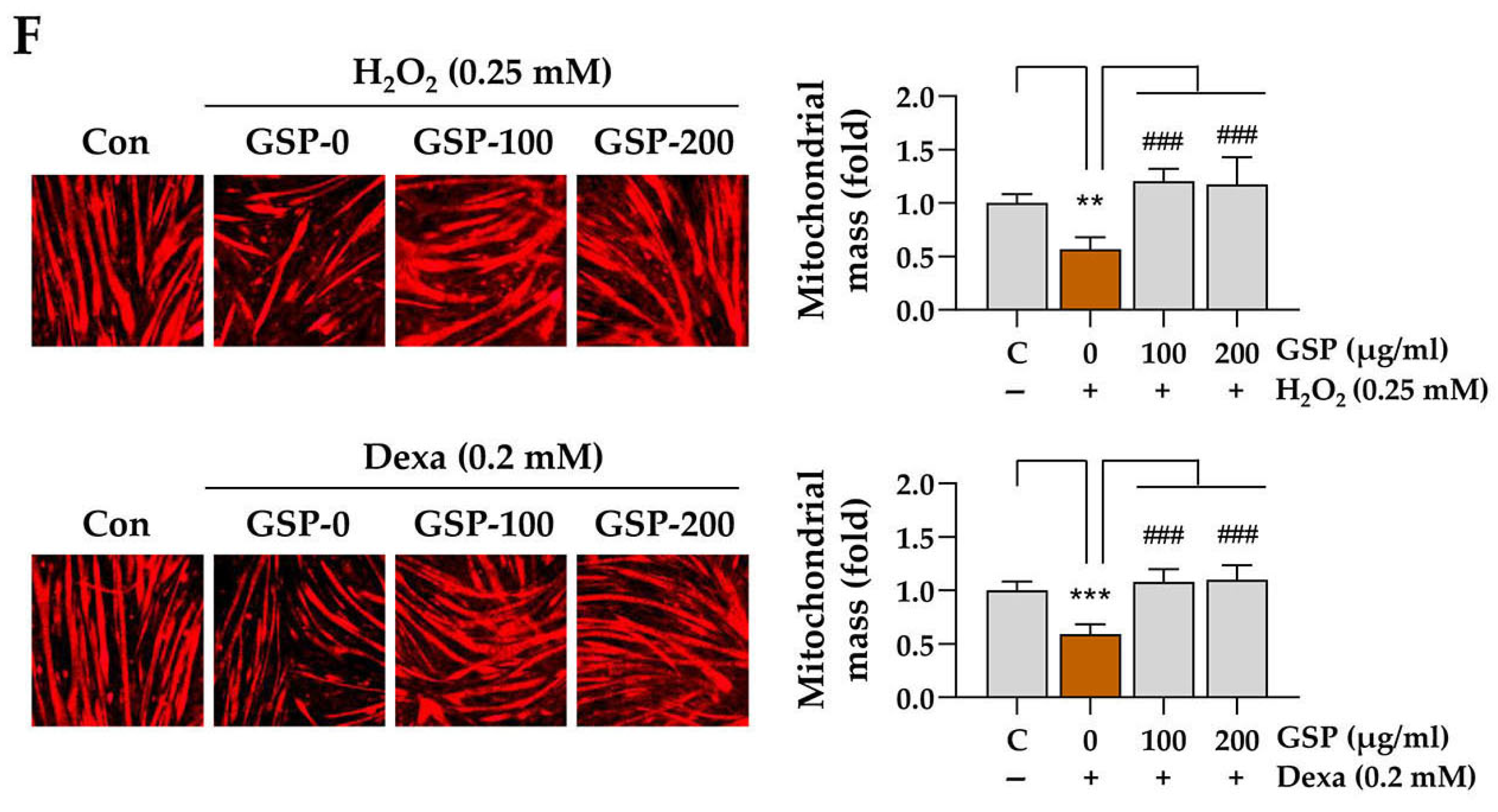
2.8. Quantitation of Mitochondrial Density
C2C12 myotubes were pretreated with GSP for 12 h, followed by exposure to H2O2 for 24 h. After the PBS wash, cells were incubated with 50 nM MitoTracker Deep Red FM (M22426; Thermo Fisher Scientific) at 37 °C for 30 min. Mitochondrial red fluorescence was visualized using a fluorescence microscope (Olympus TH4-200, Olympus Optical Co., Tokyo, Japan), and fluorescence intensity was quantified by ImageJ software, version 1.54f.
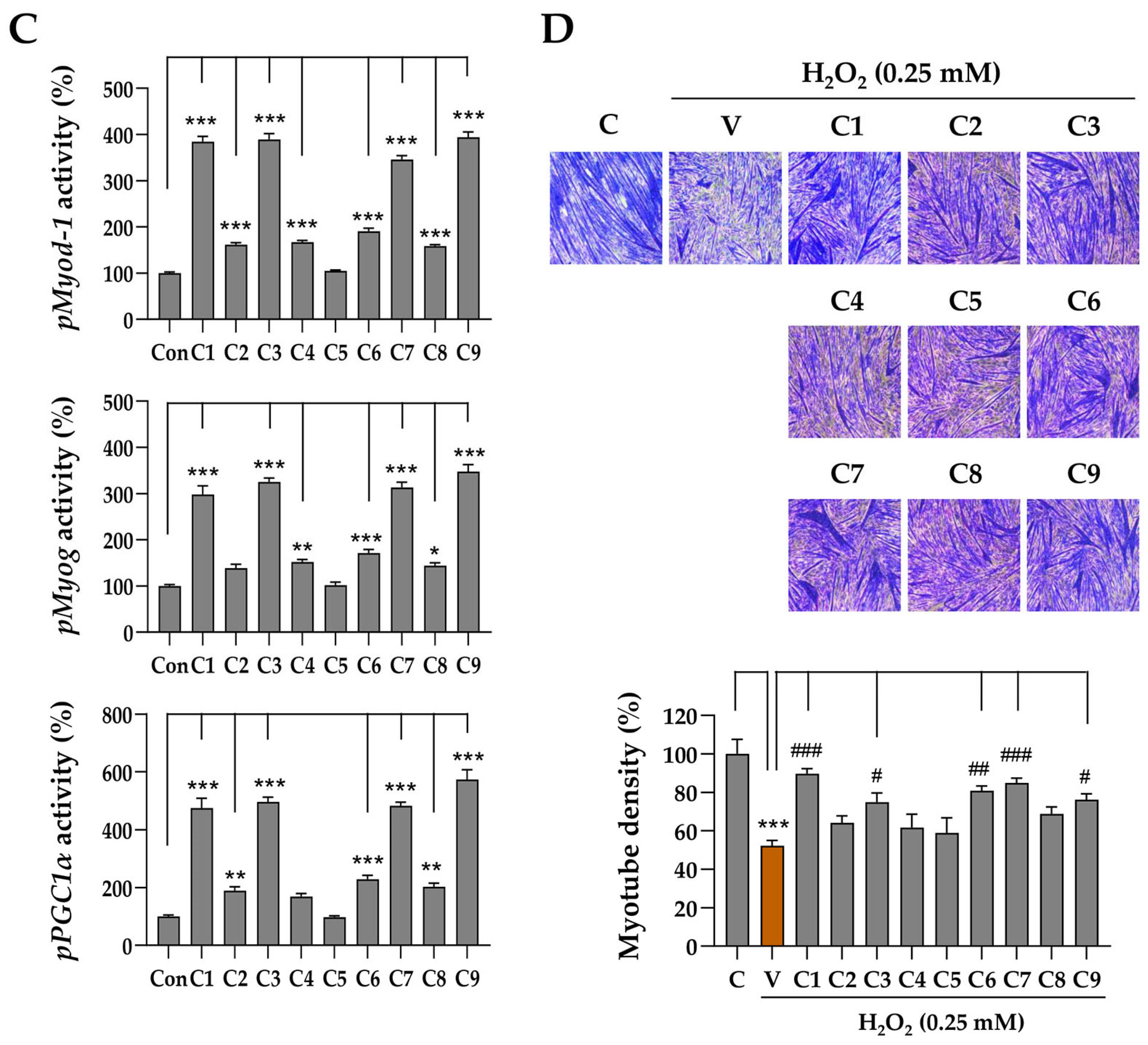
2.9. Promoter Activity Assay
Reporter plasmids for human
Myod1 and
Myog and murine
PGC1α promoter were constructed as described in our previous study [
26]. GSP- and flavonoid-modulated promoter activities were determined using a luciferase assay system. In brief, C2C12 myoblast cells cultured in 60 mm dishes were transfected with 5.5 µg of each reporter plasmid using Lipofectamine 3000 (Thermo Fisher Scientific). After overnight, cells were trypsinized, resuspended in GM without antibiotics, and replated in a 96-well plate at 25,000 cells/cm
2. After another 24 h, the cells were exposed to GSP or flavonoids and were additionally incubated for 24 h. After washing the cells with ice-cold PBS, cell lysates were prepared, and intracellular luciferase activity was determined using a commercial luciferase assay system (E1960, Promega, Madison, WI, USA).
2.10. Detection of Intracellular Reactive Oxygen Species (ROS) Level
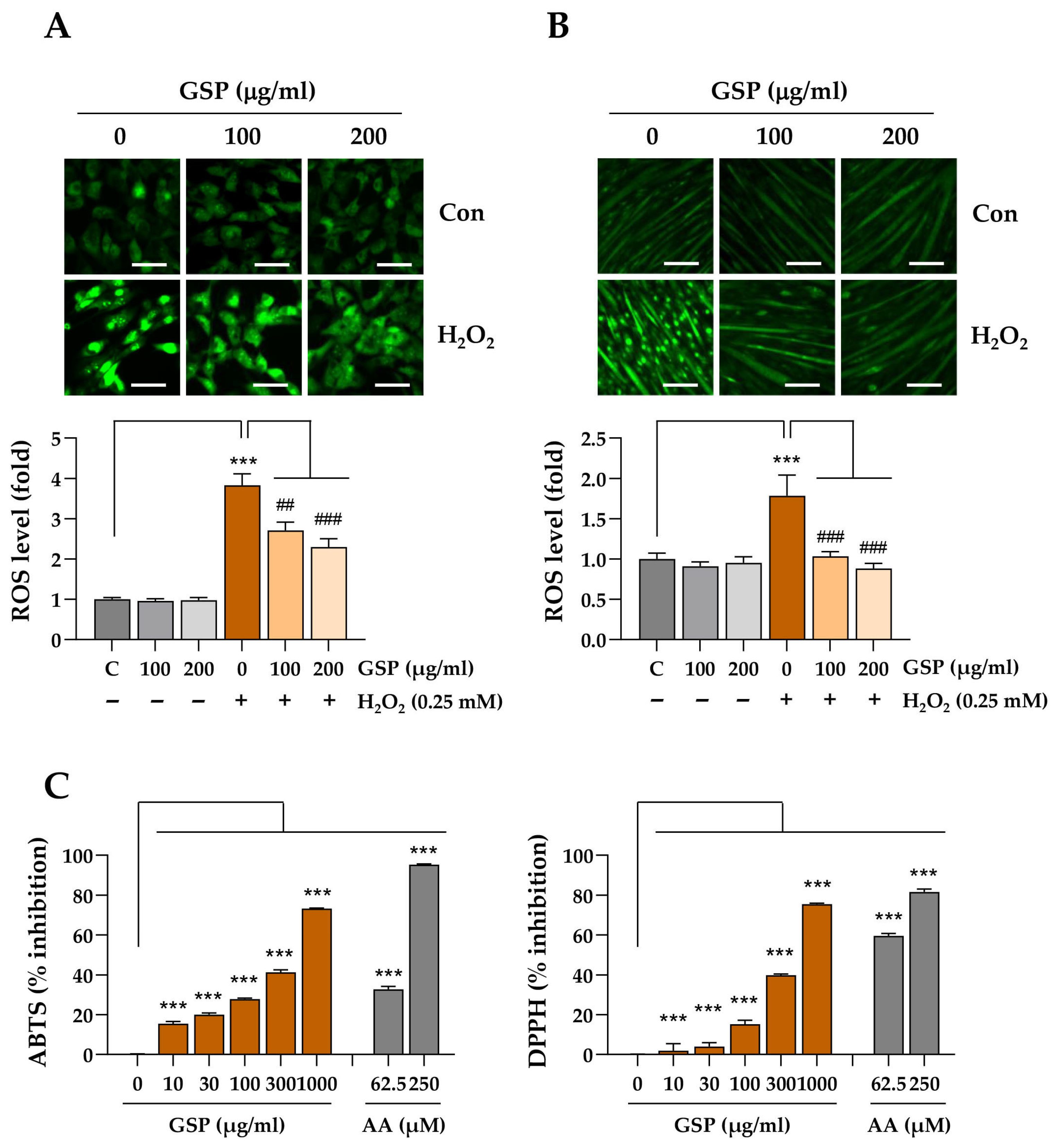
Intracellular ROS in live cells was detected with CellROX™ Green reagent (C10444, Thermo Fisher Scientific). C2C12 myoblasts or differentiation day 5 myotubes were cultured in glass-bottom dishes. The cells were pretreated with GSP or vehicle for 12 h, followed by exposure to 0.25 mM H2O2 for 6 h. Subsequently, the cells were washed with Hanks’ Balanced Salt Solution (HBSS, #14175, Thermo Fisher Scientific) and incubated with 5 μM CellROX™ Green reagent for 15 min at 37 °C. Fluorescence images of the cells were captured after rewashing the cells with HBSS.
2.11. Free Radical-Scavenging Assay
The free radical-scavenging activities of GSP and the flavonoids were determined using ABTS and DPPH assays [
30]. For the ABTS assay, the ABTS free radical stock solution was arranged by mixing equal volumes of ABTS (7.4 mM) and potassium persulfate (2.6 mM) solutions for 2 h, protected from light. The ABST working solution was prepared by diluting deep blue ABTS stock solution with water to an optical density of ~0.7 at 734 nm (OD734). Various concentrations of GSP or flavonoids were mixed with equal volumes of the ABTS working solution in a 96-well assay plate (#439454, Thermo Fisher Scientific). The assay mixture was incubated in the dark for 7 min at 25 °C, and the optical density was measured at OD734 using a SpectraMax i3 microplate reader. For the DPPH assay, a working solution was prepared by dissolving 0.2 mM DPPH in absolute methanol in the dark. The assay was initiated by mixing equal volumes of serially diluted GSP or flavonoids with brown DPPH working solutions. The assay mixture was incubated in the dark for 30 min at 25 °C, and then the optical intensity was determined at OD514. Ascorbic acid was used as a positive control in both the ABTS and DPPH assays. The free radical-scavenging activities of GSP, flavonoids, and ascorbic acid were calculated using the formula: inhibition (%) = (1 − OD of GSP, flavonoids, or ascorbic acid/OD of distilled water) × 100.
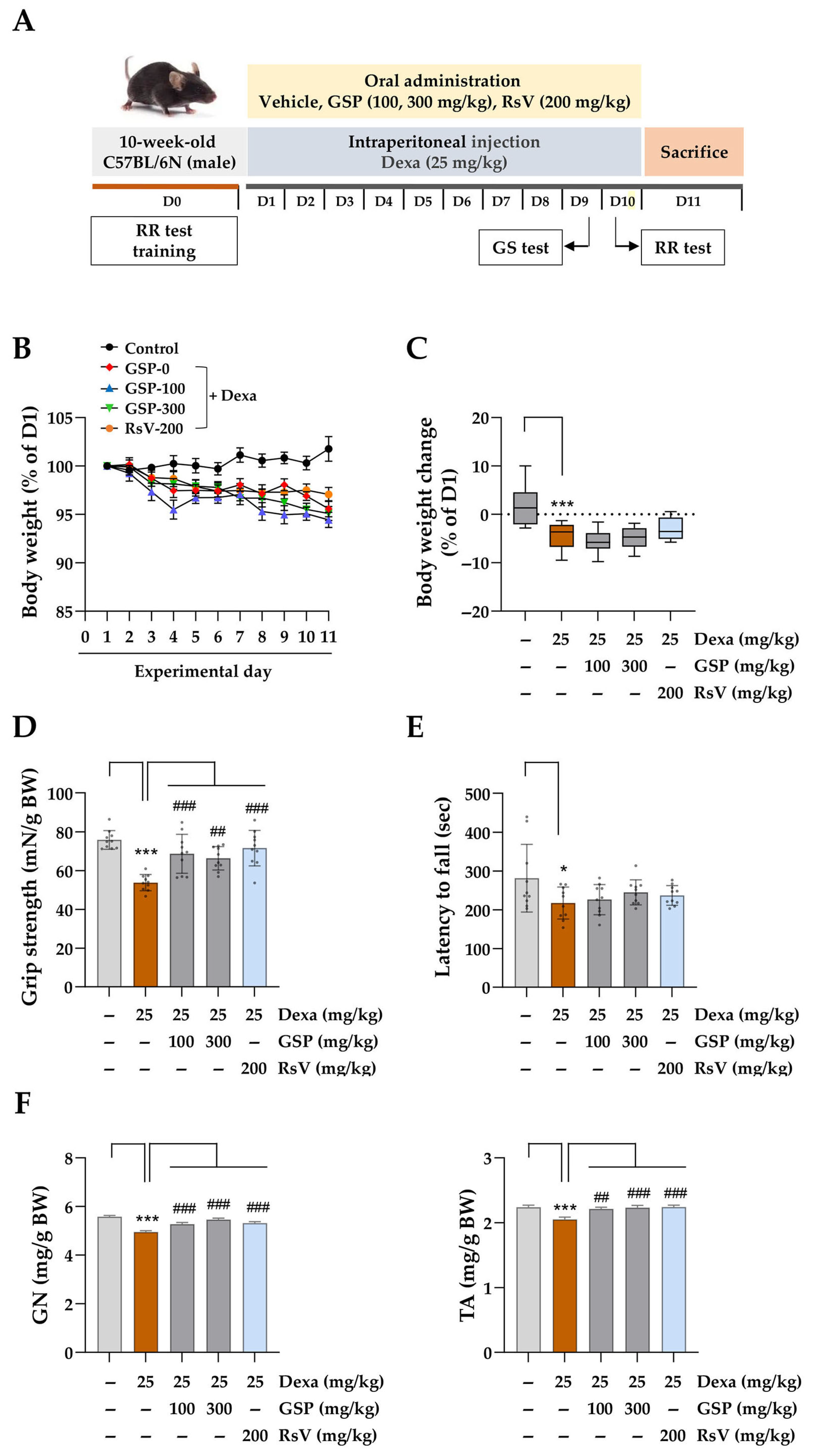
2.12. Induction of Muscle Atrophy and GSP Administration Using C57BL/6N Mice
All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals from the Ministry of Food and Drug Safety, Republic of Korea. The study protocol was approved by the Institutional Animal Care and Use Committee of the Korea Institute of Oriental Medicine (Protocol No. 23-081, approved on 21 August 2023). Nine-week-old male C57BL/6N mice (25–30 g) were purchased from DaeHan BioLink (Samseong-myeon, Republic of Korea) and housed in a specific pathogen-free facility under controlled conditions (22 ± 2 °C, 45 ± 10% humidity, 12/12 h light–dark cycle). Mice had unrestricted access to a standard diet and water. After one week of acclimatization, they were randomly assigned to five groups (n = 10/group) and trained for behavioral assessments on day 0 (D0). Water-soluble Dexa was dissolved in PBS and filtered through a 0.22 µm syringe filter (#17761-ACK, Sartorius Korea, Seongnam, Republic of Korea). GSP and RsV were resuspended in sterile 0.5% CMC-Na in PBS. GSP and positive control solutions were stored at −80 °C until use. Starting on experimental day 1 (D1), the mice received oral GSP at 100 mg/kg (group 3) or 300 mg/kg (group 4) daily for 10 days. The positive control group (group 5) received 200 mg/kg RsV, whereas groups 1 and 2 received 0.5% CMC-Na in PBS. Thirty minutes after oral administration, the mice were intraperitoneally injected with sterile PBS (group 1) or 25 mg/kg Dexa (groups 2–5) following established protocols.
2.13. Grip Strength Test and Rotarod Test
On experimental day 9 (D9), a grip strength test was conducted with a grip strength meter (DJ-356, Daejong Instrument Industry Co., Seoul, Republic of Korea) to evaluate muscle strength in mice. The mice were placed on a wire grid, and their grip strength was measured by pulling them back by their tails until they released the wire. This was repeated five times for each mouse, and the average was calculated. On experimental day 10 (D10), motor coordination and balance were evaluated using ROTA-ROD (B.S. Technolab Inc., Seoul, Republic of Korea). Initially, the mice were acclimated to the rod for 30 s to allow walking and balancing. The rotational speed of the rod was then increased according to the following program: holding at 4 rpm for 1 min, followed by gradual acceleration from 4 to 40 rpm over 5 min and maintenance at 40 rpm for 5 min. The time until the mouse fell off the rod or exhibited repeated passive rotation was recorded, and the average time of three trials was calculated.
2.14. Isolation of Muscle Tissue
On experimental day 11 (D11), the mice were euthanized by intraperitoneal injection of 2% avertin in PBS at 240 mg/kg. Gastrocnemius (GN) and tibialis anterior (TA) muscle tissues were excised from hind limbs and weighed. The tissues were snap-frozen in a dry ice–acetone bath for 10 s, transferred to pre-cooled 1.8 mL cryogenic tubes (#375418PK, Thermo Fisher Scientific) containing cotton pre-wetted with PBS to minimize tissue dryness, and stored at −80 °C for histological analysis.
2.15. Cryosectioning and Hematoxylin and Eosin (H&E) Staining of Muscle Tissues
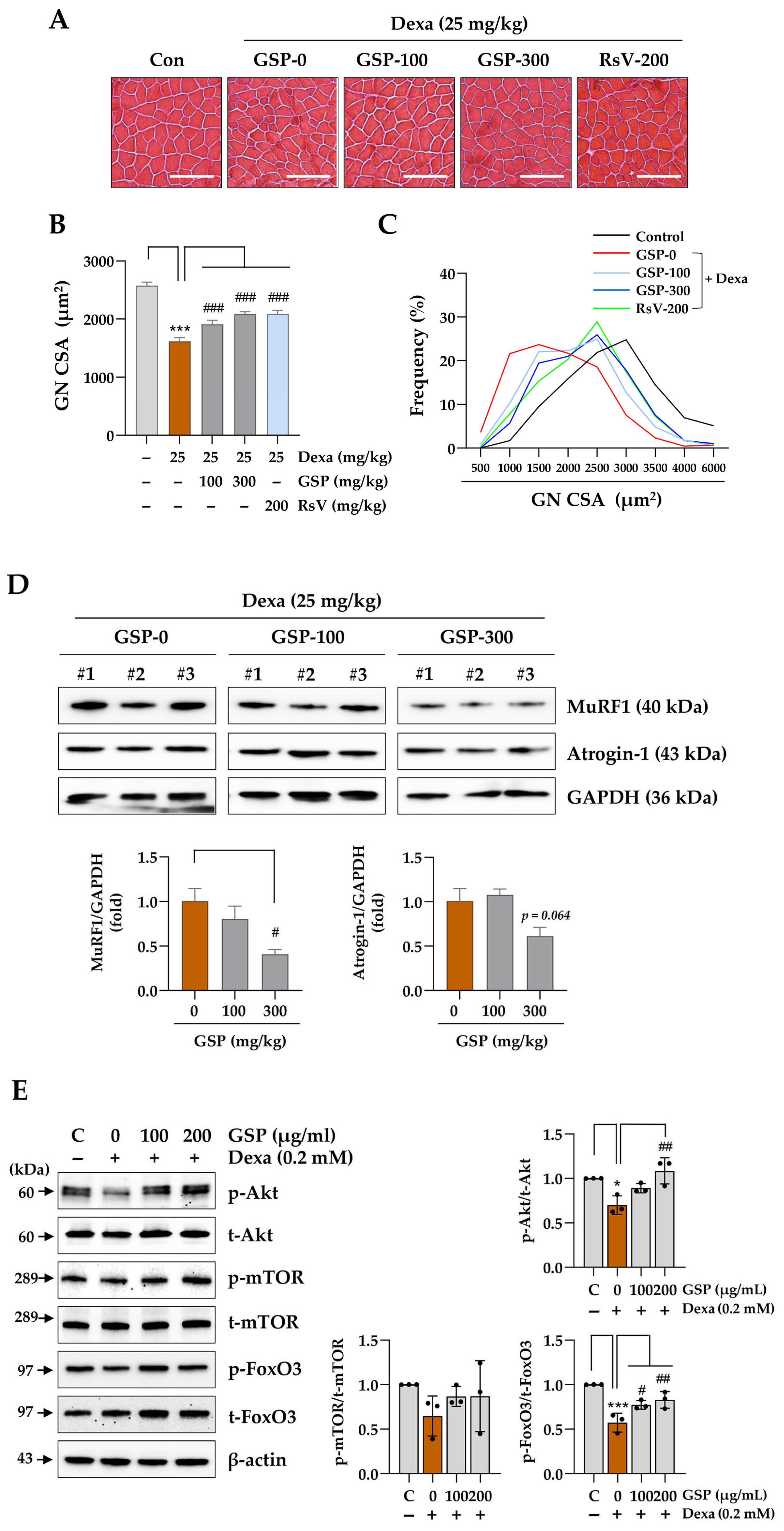
Prior to cryosectioning, the cryostat (CM3050S, Leica Biosystems) and blade were pre-cooled to −22 ± 2 °C. Frozen tissues were equilibrated within the cryostat chamber for 10 min, affixed to metallic holders with O.C.T. compound, and rapidly frozen on dry ice. A 30 µm thick section was obtained on a positively charged slide pre-equilibrated at 22–24 °C and stored at −80 °C. For H&E staining, the slides were equilibrated at 22–24 °C for 30 min and stained with hematoxylin for 10 min. After rinsing with running tap water, they were counterstained with eosin for 1 min and washed thoroughly with distilled water. They were then dehydrated using ethanol with serially increasing concentrations: 70% for 1 min, 80% for 1 min, 90% for 1 min, and 100% for 1 min. Subsequently, the slides were cleared in a 1:1 solution of ethanol and xylene for 3 min and immersed in 100% xylene for 3 min, then mounted with a xylene-based mounting solution and allowed to dry overnight under a fume hood. Images were captured using a BX43 microscope with a DP73 digital camera (Olympus, Tokyo, Japan), and the cross-sectional area (CSA) of more than 100 muscle fibers was quantified using ImageJ software, version 1.54f.
2.16. Western Blotting
Total protein was extracted from cells or frozen tissues using M-PER™ Mammalian Protein Extraction Reagent (#78501, Thermo Fisher Scientific) or T-PER™ Tissue Protein Extraction Reagent (#78510, Thermo Fisher Scientific), both of which contain Halt™ Protease and Phosphatase Inhibitor Cocktail (#78444, Thermo Fisher Scientific). Tissues were homogenized using the PreCellys instrument (Bertin Instruments, Montigny-le-Bretonneux, France), then centrifuged at 13,000 rpm for 15 min at 4 °C. Clear lysates were analyzed to determine the protein concentration using bicinchoninic acid assay (#23227, Thermo Fisher Scientific). Proteins of equal amounts were separated by SDS-PAGE and transferred to an Immobilon-P PVDF membrane (IPVH00010, Millipore, Bedford, MA, USA). After blocking the membrane with EzBlock Chemi Solution (AE-1475, ATTO Korea, Daejeon, Republic of Korea), it was incubated overnight at 4 °C with a primary antibody (1:1000 dilution). After rinsing the membrane with 0.1% Tween 20 detergent in Tris-buffered saline, it was treated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:4000 dilution) for 1 h at 25 °C. Target proteins were detected using SuperSignal West Femto Maximum Sensitivity Substrate (#34096; Thermo Fisher Scientific) and ImageQuant LAS4000 Mini (GE Healthcare, Piscataway, NJ, USA). Protein levels were quantified using ImageJ software, version 1.54f after normalization to β-actin or glyceraldehyde 3-phosphate dehydrogenase levels. The antibodies employed in this study included anti-MyHC (MAB4470), anti-muscle atrophy F-box (MAFbx/Atrogin-1, ab168371), anti-phosphorylated FoxO3 (p-FoxO3; s253, ab154786), heme oxygenase 1 (HMOX1, ab13248), glutathione peroxidase 1 (GPX1, ab22604), superoxide dismutase 1 (SOD1, ab51254), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α, 66369-lg), anti-muscle ring-finger protein-1 (MuRF1, sc-398608), anti-β-actin (sc-47778), myogenin (MYOG, sc-52903), MYOD1 (sc-32758), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, sc-32233), sourced from R&D Systems, Abcam (Cambridge, UK), Proteintech (Rosemont, IL, USA), and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Antibodies against nuclear factor-like 2 (NRF2, #12721), phosphorylated Akt (p-Akt; s473, #9271), Akt (#4685), phosphorylated mTOR (p-mTOR; s2448, #2971), mTOR (#2983), FoxO3 (#9467), HRP-conjugated anti-rabbit IgG (#7074), and anti-mouse IgG (#7076) were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.17. Statistical Analysis
Data analysis was performed using GraphPad Prism (version 9.5.1, GraphPad Software, San Diego, CA, USA). The results are presented as the mean ± standard error of the mean (SEM) derived from multiple trials. Group differences were evaluated using one-way analysis of variance followed by Dunnett’s multiple comparison test, with statistical significance set at p < 0.05.
4. Discussion
Skeletal muscle atrophy results from an imbalance between the rates of muscle protein synthesis and degradation. It is frequently associated with natural aging (sarcopenia), malnutrition, prolonged disuse, and, most importantly, pathological conditions such as diabetes, obesity, cachexia, and Alzheimer’s disease [
2,
3,
34,
35,
36]. Muscle atrophy can lead to poor quality of life, increased risk of fractures, and reduced basal metabolic rate [
37]. To date, Food and Drug Administration-approved treatments for muscle atrophy, such as nusinersen and risdipam, are primarily used for specific muscle-related conditions, such as spinal muscular atrophy [
38]; however, limited medications are available for preventing or treating general muscle loss or muscle atrophy. This study provides compelling and robust evidence that an ethanolic extract of GSP, which is prepared from fermented black soybeans, exerts protective effects against oxidative stress-induced and glucocorticoid-induced muscle atrophies, which contribute significantly to muscle degeneration and dysfunction.
Dexa, an anti-inflammatory and immunosuppressive agent, is used clinically to manage various pathological conditions, including chronic obstructive pulmonary disease, asthma, rheumatoid arthritis, cancer, and allergic responses. However, prolonged high-dose Dexa administration may result in a range of adverse effects, particularly muscle weakness accompanied by reduced muscle mass [
4,
39]. Therefore, Dexa is frequently used to establish preclinical muscle atrophy models. Dexa can reliably induce muscle atrophy by suppressing the PI3K/Akt anabolic pathway and upregulating MuRF1- and Atrogin-1-mediated catabolic muscle degradation, which mimics clinical situations [
40]. Therefore, we used experimental animals with Dexa-induced muscle atrophy to investigate the protective potential of GSP against glucocorticoid therapy-associated muscle loss.
Soybeans are an important ingredient in Korean cuisine and are considered a good source of vegetable proteins. They are rich in antioxidants, including anthocyanins, polyphenols, and flavonoids, which may reduce the risk of chronic diseases related to oxidative stress [
41]. Bioconversion processes, such as soybean fermentation, can enhance the bioavailability of nutrients in soybeans and increase the content of isoflavone aglycones, including daidzein, glycitein, and genistein, compared to non-fermented soybeans. Isoflavone aglycones have higher antioxidant potential than isoflavone glucosides such as daidzin, glycitin, and genistin [
42]. Other studies have shown that soybean fermentation increases anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines, nitric oxide, and prostaglandin E2 by lipopolysaccharide-activated immune cells [
43], thereby relieving inflammatory pain in experimental mice. These findings suggest that bioconversion processes such as fermentation can boost the nutraceutical value of soybeans [
43].
In this study, we evaluated the in vitro and in vivo protective effects of the ethanolic extract of GSP, which is prepared from fermented black soybeans, using Dexa-induced muscle atrophy models. The in vitro experiments demonstrated that GSP can protect undifferentiated C2C12 myoblasts (
Figure 1) and differentiated C2C12 myotubes (
Figure 2) from H
2O
2-mediated oxidative stress and cytotoxicity of Dexa at high concentrations, which induced muscle atrophy in C2C12 cells. The maintenance of myotube integrity was further supported by the preservation of MyHC expression, inhibition of muscle degradation-associated proteins such as Atrogin-1 and MuRF1, and upregulation of mitochondrial biogenesis. Such protective effects may be related to its antioxidant potential in terms of free radical-scavenging activity and decreased intracellular ROS production in C2C12 myoblasts and myotubes following GSP pretreatment and H
2O
2 treatment (
Figure 3), which may be ascribed in part to the upregulation of antioxidant activity-related genes (
Supplementary Figures S2 and S3). The muscle-protective potential of GSP was also supported by the downregulation of muscle degradation marker proteins MuRF1 and Atrogin-1 following H
2O
2-mediated oxidative stress challenge (
Figure 2E). In addition, GSP itself enhanced the expression of genes related to myogenesis (
Myod1 and
Myog) and mitochondrial energetics (
Pgc1α) (
Figure 1E and
Supplementary Figure S1). MYOD1 and MYOG are transcription factors essential for the initiation and progression of myogenesis, while PGC1
α is a crucial regulator of mitochondrial biogenesis and muscle fiber differentiation. The promotion of the expression of these genes by GSP indicates that it not only protects muscle cells from cytotoxicity but also enhances myoblast differentiation, a critical process in muscle regeneration. These findings suggest that GSP promotes muscle regeneration and repair under stressful conditions such as exposure to oxidative stress or glucocorticoids, contributing to muscle health maintenance and recovery.
Animal studies using mice with Dexa-induced muscle atrophy demonstrated that oral GSP administration significantly reduced both muscle wasting and dysfunction associated with the chronic Dexa toxicity (
Figure 4). Although the effects of GSP co-administration were not statistically significant, GSP-treated mice showed improved motor coordination in the rotarod test, further supporting the hypothesis that GSP has a beneficial effect on muscle function under muscle atrophy. The muscle-protective effect of GSP was related to the restoration of muscle fiber integrity and downregulation of the muscle degradation marker proteins MuRF1 and Atrogin-1 (
Figure 5), which are hallmarks of glucocorticoid-induced muscle atrophy. In addition, the proportion of fibers within the larger CSA range was significantly increased compared to that in the Dexa-only-treated group, whereas the proportion of smaller fibers decreased. This shift in muscle fiber distribution suggests that GSP helped to preserve larger muscle fibers, which are essential for maintaining overall muscle mass and strength. This protective effect of GSP is consistent with the results of previous studies reporting the beneficial effects of soybeans on muscle health. For example, soy protein supplementation has been shown to enhance the repair of damaged muscles, increase muscle growth, delay fatigue from high-intensity exercise, and enhance physical performance, which are all associated with improved lean body mass and reduced oxidative stress [
44].
Oxidative stress, caused by an imbalance between ROS production and the antioxidant defense system of our body, can trigger NF-κB pro-inflammatory signaling pathways, which accelerate muscle protein degradation by activating ubiquitin–proteasome and autophagy–lysosomal pathways [
45,
46]. In experimental mice, chronic administration of Dexa resulted in the accumulation of oxidative stress markers and significantly induced the expression of the oxidative stress-sensitive ubiquitin ligase Cbl-b [
47]. In contrast, the alleviation of oxidative stress successfully attenuates Dexa-induced muscle atrophy in experimental mice [
48], suggesting the therapeutic potential of antioxidants in muscle atrophy induced by chronic glucocorticoid use. This is consistent with the results of the present study showing the muscle-protective effect of GSP via antioxidant activity against Dexa-induced muscle atrophy. Some isoflavones with antioxidant activity in soybeans prevent sarcopenic muscle loss by blocking the expression of MuRF1, a protein marker responsible for muscle degradation, and androgen receptors related to muscle mass growth [
49]. Genistein and daidzein protect C2C12 myotubes from pro-inflammatory cytokine tumor necrosis factor-alpha-mediated myotube atrophy via upregulation of the AMP-activated protein kinase-Sirtuin 1 axis [
50]. In our cell-free ABTS and DPPH free radical-scavenging assays, genistein (C9) showed higher scavenging activity against ABTS free radicals but similar activity against DPPH free radicals compared to its glycoside genistin. In contrast, daidzein (C7) showed lower scavenging activity against ABTS free radicals but similar activity against DPPH free radicals compared to its glycoside daidzin (C1) (
Supplementary Figure S6), consistent with the results of a previous study [
51]. However, daidzein showed comparable or higher antioxidant potential than daidzin in other antioxidant activity assays, such as the copper-chelating activity assay [
52]. Other unidentified components in the GSP extract may also contribute to its antioxidant potential.
It is well known that isoflavones from soybeans, such as daidzein, genistein, and glycitein, may reduce muscle atrophy by blocking the expression of MuRF1 or by regulating androgen receptors [
49,
50]. To identify the components responsible for the promoter-enhancing activity of GSP related to myogenesis and mitochondrial bioenergetics (
Figure 1E), nine individual GSP components were evaluated for their promoter-enhancing potential in C2C12 myoblasts. The results demonstrated that daidzin (C1), genistin (C3), and their aglycones daidzein (C7) and genistein (C9) exhibited greater potential in enhancing the activity of the
Myod1,
Myog, and
Pgc1α promoters compared to other isoflavones (
Figure 6D). The promoter-enhancing activity of these components was associated with their protective effects on C2C12 myotubes exposed to high doses of Dexa (
Figure 6D). Myogenesis involves the activation and differentiation of quiescent satellite cells in response to muscle damage and therefore plays a key role in the repair process following muscle injury [
53]. Additionally, mitochondrial dysfunction can accelerate muscle atrophy by limiting the energy supply and increasing ROS levels [
54]. Therefore, enhancing the promoter activity of genes associated with myogenesis and mitochondrial energetics may help recover from or protect against muscle damage caused by chronic high-dose administration of glucocorticoids. Bioactive compounds from GSP, which significantly enhanced the promoter activity of
Myod1,
Myog, and
PGC1α, reinforce the notion that flavonoids play a crucial role in mediating the beneficial effects of GSP on muscle health. Future studies should investigate the individual and synergistic bioavailabilities and pharmacokinetic contributions of these flavonoids to muscle preservation.