Abstract
Diabetic retinopathy (DR), a leading cause of blindness in working-age adults, arises from chronic hyperglycemia-induced oxidative stress, inflammation, and vascular dysfunction. Current therapies such as laser photocoagulation, intravitreal anti-vascular endothelial growth factor (VEGF) agents, and steroids target advanced stages but fail to prevent early neuronal and microvascular damage. Emerging evidence highlights oxidative stress as a key driver of DR pathogenesis, disrupting the blood-retinal barrier (BRB), promoting neurodegeneration and angiogenesis. Advances in imaging, particularly optical coherence tomography angiography (OCTA), enable earlier detection of neurodegeneration and microvascular changes, underscoring DR as a neurovascular disorder. Polyphenols, such as resveratrol, curcumin, and pterostilbene, exhibit multitarget antioxidant, anti-inflammatory, and anti-angiogenic effects, showing promise in preclinical and limited clinical studies. However, their low bioavailability limits therapeutic efficacy. Nanotechnology-based delivery systems enhance drug stability, tissue targeting, and sustained release, offering potential for early intervention. Future strategies should integrate antioxidant therapies and precision diagnostics to prevent early irreversible retinal damage in diabetic patients.
1. Introduction
Diabetes mellitus (DM), a chronic metabolic disease characterized by sustained hyperglycemia that damages multiple organ systems, is among the top ten causes of death worldwide [1,2]. Its prevalence continues to rise, with an estimated 46% increase projected by 2045, according to the International Diabetes Federation [3]. The mortality associated with DM, along with reduced quality of life and productivity, is largely driven by its microvascular and macrovascular complications [4]. Diabetic retinopathy (DR) is considered one of the most common microvascular consequences of DM. DR is a progressive, asymptomatic neurovascular complication that leads to irreversible retinal damage [5], which represents the leading cause of vision loss among working-age adults (20–65 years) and the fifth leading cause of blindness worldwide [6,7]. In its early stages, chronic hyperglycemia disrupts the integrity of the blood-retinal barrier (BRB), increasing vascular permeability and potentially leading to capillary occlusion, which characterizes non-proliferative diabetic retinopathy (NPDR). In advanced stages, retinal hypoxia induces pathological neovascularization, delineating the transition to proliferative diabetic retinopathy (PDR). Additionally, fluid accumulation in the macula can result in diabetic macular edema (DME), compromising central visual acuity [8,9,10].
Approximately one-third of individuals with DM will develop DR, with a prevalence reaching 77.3% in DM type 1 (DM1) and 25.1% in DM type 2 (DM2) [11,12]. Among DM2 patients, 6.99% of cases occur in pediatric populations [13]. Notably, DR is the only cause of blindness whose prevalence has steadily increased over the past four decades (1980–2018) [14]. Moreover, its prevalence is expected to continue rising in the absence of effective therapeutic strategies, with a growing burden in low- and middle-income regions [15,16].
DR remains predominantly asymptomatic in its early stages, delaying diagnosis and allowing progression to advanced stages with irreversible damage [17]. Throughout this process, oxidative stress induced by chronic hyperglycemia plays a crucial role in both vascular and neuronal deterioration of the retina [18,19,20,21]. In this review, our aim is to highlight the limitations of currently used conventional therapies and, based on the oxidative etiology of DR, to explore in depth the prevention of disease progression through the use of natural polyphenols.
2. Conventional Therapies and Therapeutic Effects
Visual impairment in patients with DR can be caused by the development of DME, which implies the presence of fluid in the macula (the central area of the retina) and can occur at any stage of the disease. Visual loss may also result from bleeding or tractional retinal detachments due to the presence of neovessels; in the context of PDR, this is the most advanced stage of DR. According to a 2021 meta-analysis, the prevalence of DME was estimated to be approximately 4.07% among the diabetic population, while vision-threatening DR affected about 6.17% of people in this category [7]. Most patients may remain visually asymptomatic for long periods of time if they do not develop any of the mentioned situations. For this reason, screening visits are recommended, generally involving periodic fundus photographs, which should be performed three to five years after the diagnosis of DM1 and from the time of diagnosis in DM2 [22,23].
In routine clinical practice, treatments applied according to the DR management guidelines are laser retinal photocoagulation, intravitreal injections, and vitreoretinal surgery. Figure 1 summarizes the indications for each treatment based on the degree of DR according to the International Classification of Diabetic Retinopathy Severity Scale [24]. These conventional therapies are used in cases of severe NPDR, PDR, or DME [22,23].
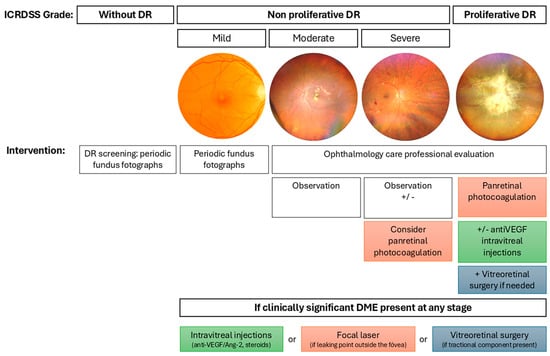
Figure 1.
Management of DR according to severity based on the International Classification of Diabetic Retinopathy Severity Scale. The figure outlines the clinical approach to DR across its progressive stages: no apparent DR, NPDR (mild, moderate, severe), and PDR. Representative fundus photographs illustrate the retinal appearance at each stage. Recommended interventions are indicated for each level of severity, ranging from periodic fundus imaging and observation to panretinal photocoagulation, intravitreal injections (anti-VEGF, angiopoietin-2 (Ang-2) inhibitors, corticosteroids), and vitreoretinal surgery when necessary. Additionally, treatment options for clinically significant diabetic macular edema (DME) are specified, depending on the presence and anatomical characteristics of the edema.
Laser photocoagulation is indicated in any case of PDR and in cases of severe NPDR where rapid progression to PDR is expected, when the following risk factors are present: pregnancy, contralateral PDR, non-compliance with appointments, poor glycemic control, large ischemic areas, or the presence of cataract-impeding laser application in the short or medium term. In these cases, a panretinal photocoagulation (PRP) is performed, applying laser to the entire peripheral retina, while sparing the macular area, which is responsible for visual acuity (VA) [22,23]. Conventional argon laser treatment causes thermal burns in the outer retina. This reduces the oxygen demands from the ischemic retina while allowing oxygen supply from the choriocapillaris to diffuse into the inner retina, thereby decreasing vascular endothelial growth factor (VEGF) production. This promotes the regression of existing neovascularization and prevents the formation of further erratic neovessels [25]. Side effects would include peripheral visual field loss [22,25]. New instruments or techniques with less aggressive parameters are currently used to minimize retinal tissue damage. These include Pattern Scanning Laser Photocoagulation, subthreshold diode micropulse photocoagulation, navigated laser photocoagulation, targeted retinal photocoagulation, photobiomodulation therapy, and photo-mediated ultrasound therapy. However, further randomized clinical trials are necessary to determine their applications and effectiveness in comparison to conventional argon laser strategy [25,26].
A second indication for laser is the presence of non-center-involving DME when a leaking microaneurysm is evident as the origin of the macular fluid, located at least 500 microns away from the fovea, the center of the macula. An impact is applied directly to the leaking point to occlude it by generating a scar [23,27].
Intravitreal injections are the gold-standard treatment for center-involved DME when VA is worse than 20/25 [28]. Currently available intravitreal drugs focus on reducing inflammatory cytokines, inhibiting VEGF and, more recently, the angiopoietin-2 (Ang-2). These drugs make it possible to stop the leaking of fluid within the macula, restoring VA. Table 1 shows the drugs approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of DME and the therapeutic targets on which they act [29]. Due to the high cost of these injections, biosimilar medicines have begun to be developed to reduce the economic burden of this pathology [30].

Table 1.
Available intravitreal drugs for diabetic macular edema.
For anti-VEGF injections, there are several treatment regimens. In cases where close monitoring of the patient is possible, a pro re nata strategy can be used, which consists of administering a single intravitreal injection followed by monthly monitoring to assess whether the patient requires another injection. The current strain on health systems, with the inability to conduct exhaustive check-ups on all patients, led to the establishment of treat-and-extend regimens. These involve three to five initial monthly injection procedures, followed by new doses at longer intervals (extending to every 8 or 12 weeks, or, in the case of drugs more recently approved by the FDA/EMA, up to every 16 weeks for faricimab [31] or aflibercept (8 mg)) [22].
A meta-analysis evaluating different anti-VEGF agents in 2023 (including bevacizumab, ranibizumab, aflibercept (2 mg), brolucizumab, and faricimab) determined a mean number of injections ranging from seven to 12 within the first year of treatment. Every treatment improved VA at 24 months, although the difference when comparing anti-VEGF agents was unlikely to be significant [32]. Brolucizumab and faricimab demonstrated longer durability, requiring fewer injections. However, caution must be taken with brolucizumab, given the higher incidence of intraocular inflammation events; close monitoring is required to detect them [33,34]. The authors of the meta-analysis warned that the results of the included randomized clinical trials may differ from real-world settings, as treatment regimens may be less strict [32]. Indeed, a multicenter real-world data review from the Fight Retinal Blindness Study Group evaluating bevacizumab, ranibizumab, and aflibercept (2 mg) reported in 2024 a frequency of four to seven injections during the first 12 months of treatment [35].
Other uses for anti-VEGF injections include their administration less than five days prior to vitreoretinal surgery in order to reduce intra- and postoperative vitreous cavity bleeding [36]. Their application in cases of moderate to severe NPDR and PDR to eliminate or prevent neovascular formation is also being studied and does not meet with approval in this context worldwide. The Diabetic Retinopathy Clinical Research Network determined that patients with moderate to severe NPDR treated with prophylactic aflibercept (2 mg) for 2 years, and if needed between the second and the fourth year, presented statistically significant milder stages of DR after 4 years, but there were no benefits in terms of VA [37]. Due to the high cost of injections and the need for extensive monitoring, these treatments have not yet demonstrated their definite applicability in patients without center-involved DME. For PDR, the Diabetic Retinopathy Clinical Research Network reported that ranibizumab given monthly for 24 weeks, allowing for deferral from the sixteenth week if neovascularization regressed, was not inferior to PRP after 5 years of follow-up, with similar VA [38]. Nevertheless, treatment with ranibizumab between the fifth and the tenth year was only cost-effective for patients with center-involved DME [39]. The CLARITY study demonstrated that aflibercept was not inferior to PRP after 1 year, reducing complications in terms of DME, vitreous cavity bleeding, the need for vitrectomy, or visual loss [40]. However, anti-VEGF injections do not produce reperfusion of the retina [41], and stopping injections may significantly worsen the DR. It has been demonstrated that patients with PDR treated with anti-VEGF injections and then loss to follow-up present a higher incidence of tractional retinal detachment and iris neovascularization in comparison to PRP [42], meaning that the long-term application of anti-VEGF in PDR must be applied with caution.
In the case of intravitreal steroids, the biodegradable implant Ozurdex® provides an intraocular duration of up to 6 months, while Iluvien® is a non-biodegradable sustained-release drug delivery system that lasts for 36 months. These drugs are particularly indicated in cases where DME shows predictive inflammatory biomarkers on retinal imaging of the macula [43]. The treatment regimen for these implants begins with a single dose of Ozurdex® to assess its effect and potential side effects, such as an increase in intraocular pressure. After 4 months, the need for another injection is evaluated. If recurrent DME responds to Ozurdex®, Iluvien® is available as a long-term treatment [22].
When considering the cost of the intravitreal injections, including medication, procedure, and follow-up evaluation, a French study estimated that the average annual cost during the first year of treatment was €5782 for aflibercept (2 mg), €2779 for dexamethasone, and €5536 for ranibizumab [44]. These findings are consistent with a prior German systematic review, which reported 3-year costs of €17,540 for ranibizumab, €15,894 for aflibercept, €10,822 for the fluocinolone acetonide implant, and €12,363 for the dexamethasone implant [45]. In the United States, where off-label use of bevacizumab is more prevalent, and often required by insurance companies, the Diabetic Retinopathy Clinical Research Network reported 2-year costs of $13,929 for a bevacizumab-first strategy, compared to $26,504 for aflibercept monotherapy. Notably, 70% of patients initially treated with bevacizumab needed to switch to aflibercept by the end of the 2-year period [46]. Biosimilar products offer the potential for more affordable treatment options, typically reducing costs by approximately 30% compared to the reference drug, though they remain more expensive than off-label alternatives like bevacizumab [30].
The last conventional therapy that deserves to be mentioned is the pars plana vitrectomy, which is indicated in cases of PDR when there is a non-clearing vitreous hemorrhage (especially without prior PRP), in cases of tractional retinal detachment involving the macula, combined tractional-rhegmatogenous retinal detachment, or when a dense pre-macular subhyaloid hemorrhage is present [22]. A second scenario is the presence of DME influenced by vitreomacular traction, with the aim of reducing macular thickness [47].
As mentioned, the set of therapies currently used focuses on addressing the disease in advanced stages to prevent its progression to blindness [21]. Due to the inability to regenerate neural and vascular tissue appropriately, it would be more appropriate to prevent the tissue damage induced by diabetes from occurring in the first place. With this goal, efforts are made to raise awareness among patients about the importance of maintaining good glycemic control, but in many cases, it has little effect on the population. This situation leads us to consider other approaches that can protect retinal tissue, for which an in-depth study of the disease and the molecular causes that induce the damage is necessary.
3. Oxidative Stress and Classical Biochemical Mechanisms as Inducers of Diabetic Retinopathy
The human retina requires approximately 300% and 600% more oxygen than the cerebral cortex and cardiac muscle, respectively [48]. Due to this high demand, both the retina and its vascular system are particularly susceptible to oxidative stress [49]. Oxidative stress arises from an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant capacity to neutralize them [18]. Through glycolysis, glucose is metabolized into pyruvate, which subsequently enters the mitochondria to produce adenosine triphosphate (ATP) via the respiratory chain [18,50]. Chronic hyperglycemia and the resultant metabolic overload trigger various pathological alterations that contribute to microvascular and neuronal damage through ischemic and hyperosmotic mechanisms, while also exacerbating oxidative stress [49,51].
Key metabolic pathways activated in DR include the polyol pathway [50], the hexosamine pathway [52], protein kinase C (PKC) signaling [53], and the accumulation of advanced glycation end products (AGEs) [54]. These pathways promote the activation of proliferative growth factors, such as VEGF, and exacerbate ROS overproduction, inflammation, cellular apoptosis, and BRB disruption, ultimately leading to vascular and neuronal damage in the retina [55,56]. In parallel, hyperglycemia compromises endothelial function [57] and disrupts vascular homeostasis, while extracellular matrix alterations and neuronal dysfunction further contribute to retinal deterioration [58]. Moreover, inflammation and immune responses, characterized by leukocyte adhesion, chemokine overexpression, and increased inflammatory mediators, play a key role in disease pathogenesis [59].
The polyol pathway converts excess glucose, which cannot be phosphorylated by hexokinase due to saturation, into sorbitol via aldose reductase, using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. Sorbitol is subsequently converted into fructose by sorbitol dehydrogenase with the aid of NAD+ [50,60]. Excessive activation of aldose reductase has been associated with damage to various retinal cell types, including endothelial cells [61], pericytes [62], ganglion cells [63], Müller glial cells [63], retinal pigment epithelial cells [63], photoreceptors, and amacrine cells [64]. The deleterious effects of the polyol pathway on the viability of multiple retinal cell types in DR arise from several mechanisms. First, the consumption of NADPH and NAD+ reduces glutathione (GSH) regeneration, weakening antioxidant defenses and promoting ROS accumulation, leading to oxidative stress [60]. Second, intracellular sorbitol accumulation, due to its low permeability across cell membranes, induces osmotic stress, increasing water retention and causing cellular damage [65,66]. Third, fructose produced in this pathway is converted into fructose-3-phosphate, which degrades into compounds that promote the formation of AGEs [67].
The hexosamine biosynthetic pathway is activated when excess fructose-6-phosphate, a glycolytic intermediate, is diverted from glycolysis [52]. This pathway contributes to DR through three main mechanisms: the inhibition of insulin/Protein Kinase B (Akt) signaling [68], promoting neuronal death; the enhancement of inflammation via increased O-GlcNAcylation of nuclear factor κ B (NF-κB) p65, leading to ganglion cell injury; and the induction of pericyte apoptosis through p53 modification, resulting in vascular dysfunction and capillary instability [69].
PKC activation is another key mechanism in DR progression. Hyperglycemia elevates levels of glyceraldehyde-3-phosphate (GA-3P), a glycolytic intermediate that enhances diacylglycerol production and activates PKC isoforms in the retina [53]. Diacylglycerol accumulation activates PKC-β, which alters enzymatic activities of nitric oxide synthase (NOS), as well as the expression of endothelin-1, and VEGF in endothelial cells, leading to vascular dysfunction [70,71]. Additionally, PKC-δ activation promotes pericyte loss by increasing oxidative stress and downregulating the platelet-derived growth factor survival signaling pathway [53].
Chronic hyperglycemia also promotes AGE formation and accumulation in the extracellular matrix and endothelial cells of retinal capillaries [54]. AGEs contribute to retinal damage by inducing aberrant cross-linking of extracellular matrix proteins, increasing vascular stiffness, and compromising structural and functional integrity [72]. Moreover, the interaction of AGEs with their receptor (RAGE) initiates intracellular signaling cascades that amplify the expression of pro-inflammatory cytokines, pro-angiogenic factors, endothelial dysfunction, pericyte apoptosis, and the breakdown of the inner BRB [54,72,73].
In addition, hyperglycemia-induced ROS production activates poly (ADP-ribose) polymerase, which inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [74]. This inhibition facilitates further activation of the polyol pathway, enhances AGE formation via methylglyoxal, activates PKC and NF-κB, and increases flux through the hexosamine pathway [75]. Within this pathway, glutamine:fructose-6-phosphate amidotransferase regulates transforming growth factor β (TGF-β) expression and PKC activation in response to glucose [76]. Concurrently, excess glucose is converted into sorbitol, inducing osmotic stress before being metabolized to fructose [65,66]. The byproducts of this process, including fructose-3-phosphate and 3-deoxyglucosone, are potent glycation agents that further promote AGE formation. In turn, AGEs enhance oxidative stress and reinforce PKC activation, perpetuating the cycle of retinal damage in DR [77].
Beyond metabolic alterations, abnormal epigenetic modifications, NF-κB activation, decreased nuclear factor erythroid 2- related factor 2 (Nrf2) activity, and hyperglycemia-induced mitochondrial dysfunction contribute to ROS overproduction in DR [55]. Importantly, oxidative stress resulting from epigenetic changes can persist even after glycemic control is achieved, a phenomenon termed “metabolic memory” [78]. Among the relevant epigenetic alterations involved in DR, an increase in S-adenosylmethionine (SAM)-mediated DNA methylation has been observed in patients, particularly affecting the matrix metalloproteinase 9 (MMP-9) gene, which has been closely linked to the disease [79]. Additionally, acetylation of NF-κB p65 subunit regulates MMP-9 expression [80], while aberrant CpG island methylation disrupts critical pathways involved in disease progression [81].
The oxidative stress-driven pathogenesis of DR involves mitochondrial dysfunction, apoptosis, and autophagy dysregulation, modulated by pathways such as mechanistic target of rapamycin (mTOR)/AMP-activated protein kinase (AMPK) and mediated by VEGF, intercellular adhesion molecule-1 (ICAM-1), NO, and lipid peroxidation. Hyperglycemia generates excess ROS, damaging mitochondrial DNA, promoting the activation of MMP-2 and MMP-9, and facilitating cytochrome c release, leading to cell death [20,66,82,83,84]. Furthermore, NF-κB activation promotes pro-inflammatory and pro-apoptotic responses that intensify cellular damage [85,86,87]. Lipid peroxidation compromises cellular membrane integrity and generates aldehyde byproducts that contribute to neurodegeneration [88,89,90]. These interconnected processes culminate in BRB breakdown, inflammation, and cell death, fueling the progression of DR [19].
4. Inflammation and Vascular Alterations
Studies in animal models and diabetic patients have demonstrated that low-grade, chronic inflammation is present throughout multiple stages of DR [91] and represents a pivotal contributor to disease progression, particularly during the early stages [92].
In DR, inflammation is initiated by various stimuli, including hyperglycemia, growth factors, AGEs, circulating cytokines and chemokines, and ROS [93]. These molecular signals are detected by the innate immune system via pattern recognition receptors, such as Toll-like receptors, which recognize pathogen-associated molecular patterns and damage-associated molecular patterns [94]. This activation induces intracellular pathways that promote the expression of pro-inflammatory cytokines (e.g., tumor necrosis factor α (TNF-α), interleukin 1 β (IL-1β), IL-6 and monocyte chemoattractant protein-1 (MCP-1)) [9,21,95].
This pro-inflammatory environment facilitates leukocyte adhesion (leukostasis) to retinal microvasculature, elevates oxidative stress levels, and enhances the expression of adhesion molecules (ICAM-1, VCAM-1), collectively contributing to BRB breakdown, pericyte degeneration, increased vascular permeability, and pathological angiogenesis [96]. Inflammation and angiogenesis exhibit a reciprocal interplay, where each process potentiates the other [97]. ROS, such as hydrogen peroxide (H2O2) and superoxide anion (), enhance the production of pro-inflammatory transcription factors like NF-κB, which promotes mediators that upregulate VEGF expression [98]. In turn, VEGF activates and translocates NF-κB to the nucleus, inducing the expression of pro-inflammatory molecules such as ICAM-1, VCAM-1, MCP-1, and cyclooxygenase-2 (COX-2). COX-2, through the synthesis of prostaglandins, stabilizes hypoxia-inducible factor-1 (HIF-1), and further reinforcing VEGF production and NF-κB activation, perpetuating a chronic inflammatory feedback loop [99]. Moreover, VEGF-mediated activation of NF-κB, enhances the production of inflammatory mediators and promotes the NOD-like receptor pyrin domain containing protein 3 (NLRP3) inflammasome activation, leading to IL-1β and IL-18 secretion and contributing to retinal neurodegeneration via pyroptotic mechanisms in Müller glial cells [100].
In parallel, the transcription factor signal transducer and activator of transcription 3 (STAT3) also plays a key role in DR-associated inflammation and angiogenesis. STAT3 activation, induced by pro-inflammatory cytokines and VEGF, promotes endothelial cell proliferation and survival, facilitating neovascularization. Furthermore, STAT3 acts as a central regulator in the amplification of chronic inflammation by inducing the expression of cytokines such as IL-6 and TNF-α, thereby exacerbating vascular dysfunction and increasing BRB permeability. Pharmacological inhibition of STAT3 in rodent models of DR has been shown to attenuate vision loss and reduce retinal damage, underscoring its potential as a therapeutic target [101,102].
DME, a major complication of DR, arises from the compromise of BRB integrity and subsequent accumulation of intra- and extracellular fluid in the macular region [103]. The inner BRB, composed of endothelial cells connected by tight junctions and stabilized by pericytes and glial cells, undergoes alterations induced by hyperglycemia, oxidative stress, and chronic inflammation. The progressive loss of pericytes, which are essential for maintaining capillary integrity, weakens endothelial junctions, increasing vascular permeability and fluid extravasation into the retina. Additionally, the activation of adhesion molecules such as ICAM-1 and leukostasis exacerbates endothelial damage, further disrupting the BRB. As fluid accumulates in the extracellular space, the excessive burden on the retinal pigment epithelium (RPE) impairs its pumping function and homeostatic regulation, compromising the outer BRB. Prolonged inflammation and hypoxic conditions induce VEGF overexpression and dysregulation of RPE ion transport mechanisms, perpetuating fluid accumulation and further compromising BRB function [104,105,106]. This process ultimately leads to the development of DME, the leading cause of vision loss in diabetes, occurring more frequently in advanced stages of the disease [107,108].
5. Polyphenols in the Treatment of Diabetic Retinopathy
5.1. Oxidative Stress and Antioxidant Defences in DR
The eye is an organ with effective antioxidant defenses that protect it from the oxidative attacks to which it is continuously subjected. The primary source of these attacks lies in the high metabolic activity and oxygen consumption occurring in the retina, as well as in exposure to UV radiation in the anterior portion of the eye. During the physiological activity of the retina, oxidative metabolism in the mitochondria and endoplasmic reticulum produces ROS and reactive nitrogen species [109,110]. The cornea and ocular lenses have the ability to absorb a significant portion of the incident light, thereby protecting the retina from the effects of ultraviolet radiation. However, the production of ROS on the ocular surface can induce molecular changes in deeper structures [111]. Additionally, the production of ROS and reactive nitrogen species can be enhanced by inflammation, exposure to environmental pollutants, genetic factors, aging, or a reduction in antioxidant defenses. Due to their high reactivity, in order to counteract the damage that they can cause to cellular biomolecules with pathophysiological effects, the retina has developed a complex antioxidant defense system that is essential for maintaining ocular homeostasis. However, the imbalance between the production and elimination of these species, which can occur in pathologies such as DM due to exposure to chronic hyperglycemia, triggers diseases such as DR.
The cellular antioxidant defense system is typically classified into two major groups: the enzymatic system, primarily composed of superoxide dismutase (SOD), catalase (CAT), GSH peroxidase, and heme oxygenase, and the non-enzymatic antioxidant system, consisting of molecules capable of reducing or chelating oxidizing species such as GSH [112]. Furthermore, exogenous antioxidants such as vitamins (C, E), carotenoids (lutein, zeaxanthin), flavonoids, coenzyme Q10, and alpha-lipoic acid may also participate in retinal protection mechanisms [113].
is one of the major reactive species involved in retinal damage. It has been shown that its levels increase in cell culture experiments at high glucose concentrations and in retinal tissue in various animal models of diabetes compared to healthy animals [114,115,116,117]. In the diabetic retina, is primarily generated at the mitochondrial level and through the activity of NADPH oxidase (Nox) [118]. The removing of this free radical is catalyzed by SOD, which transforms to a less reactive molecule, hydrogen peroxide (H2O2) [119]. The action of peroxidases and CAT avoid the oxidative damage caused by the accumulation of H2O2. In different DR animal models, the modulation of these ROS has been related with retinal damage reduction. In fact, a reduction in SOD and CAT activity has been observed in the diabetic rat and rabbit retinas [117,120]. Overexpression of SOD2 protected retinas of diabetic mice against the development of acellular retinal capillaries [121]. Therefore, although chronic exposure to glucose is a potent inducer of ROS capable of overcoming the antioxidant defenses of the retina, interventions that stimulate the endogenous antioxidant capability may be a promising therapeutic approximation to avoid the development of DR. However, to ensure the success of this kind of therapeutic approach, it seems critical both start the interventions as soon as possible, before irreparable damage has occurred and phenotypic consequences, such as angiogenesis, are observable, and to sustain the intervention over time.
5.2. Polyphenol Supplements Against DR
Between the late 20th century and the early 21st century, scientific interest in the beneficial effects of natural antioxidants has steadily increased. Since the proposal of the French paradox and the attribution of the cardiovascular protective effects of resveratrol found in wine [122], numerous studies, both in vitro and in vivo, have been conducted, delving into the antioxidant properties of a wide range of phytochemicals, such as polyphenols, which are present in the diet. In parallel, a variety of techniques have been developed to assess antioxidant capacity [123] and its implications in pathologies associated with increased oxidative stress. For instance, polyphenols such as resveratrol, curcumin, pterostilbene, quercetin, and epigallocatechin gallate have shown beneficial effects in diseases characterized by oxidative imbalance, including cancer [124,125,126,127,128], diabetes [129,130,131,132,133], and Alzheimer’s disease [134,135,136,137,138].
In the development of DR, as previously discussed, oxidative stress induced by chronic hyperglycemia is a key contributor. The imbalance between antioxidant defenses and oxidative insults triggers subclinical damage that progressively worsens, such as ganglion cell neurotoxicity and pericyte cell death. These early effects, which are etiologically linked to ROS, do not result from a single molecular pathway but rather from a broad cascade of reactions that exacerbate retinal damage. As there is no unique disrupted pathway, and unless the root cause, diabetes, is cured, treatments targeting only one molecular mechanism are unlikely to be effective. A large body of literature highlights the complex protective role of polyphenols in DR, acting on multiple molecular targets. This section provides a comprehensive review of the principal polyphenols and the molecular mechanisms by which they confer therapeutic benefits in DR.
5.2.1. Resveratrol
Among the numerous studies conducted on animal models, resveratrol has been shown to mitigate the diabetes-induced downregulation of occludin and the upregulation of pro-inflammatory HMGB1 and RAGE in the retinas of diabetic rats, as well as the degradation of the BRB. These findings are particularly relevant, as the same study also demonstrated increased HMGB1 expression in the vitreous humor of patients with PDR [84]. Resveratrol’s anti-inflammatory, antioxidant, and anti-apoptotic activities in experimental DR animal models include: promoting AMPK activity [139], inhibiting SIRT1 deactivation [84,139], suppressing NF-κB phosphorylation [139], preventing apoptosis in retinal neurons [140], Müller glial cells [141], RPE cells [142], protecting against ferroptosis regulating the Nrf2/glutathione peroxidase 4 (GPx4)/PTGS2 pathway [143], and reducing inflammatory factors such as IL-1β, IL-6, TNF-α, VEGF, Interferon-γ, MCP-1 [144], COX-2 [145], and TGF-β1, IL8 [146], along with the GSSG/GSH ratio and lipid peroxidation index [147].
5.2.2. Piceatannol
Although experiments were not conducted under high-glucose conditions, piceatannol, a natural stilbene derived from resveratrol, has been shown to enhance antioxidant defenses and prevent H2O2- induced cell death in ARPE-19 cells through the activation of the phosphoinositide 3-kinase (PI3K)/Akt–Nrf2/HO-1 signaling pathway [148].
5.2.3. Quercetin
Despite substantial preclinical evidence, the clinical use of quercetin in DR remains limited, mainly due to its poor bioavailability, the lack of suitable ocular formulations, and rapid degradation [149]. In human retinal microvascular endothelial cells, quercetin reduces cell migration and tube formation, as well as the expression of NLRP3, apoptosis-associated speck-like protein (ASC), caspase-1, IL-1β, IL-18, microtubule-associated proteins 1A/1B light chain 3B (LC3-B), Beclin-1, and autophagy under high-glucose conditions, in a dose-dependent manner [150].
In diabetic rat models, intraperitoneal administration of quercetin decreases cell apoptosis, improves retinal histopathology, increases the number of ganglion cells, and suppresses the overexpression of pro-inflammatory cytokines (IL-1β, IL-18, IL-6, TNF-α), HMGB-1, and NLRP3, as well as proangiogenic factors such as VEGF, ICAM-1 [151], and MMP9 [152]. Simultaneously, it promotes the expression of neurotrophic factors, including brain-derived neurotrophic factor and nerve growth factor, likely through the induction of heme oxygenase-1 (HO-1) [151].
5.2.4. Anthocyanins
Anthocyanins, a class of flavonoids found in fruits such as blueberries, blackberries, and black grapes, have demonstrated notable protective effects in DR. These compounds reduce the production of ROS and malondialdehyde (MDA), a key oxidative stress and lipid peroxidation marker, in diabetic rat retinas, and lower VEGF and IL-1β levels in serum. Concurrently, they increase GSH content and the expression of the antioxidant enzyme GPx, accompanied by an upregulation and nuclear translocation of Nrf2 and HO-1 [153].
In vivo studies have shown that blueberry-derived anthocyanins mitigate diabetes-associated outcomes such as weight loss, hyperglycemia, and retinal cell apoptosis. Moreover, in diabetic mice, they reduce plasma IL-1β levels and attenuate the inflammatory response in a dose-dependent manner, improve retinal architecture, and prevent early retinal damage. These effects are associated with enhanced phosphorylation of AKT and glycogen synthase kinase 3 beta, stimulation of glucagon-like peptide-1 release, and increased retinal expression of the glucagon-like peptide-1 receptor, suggesting a role in restoring insulin signaling and offering neuroprotective and anti-inflammatory benefits [154].
5.2.5. Epigallocatechin Gallate
Epigallocatechin gallate, the most abundant and bioactive polyphenol in green tea, has shown beneficial effects in diabetic rat retinas by restoring GSH levels, enhancing the activity of antioxidant enzymes such as SOD and CAT, and reducing TNF-α, VEGF, and capillary basement membrane thickening [155]. Beyond its antioxidant action, epigallocatechin gallate prevents the overexpression of glial fibrillary acidic protein (GFAP) and the loss of occludin, while positively modulating neuronal NOS and the AKT/endothelial NOS pathway, contributing to the preservation of BRB integrity by limiting VEGF-induced permeability [156].
In vitro, epigallocatechin gallate inhibits ARPE-19 cell migration and adhesion by blocking PI3K/Akt, MEK/extracellular signal-regulated kinase 1/2 (ERK1/2), and p38 mitogen-activated protein kinase (MAPK) pathways [157]. Additionally, in high-glucose-treated Müller cells, EGCG promotes autophagy by inhibiting the mTOR pathway, sustaining LC3-B and Beclin-1 levels, and decreasing p62 expression, thereby facilitating autophagosome and autolysosome formation [158].
5.2.6. Naringenin
Naringenin, a citrus-derived flavonoid, exhibits both antioxidant and anti-inflammatory properties in experimental models of DR. In streptozotocin-induced diabetic rats, naringenin improves redox balance, as evidenced by decreased lipid peroxidation [159] and increased levels of GSH, SOD, and CAT [159,160]. It downregulates the expression of NF-κB and pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [160].
In the diabetic retina, naringenin enhances the expression of neuroprotective markers, including synaptophysin and brain-derived neurotrophic factor, along with its receptor tropomyosin receptor kinase B, and exerts antiapoptotic effects, contributing to retinal protection [159]. In treated diabetic rats, it also improves body weight in a dose-dependent manner, reduces serum glucose levels, increases ganglion cell layer thickness and cell number, and lowers GFAP expression [160].
5.2.7. Eriodictyol
Eriodictyol, a polyphenol found in various fruits and vegetables, has demonstrated notable anti-inflammatory and antioxidant effects [161,162]. In diabetic rat retinas, eriodictyol treatment significantly reduces TNF-α, ICAM-1, VEGF, and endothelial NOS levels in a dose-dependent manner. It also lowers retinal lipid peroxidation and mitigates BRB breakdown [163].
Lv et al. further explored its role in DR through in vitro experiments on ganglion cells. Eriodictyol treatment under high-glucose conditions decreased apoptosis and reduced TNF-α and IL-8 production. Additionally, it enhanced the expression of antioxidant enzymes such as SOD, GPx, and CAT, thereby strengthening the retinal antioxidant defense system. These effects were associated with the activation of the Nrf2/HO-1 pathway [164].
5.2.8. Myrecetin/Galic Acid
Myricetin is a polyphenol with antioxidant and anti-inflammatory properties, present in vegetables, fruits, nuts, berries, tea, and red wine. Although in vivo evidence is limited, several in vitro studies support its potential in DR. In ARPE-19 cells exposed to oxidative stress, myricetin promotes cell survival by activating SOD and Nrf2, while downregulating inducible NOS at the transcriptional level [165]. In retinal pericytes, myricetin inhibits AGE-induced cell migration by modulating the RAGE-Src-ERK1/2-FAK-paxillin signaling cascade, a key process in microvascular destabilization [166].
In diabetic rats, co-treatment with myricetin and gallic acid lowers hyperglycemia, improves histopathological retinal features, suppresses TNF-α and prostaglandin-E2 overexpression, reduces vascular leakage and neovascularization, and lowers the MDA/GSH ratio [167].
5.2.9. Scutellarin
Scutellarin, a flavone extracted from Scutellaria baicalensis, has demonstrated promising effects in DR. It significantly increases the activity of GPx, SOD, and CAT, reduces retinal inflammation, and ameliorates histopathological alterations induced by chronic hyperglycemia in diabetic rats, potentially through inhibition of NLRP3 inflammasome activation [168]. Furthermore, scutellarin protects retinal ganglion cells from pyroptosis by inhibiting caspase-1, gasdermin D, NLRP3, IL-1β, and IL-18 [169].
In addition, scutellarin reduces proliferation, migration, and tube formation in human retinal endothelial cells. In vivo, it attenuates microvascular neovascularization in type II diabetic rats by suppressing VEGF, p-ERK, p-FAK, and p-Src expression [170]. In diabetic mouse models, it also protects against oxidative stress-induced BRB damage by reducing claudin-1 and claudin-19 expression, downregulating microglial activation, inhibiting NF-κB and TNF-α expression, and suppressing p-ERK signaling both in vivo and in vitro [171].
5.2.10. Rhaponticin
Rhaponticin, a natural stilbenoid glucoside compound, exhibits potent antioxidant activity in vivo by activating the Nrf2/HO-1/NF-κB signaling pathway, thereby enhancing the retinal expression of key antioxidant enzymes, including HO-1, SOD, CAT, GPx, and NAD(P)H quinone dehydrogenase 1. Additionally, rhaponticin reduces levels of MDA, TNF-α, and MMP-2, while increasing IL-10 and tissue inhibitor of metalloproteinases-1, indicating a favorable shift toward an anti-inflammatory and redox-balanced retinal microenvironment [172].
5.2.11. Curcumin
Curcumin enhances the expression of the antioxidant transcription factor Nrf2 [173], reduces retinal lipid peroxidation [174,175], increases SOD levels, alleviates oxidative stress [175], decreases VEGF expression, reduces basement membrane thickness of retinal capillaries [176], and lessens vascular tortuosity and microaneurysm formation [177].
For instance, administration of piperine/curcumin (1 g/day) for 12 weeks to 30 NPDR patients increased their total antioxidant capacity and SOD activity while reducing lipid peroxidation, as assessed by MDA levels. However, no macroscopic ocular improvements were observed via optical coherence tomography (OCT) or OCTA compared to 30 placebo-treated patients [178].
5.2.12. Ferulic Acid/Chlorogenic Acid/Arctiin/Rutin
In both in vitro and in vivo experimental models, the He-Ying-Qing-Re formula, whose main active constituent is ferulic acid, has demonstrated beneficial effects on DR by modulating multiple pathophysiological pathways [179]. In human umbilical vein endothelial cells, ferulic acid inhibits the formation of AGEs and attenuates the associated inflammatory response by suppressing the activation of the NF-κB and p38 MAPK signaling pathways [180]. Furthermore, the combination of ferulic acid with other polyphenols such as chlorogenic acid and arctiin prevents degeneration of the retinal vasculature and preserves the integrity of the BRB. This is achieved by maintaining the expression of tight junction proteins, including claudin-1 and ZO-1, without significantly affecting hyperglycemia. In addition, this polyphenolic combination suppresses activation of the AGEs receptor and its downstream signaling via Akt [181].
The combination of ferulic acid, chlorogenic acid, and rutin has also been shown to attenuate retinal ganglion cell loss and retinal thinning in diabetic mice. Moreover, it inhibits both endoplasmic reticulum stress and mitochondrial oxidative stress in retinal ganglion cells in vitro by downregulating ATF4-dependent pathways, thereby reducing apoptosis [182]. Notably, prolonged treatment with ferulic acid in a diabetic murine model (C57BL/KsJ db/db) protects against retinal structural deterioration, preserving critical layers such as the ganglion cell layer, inner nuclear layer, and inner plexiform layer [183].
5.2.13. Pterostilbene
Our group demonstrated that pterostilbene, via NRF2, one of the key regulators of redox homeostasis, can prevent ganglion cell damage in the early stages of DR without affecting glycemia or body weight in experimental animals. Polyphenol treatment restored the reduced activities of SOD, CAT, and GPx observed in the retinas of diabetic rabbits, alongside a decrease in H2O2 levels. Furthermore, the GSH/GSSG ratio, which was reduced in diabetic retinas, was normalized after pterostilbene treatment [117]. Decreased GSH levels have also been reported in other experimental models, such as diabetic rat retinas [184] or pericyte cultures exposed to high glucose levels [185]. Additionally, reduced levels of 4-HNE were observed [117], and modulation of the antioxidant machinery by pterostilbene supplementation prevented early neuroretinal damage and lipid peroxidation of the most abundant retinal fatty acids: docosahexaenoic acid (DHA; 22:6n-3), arachidonic acid (20:4n-6), and adrenic acid (22:4n-6) [186,187,188]. These fatty acids play a physiological role in protecting against the development of various retinal disorders, including DR [189]. The increase in products derived from their lipid peroxidation has been associated with neuronal damage [190] and the development of neovascularization in DR [191,192].
Spectrometry analyses have shown that lipid peroxidation in the retinas of diabetic rabbits increases the levels of: PGE2, PGF2α, isoprostanes (8-iso-PGE2, 8-iso-15-keto-PGF2α, and 8-iso-15-(R)-PGF2α), 17-F2t-dihomo-IsoP, Ent-7(RS)-7-F2t-dihomo-IsoP, 17-epi-17-F2t-dihomo-IsoP, 17(RS)-10-epi-SC-∆15-11-dihomo-IsoF, 10-epi-10-F4t-NeuroP, 4(RS)-4-F4t-NeuroP, and 14(RS)-14-F4t-NeuroP. Pterostilbene treatment was able to reduce the levels of these lipid peroxidation products in diabetic rabbits to values similar to those in controls [193].
As previously mentioned, current therapeutic approaches against DR are typically implemented when irreversible damage has already occurred, such as induced neurotoxicity or abnormal blood vessel formation. Targeting a specific molecule like VEGF or photocoagulating blood vessels are palliative strategies aimed at temporarily halting the progression of irreversible retinal damage. For this reason, the pleiotropic effects [194] of polyphenols may underlie the clinical benefits of their use, benefits that go beyond a singular, directed, and dominant primary effect. However, to increase the likelihood of success, early intervention is crucial. Thus, in order to prevent DR development, these compounds should be administered at the time of diabetes diagnosis. Both we and other researchers, for example [113], think that although human studies are limited; this hypothesis is supported by numerous in vitro and in vivo investigations and by the beneficial outcomes observed in patients with DR following polyphenol supplementation.
Recently, our group demonstrated in a pilot clinical study that pterostilbene treatment for one year improved systemic redox status and reduced the progression of retinal vascular alterations. Moreover, after a six-month treatment interruption, both the redox imbalance and retinal damage indicators reappeared [195]. These studies are temporally limited and therefore do not capture the evident macroscopic progression of the disease due to its slow development in patients with good glycemic control. Nevertheless, the results suggest that polyphenol treatment may further delay disease progression, offering clear advantages for patients.
5.3. Nanotechnology for Polyphenol Delivery
Among the evident limitations of polyphenol-based treatments are their low bioavailability, difficulty in reaching biologically effective concentrations, and poor localization in target organs. Conventional therapies against DR, as previously discussed, also present several limitations and side effects. The innovative development of nanomedicine is enabling new strategies to overcome these challenges. In recent years, new materials with evident advantages for the treatment of DR have been developed. Among their key characteristics are small particle size, high penetration through the BRB, good biocompatibility, the ability to reduce the elimination rate of therapeutics by allowing sustained release, and favorable physicochemical properties of the nanoparticles [196].
Although the progress and clinical validation of this approach still require further efforts, the use of nanomaterials as carriers enables controlled, localized, and sustained delivery of therapeutic agents to the retinal tissue. This reduces the side effects associated with conventional treatments and represents a significant advancement toward personalized and precision medicine for DR [196,197,198]. Recently, Baghban et al. published an excellent review presenting numerous studies that employ nanotechnology to improve the diagnosis and treatment of DR [196].
In the treatment of DR at more advanced stages, nanoparticles carrying various types of anti-angiogenic molecules have shown promising results. For example, in an effort to reduce the frequency of intraocular anti-VEGF injections, Du Toit et al. designed a nanosystem for the controlled release of the anti-angiogenic peptide p11. The nanoparticles developed enabled sustained release of the peptide for up to 60 days, with a loading efficiency of 67% and biocompatibility ranging from 97% to 99% [199]. Similarly, encouraging results have been obtained by encapsulating other anti-angiogenic drugs into various types of nanoparticles, such as Apatinib, a VEGFR-2 inhibitor [200,201]; Bevacizumab, a monoclonal antibody against VEGF [202]; and nanoparticles loaded with small interfering RNA targeting the human antigen R (HuR), an RNA-binding protein that stabilizes VEGF mRNA and promotes its expression, an essential factor in the progression of DR [203]. Additionally, lipid-encapsulated natural compounds such as naringenin, a polyphenol abundant in citrus fruits, have demonstrated anti-angiogenic effects in animal models of retinopathy comparable to those of bevacizumab [204] (Table 2). Another example of liposomes encapsulation is ellagic acid, a polyphenol capable of downregulating VEGF, GFAP, HIF-1α, and VEGFR-2 expression in murine models of DR [205] (Table 2).
Nevertheless, despite fewer studies in this area, it may be of greater interest to focus on protecting the diabetic retina from oxidative stress and inflammation, which are the underlying causes of vascular alterations observed during disease progression. Treatment with nanoparticles co-loaded with curcumin and insulin halted disease progression, stabilized blood glucose levels, improved liver function, reduced AGEs, enhanced ATP synthesis, and protected the retina from vascular damage induced by hyperglycemia. Moreover, this therapy did not exhibit any nanoparticle-induced toxicity in experimental animals [206]. In addition, new advanced encapsulation strategies for curcumin have been explored, such as proniosomal gels, transferosomes, and cyclodextrin complexes, which have enhanced its stability and therapeutic efficacy in deep ocular tissues, strengthening its antioxidant action in DR [207] (Table 2).
Nanoparticles encapsulating pyrrolidine dithiocarbamate and triamcinolone acetonide demonstrated antioxidant activity in a diabetic animal model by reducing protein carbonylation and lipid peroxidation, and they protected against the development of vascular damage and retinal neovascularization [208].
The encapsulation of diosmin (3’,5,7-trihydroxy-4’-methoxyflavone-7-rhamnoglucoside), a natural flavone glycoside derived from hesperidin, a flavanone glycoside abundant in citrus fruits [209], in nanostructured lipid carriers enhanced its water solubility for potential ophthalmic topical application. Studies in RPE cells confirmed its antioxidant and anti-inflammatory properties, making it a potential candidate for DR treatment [210] (Table 2).
Treatment with gold nanoparticles coated with resveratrol in diabetic rats improved various retinal parameters affected by hyperglycemia. For instance, it reduced disease-induced retinal vascular permeability, increased the thickness of the inner nuclear layer and ganglion cell layer, and decreased the expression of VEGF, TNF-α, IL-6, IL-1β, NF-κB, vascular cell adhesion molecule-1, ICAM-1, and MCP-1 [211] (Table 2).
Other natural compounds, such as green tea catechins, particularly epigallocatechin gallate, have been nanoformulated into cationic lipid-based systems, significantly enhancing ocular permeability and bioavailability [212], whereas several phenolic acids, including gallic, ferulic, caffeic, chlorogenic, and rosmarinic acids, have been encapsulated using platforms such as lipid nanoparticles, silica nanoparticles, gold nanoparticles, poly (lactic-co-glycolic acid), and chitosan-based carriers. These nanocarriers have demonstrated the capacity to modulate key neuroinflammatory and oxidative stress-related signaling pathways, including NF-κB, MMP-9, and VEGF, thereby exhibiting promising therapeutic potential for the prevention and management of neurodegenerative processes associated with DR and other oxidative stress-related pathologies [213].
An additional innovative strategy involves the use of ROS-responsive nanoparticles, such as those loaded with essential oils derived from fructus Alpiniae zerumbet. These nanocarriers have shown efficacy in attenuating Müller cell activation and mitigating oxidative stress and inflammation during the early stages of DR [214].
Moreover, fenofibrate encapsulated within biodegradable poly (lactic-co-glycolic acid) nanoparticles has been shown to effectively reduce retinal vascular leakage, inhibit retinal leukostasis, and downregulate the overexpression of VEGF and ICAM-1 in experimental DR models, thereby enhancing retinal function without inducing detectable toxicity [215].
Furthermore, myricetin, a polyphenolic flavonoid with potent antioxidant properties, has recently been incorporated into a thermosensitive in situ, forming nanoemulgel to optimize ocular delivery. This formulation exhibited markedly enhanced corneal permeation and retention, favorable ocular biocompatibility, and a significant antioxidant and antiproliferative effect comparable to bevacizumab in retinoblastoma cell lines, positioning it as a promising non-invasive therapeutic candidate for the treatment of DR [216] (Table 2).
Antioxidant strategies based on nanotechnology have also expanded to include other natural flavonoids such as rutin and quercetin. Treatment with phyto-reduced nanoparticles loaded with rutin in an early-stage DR and cataract animal model showed superior antioxidant capacity compared to treatment with insulin and rutin, as indicated by analyses of MDA, CAT, and SOD levels [217] (Table 2). Likewise, the combination of quercetin with low-toxicity iron ions has emerged as a promising treatment, capable of mimicking antioxidant enzyme activity. Beyond their action against ROS, these compounds exhibit multitarget effects that confer both anti-inflammatory and anti-angiogenic properties relevant to DR [218].

Table 2.
Polyphenols and nanotechnology: Mechanisms, therapeutic effects, and limitations.
Table 2.
Polyphenols and nanotechnology: Mechanisms, therapeutic effects, and limitations.
| Polyphenol | Antioxidant Mechanism | Anti-Inflammatory Pathways Modulated | Comparative Effect vs. Conventional Therapies | Nanoparticle Type and Effect on Bioavailability | Limitations/Future Directions | Refs |
|---|---|---|---|---|---|---|
| Naringenin | VEGF inhibition, anti-angiogenic activity | Potential indirect inhibition of inflammatory mediators | Comparable efficacy to bevacizumab in animal models | Liposomal/solid lipid carriers; improved retinal penetration | Requires clinical validation; limited in vivo studies | [204] |
| Ellagic acid | Downregulation of VEGF, HIF-1α, GFAP | Potential neuroprotective anti-inflammatory effects | Promising multitarget anti-angiogenic activity | Liposomes; adequate tissue retention | Few direct comparisons; limited in vivo evidence | [205] |
| Curcumin | Reduces AGEs, enhances ATP synthesis | Decreases oxidative stress and pro-inflammatory cytokines | Complementary to insulin; systemic metabolic effects | Co-encapsulated in nanogels, liposomes, cyclodextrins | Low stability; lacks extensive clinical trials | [206,207] |
| Resveratrol | ROS scavenging, lipid peroxidation inhibition | NF-κB, IL-6, IL-1β, TNF-α, VCAM-1, ICAM-1 | Multitarget effects; comparable to anti-VEGF in some aspects | Gold nanoparticles; markedly enhances ocular bioavailability | Formulation standardization needed | [211] |
| Diosmin | Antioxidant activity in RPE cells | Reduces pro-inflammatory mediators | Potential topical ocular treatment | Nanostructured lipid carriers; improves aqueous solubility | Preclinical validation still lacking | [210] |
| Myricetin | Sustained antioxidant ROS reduction | Antiproliferative and ocular anti-inflammatory effects | Comparable to bevacizumab in cell models | Thermosensitive nanoemulgel; high corneal retention and biocompatibility | Lacks in vivo human data | [216] |
| Rutin | Increases SOD, CAT; lowers MDA | Indirect antioxidant activation with anti-inflammatory potential | Greater antioxidant effect than insulin-rutin combination | Phyto-reduced nanoparticles; enhanced delivery | Preliminary studies; not compared to standard therapies | [217] |
| Quercetin | Enzyme-like antioxidant capacity | NF-κB, IL-6, VEGF (indirectly) | Combined anti-inflammatory and anti-angiogenic action | Complexed with low-toxicity iron; sustained release | Early-stage mechanistic research | [218] |
AGE(s): Advanced glycation end products; ATP: Adenosine triphosphate; CAT: Catalase; GFAP: Glial fibrillary acidic protein; HIF-1α: Hypoxia-inducible factor 1-alpha; ICAM-1: Intercellular adhesion molecule-1; IL: Interleukin; MDA: Malondialdehyde; NF-κB: Nuclear factor kappa B; ROS: Reactive oxygen species; RPE: Retinal pigment epithelial; SOD: Superoxide dismutase; TNF-α: Tumor necrosis factor Alpha; VCAM-1: Vascular adhesion molecule-1; VEGF: Vascular endothelial growth factor.
6. Relevance of Polyphenol Therapy Against DR
Although, as shown, therapeutic approaches for advanced stages of DR have been developed in recent years, the reality is that not all diabetic patients suffering from this complication have access to such treatments. Low-income countries, regions, or individuals often face barriers to therapies and specific equipment that are, in many cases, simply inaccessible. Several studies have examined the relationship between socioeconomic status and diabetes-related microvascular complications.
In the United Kingdom, a prospective study including 28,339 patients from the UK Biobank demonstrated that individuals with higher income levels and lower Townsend deprivation index scores had a lower risk of developing DR [219]. Similarly, associations between area-level socioeconomic status and the development, progression, and vision loss due to DR have been reported in Asian [220] and Canadian populations [221]. The relationship between ocular diabetes-related complications and socioeconomic status can be attributed to several factors including:
- -
- Dietary patterns linked to socioeconomic status. A pro-oxidative diet, rich in red meat, sugar, processed foods, and saturated and trans fats, and low in fruits, vegetables, and mono- and polyunsaturated fats, has been associated with the development of diabetes [222] and DR [223]. Furthermore, this association is reinforced by the fact that healthier eating habits, such as diets rich in polyphenols, are more common among people with a higher socioeconomic status [224].
- -
- Lack of trust in healthcare systems among low-income individuals, which may lead to lower engagement with healthcare services [224].
- -
- Limited capacity to attend regular screenings, either due to the geographical distance of disadvantaged neighborhoods from ophthalmology clinics [225] or due to transportation difficulties or securing time off from work [226].
- -
- Low health literacy, often tied to lower educational attainment and socioeconomic status. Understanding and appropriately applying health information is crucial for enabling individuals to make informed decisions and actively participate in disease prevention and treatment [227]. In this context, poor glycemic control, often associated with lack of awareness and financial limitations, has serious implications for the development of DR [228].
- -
- Inability to afford healthcare costs, especially when access to vision care is dependent on insurance coverage, further limits access to treatment for individuals with fewer economic resources [228,229].
The Mediterranean diet has emerged as a promising non-pharmacological intervention for the management of diabetes and DR, as it can improve glycemic control, reduce oxidative stress and inflammation, and slow the progression of DR [230]. Among its components, polyphenols stand out as the most abundant phytochemicals, with substantial evidence supporting their benefits in chronic diseases such as diabetes [231]. As previously discussed, their antioxidant and anti-inflammatory properties offer protective effects against the development and progression of DR.
Despite limitations in their bioavailability, the direct use of polyphenols or their integration into delivery systems may help address many of the challenges associated with conventional therapies against DR. First, such approaches could help avoid the adverse effects linked to some current treatments. Furthermore, due to their lower cost, they could be used by the most vulnerable population and in impoverished regions. Some polyphenols have shown potential in supporting glycemic control, a key factor in preventing diabetes-related complications. Administering them from the moment of diabetes diagnosis could help to delay or prevent the onset and progression of DR and other chronic hyperglycemia-related complications, thereby significantly reducing the economic burden on national healthcare systems. When combined with effective patient education and communication strategies, polyphenol-based interventions may also help address antioxidant deficiencies and the high cost of care among socioeconomically disadvantaged populations. Moreover, slowing disease progression could allow for longer intervals between regular screening exams, making disease monitoring more feasible and accessible.
7. Conclusions
DR is a microvascular complication resulting from chronic hyperglycemia in the context of DM. Despite notable advances in diagnostic techniques over recent years, DR is commonly diagnosed upon the appearance of clinically visible lesions, such as microaneurysms, hard exudates, and retinal hemorrhages, hallmarks of retinal vascular damage. Consequently, current therapeutic interventions primarily aim to limit the progression of microvascular alterations to prevent irreversible vision loss.
Recent developments in imaging technologies, particularly OCTA, have enabled the acquisition of high-resolution, wide-field structural images of the retina. These advancements not only allow for improved visualization of peripheral retinal areas but also facilitate quantitative assessment of retinal thickness. OCT studies have revealed a significant thinning of the inner retinal layers, specifically involving the ganglion cell layer and the retinal nerve fiber layer [232,233,234,235]. This progressive thinning, and, consequently, the loss of retinal neurons, precedes the onset of microvascular lesions [232,234], leading to the redefinition of DR as a neurovascular complication [236]. While OCTA has already demonstrated significant utility in the diagnosis of DR, its full potential has yet to be realized. The in-depth microvascular analysis capabilities of OCTA, such as vascular density measurement, fractal dimension, tortuosity, and expanded field imaging, combined with longitudinal clinical studies and the integration of artificial intelligence capable of correlating imaging data with biochemical and molecular findings, are poised to become essential tools for both the diagnosis and prognosis of DR.
Currently, there is no treatment available that can cure DR. As previously noted, existing therapeutic strategies target vascular abnormalities that appear once irreversible cellular and molecular retinal damage has occurred. Although scientific advancements have extended the lifespan of individuals with diabetes by transforming the disease into a manageable chronic condition, this increased longevity is frequently accompanied by complications such as DR. In this context, it is proposed that, in addition to strict glycemic control, early intervention strategies should be implemented, ideally prior to the onset of phenotypically detectable signs of retinal damage, and as early as the initial diagnosis of diabetes.
Given the strong association between chronic hyperglycemia, oxidative stress, and the development of DR, antioxidant therapies may offer significant benefits. Although there are a limited number of long-term clinical studies involving large patient cohorts, polyphenols have emerged as a promising therapeutic approach. Regardless of whether they are used individually or in combination, polyphenols have demonstrated efficacy at the cellular level in slowing disease progression to advanced stages. However, a major limitation of such therapeutic strategies lies in their low bioavailability and poor tissue targeting. These challenges may be overcome through the development of novel delivery systems, such as those enabled by nanotechnology.
Author Contributions
Conceptualization, Á.L.O.; methodology, V.G.-J., R.B.-S.d.l.M. and Á.L.O.; investigation, V.G.-J., R.B.-S.d.l.M. and Á.L.O.; writing—original draft preparation, V.G.-J., R.B.-S.d.l.M. and Á.L.O.; writing—review and editing, Á.L.O.; visualization, V.G.-J., R.B.-S.d.l.M. and Á.L.O.; supervision, Á.L.O.; project administration, Á.L.O.; funding acquisition, Á.L.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Generalitat Valenciana, Conselleria de Educiación, Cultura, Universidades y Empleo, CIAICO/2023/096.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AGE(s): Advanced glycation end products; AKT: Protein Kinase B; AMPK: AMP-activated protein kinase; Ang-2: Angiopoietin-2; ATP: Adenosine triphosphate; BRB: Blood-retinal barrier; CAT: Catalase; CHOP: C/EBP homologous protein; COX-2: Cyclooxygenase-2; DME: Diabetic macular edema;DM: Diabetes mellitus;DM1: Diabetes mellitus type 1; DM2: Diabetes mellitus type 2; DR: Diabetic retinopathy; ERK: Extracellular signal-regulated kinase; FDA: Food and Drug Administration; GFAP: Glial fibrillary acidic protein; GSH: Glutathione (reduced form); GSSG: Glutathione disulfide (oxidized form); H2O2: Hydrogen peroxide; HIF-1α: Hypoxia-inducible factor 1-alpha; HO-1: Heme oxygenase-1; ICAM-1: Intercellular adhesion molecule-1; ICDRSS: International Classification of Diabetic Retinopathy Severity Scale; IL: Interleukin; LC3-B: Microtubule-associated proteins 1A/1B light chain 3B; MAPK: Mitogen-activated protein kinase; MCP-1: Monocyte chemoattractant protein-1; MDA: Malondialdehyde; MMP: Matrix metalloproteinase; mTOR: Mechanistic target of rapamycin; NAD+: Nicotinamide adenine dinucleotide; NADPH: Nicotinamide adenine dinucleotide phosphate; NF-κB: Nuclear factor kappa B; NLRP3: NOD-like receptor pyrin domain containing protein 3; NOS: Nitric oxide synthase; NPDR: Non-proliferative diabetic retinopathy; NRF2/Nrf2: Nuclear factor erythroid 2–related factor 2; OCT: Optical coherence tomography; OCTA: Optical coherence tomography angiography; : Superoxide anion; PDR: Proliferative diabetic retinopathy; PI3K: Phosphoinositide 3-kinase; PKC: Protein kinase C; PRP: Panretinal photocoagulation; RAGE: Receptor for advanced glycation end products; ROS: Reactive oxygen species; SOD: Superoxide dismutase; STAT3: Signal transducer and activator of transcription 3; TGF-β: Transforming growth factor beta; TNF-α: Tumor necrosis factor Alpha; VA: Visual acuity; VEGF: Vascular endothelial growth factor.
References
- Harreiter, J.; Roden, M. Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of Diabetes: An Overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Global. Available online: https://diabetesatlas.org/data-by-location/global/ (accessed on 17 April 2025).
- Yu, M.; Ning, F.T.E.; Liu, C.; Liu, Y.-C. Interconnections between Diabetic Corneal Neuropathy and Diabetic Retinopathy: Diagnostic and Therapeutic Implications. Neural Regen. Res. 2025, 20, 2169. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Church, K.A.; Pietramale, A.N.; Cardona, S.M.; Vanegas, D.; Rorex, C.; Leary, M.C.; Muzzio, I.A.; Nash, K.R.; Cardona, A.E. Fractalkine Isoforms Differentially Regulate Microglia-Mediated Inflammation and Enhance Visual Function in the Diabetic Retina. J. Neuroinflamm. 2024, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.-T.K.S.; de Clerck, E.; Polivka, J.; Potuznik, P.; Polivka, J.; et al. Diabetic Retinopathy as the Leading Cause of Blindness and Early Predictor of Cascading Complications-Risks and Mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Cloete, L. Diabetes Mellitus: An Overview of the Types, Symptoms, Complications and Management. Nurs. Stand. 2022, 37, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tan, T.-E.; Shao, Y.; Wong, T.Y.; Li, X. Classification of Diabetic Retinopathy: Past, Present and Future. Front. Endocrinol. 2022, 13, 1079217. [Google Scholar] [CrossRef] [PubMed]
- Maniadakis, N.; Konstantakopoulou, E. Cost Effectiveness of Treatments for Diabetic Retinopathy: A Systematic Literature Review. Pharmacoeconomics 2019, 37, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cioana, M.; Deng, J.; Nadarajah, A.; Hou, M.; Qiu, Y.; Chen, S.S.J.; Rivas, A.; Toor, P.P.; Banfield, L.; Thabane, L.; et al. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e231887. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Mounirou, B.A.M.; Adam, N.D.; Yakoura, A.K.H.; Aminou, M.S.M.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian. J. Endocrinol. Metab. 2022, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, O.-M.; José Alberto, C.-G.; José, N.-P.; Ernesto Germán, C.-M.; Ana Karen, L.-C.; Luis Miguel, R.-P.; Ricardo Raúl, R.-R.; Adolfo Daniel, R.-C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative Stress, Mitochondrial Damage and Diabetic Retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy: Management and Monitoring; National Institute for Health and Care Excellence: Clinical Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2024; ISBN 978-1-4731-6324-9.
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hua, R.; Zhao, Y.; Liu, L. Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies. J. Clin. Med. 2024, 13, 5439. [Google Scholar] [CrossRef] [PubMed]
- Moutray, T.; Evans, J.R.; Lois, N.; Armstrong, D.J.; Peto, T.; Azuara-Blanco, A. Different Lasers and Techniques for Proliferative Diabetic Retinopathy. Cochrane Database Syst. Rev. 2018, 3, CD012314. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; Danis, R.P.; Bressler, S.B.; Bressler, N.M.; Browning, D.J.; Qin, H. Diabetic Retinopathy Clinical Research Network. Effect of Focal/Grid Photocoagulation on Visual Acuity and Retinal Thickening in Eyes with Non-Center-Involved Diabetic Macular Edema. Retina 2009, 29, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.R.; Baker, C.W.; Beaulieu, W.T.; Bressler, N.M.; Punjabi, O.S.; Stockdale, C.R.; Wykoff, C.C.; Jampol, L.M.; Sun, J.K.; for the DRCR Retina Network. Assessment of the DRCR Retina Network Approach to Management with Initial Observation for Eyes with Center-Involved Diabetic Macular Edema and Good Visual Acuity: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sakini, A.S.A.; Hamid, A.K.; Alkhuzaie, Z.A.; Al-Aish, S.T.; Al-Zubaidi, S.; Tayem, A.A.; Alobi, M.A.; Sakini, A.S.A.; Al-Aish, R.T.; Al-Shami, K.; et al. Diabetic Macular Edema (DME): Dissecting Pathogenesis, Prognostication, Diagnostic Modalities along with Current and Futuristic Therapeutic Insights. Int. J. Retin. Vitr. 2024, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Kaiser, P.K.; Do, D.V.; Nguyen, Q.D.; Park, K.H.; Woo, S.J.; Sagong, M.; Bradvica, M.; Kim, M.Y.; Kim, S.; et al. Biosimilars of Anti-Vascular Endothelial Growth Factor for Ophthalmic Diseases: A Review. Surv. Ophthalmol. 2024, 69, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Penha, F.M.; Masud, M.; Khanani, Z.A.; Thomas, M.; Fong, R.D.; Smith, K.; Chand, A.; Khan, M.; Gahn, G.; Melo, G.B.; et al. Review of Real-World Evidence of Dual Inhibition of VEGF-A and ANG-2 with Faricimab in NAMD and DME. Int. J. Retin. Vitr. 2024, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Curran, K.; Lucenteforte, E.; Peto, T.; Parravano, M. Anti-vascular Endothelial Growth Factor for Diabetic Macular Oedema: A Network Meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD007419. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Garweg, J.G.; Regillo, C.; Souied, E.; Wolf, S.; Dhoot, D.S.; Agostini, H.T.; Chang, A.; Laude, A.; Wachtlin, J.; et al. KESTREL and KITE Phase 3 Studies: 100-Week Results With Brolucizumab in Patients With Diabetic Macular Edema. Am. J. Ophthalmol. 2024, 260, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Barakat, M.R.; Ip, M.S.; Wykoff, C.C.; Eichenbaum, D.A.; Joshi, S.; Warrow, D.; Sheth, V.S.; Stefanickova, J.; Kim, Y.S.; et al. Efficacy and Safety of Brolucizumab for Diabetic Macular Edema: The KINGFISHER Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Gabrielle, P.-H.; Hashimoto, Y.; Kibret, G.D.; Arnold, J.; Guillaumie, T.; Kheir, W.J.; Kok, G.; Vujosevic, S.; O’Toole, L.; et al. One-Year Anti-VEGF Therapy Outcomes in Diabetic Macular Edema Based on Treatment Intensity: Data from the Fight Retinal Blindness! Registry. Ophthalmol. Retin. 2024, 8, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Dervenis, P.; Dervenis, N.; Smith, J.M.; Steel, D.H. Anti-Vascular Endothelial Growth Factors in Combination with Vitrectomy for Complications of Proliferative Diabetic Retinopathy. Cochrane Database Syst. Rev. 2023, 5, CD008214. [Google Scholar] [CrossRef] [PubMed]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Baker, C.W.; Gerstenblith, A.T.; Jampol, L.M.; Meleth, A.; Martin, D.F.; Melia, M.; Punjabi, O.S.; et al. Four-Year Visual Outcomes in the Protocol W Randomized Trial of Intravitreous Aflibercept for Prevention of Vision-Threatening Complications of Diabetic Retinopathy. JAMA 2023, 329, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Hutton, D.W.; Stein, J.D.; Glassman, A.R.; Bressler, N.M.; Jampol, L.M.; Sun, J.K.; for the DRCR Retina Network. Five-Year Cost-Effectiveness of Intravitreous Ranibizumab Therapy vs Panretinal Photocoagulation for Treating Proliferative Diabetic Retinopathy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Prevost, A.T.; Vasconcelos, J.C.; Riddell, A.; Murphy, C.; Kelly, J.; Bainbridge, J.; Tudor-Edwards, R.; Hopkins, D.; Hykin, P.; et al. Clinical Efficacy of Intravitreal Aflibercept versus Panretinal Photocoagulation for Best Corrected Visual Acuity in Patients with Proliferative Diabetic Retinopathy at 52 Weeks (CLARITY): A Multicentre, Single-Blinded, Randomised, Controlled, Phase 2b, Non-Inferiority Trial. Lancet 2017, 389, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Touhami, S.; Cicinelli, M.V.; Agapitou, C.; Dimitriou, E.; Theodossiadis, G.; Theodossiadis, P. Disentangling the Association between Retinal Non-Perfusion and Anti-VEGF Agents in Diabetic Retinopathy. Eye 2022, 36, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Obeid, A.; Gao, X.; Ali, F.S.; Talcott, K.E.; Aderman, C.M.; Hyman, L.; Ho, A.C.; Hsu, J. Loss to Follow-Up in Patients with Proliferative Diabetic Retinopathy after Panretinal Photocoagulation or Intravitreal Anti-VEGF Injections. Ophthalmology 2018, 125, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Somfai, G.M.; de Smet, M.D.; Donati, G.; Menke, M.N.; Garweg, J.G.; Ceklic, L. The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers. Int. J. Mol. Sci. 2022, 23, 7585. [Google Scholar] [CrossRef] [PubMed]
- Gascon, P.; Borget, I.; Comet, A.; Carton, L.; Matonti, F.; Dupont-Benjamin, L. Costs Comparison of Treating Diabetic Macular Edema with Aflibercept, Ranibizumab or Dexamethasone at 1 Year in France (INVICOST Study). Eur. J. Ophthalmol. 2022, 32, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.S.; Haritoglou, C.; Ulbig, M.W. Cost Comparison of Licensed Intravitreal Therapies for Insufficiently Anti-VEGF Responding Fovea Involving Diabetic Macular Edema in Germany. Klin. Monbl Augenheilkd. 2019, 236, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hutton, D.W.; Glassman, A.R.; Liu, D.; Sun, J.K.; DRCR Retina Network. Cost-Effectiveness of Aflibercept Monotherapy vs Bevacizumab First Followed by Aflibercept If Needed for Diabetic Macular Edema. JAMA Ophthalmol. 2023, 141, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.K.; Wilson, D.J.; Flaxel, C.J.; Pope, S.I.; Lundquist, A.D.; Toomey, M.D.; Ira, S.D.; Vahrenwald, D.R.; Nolte, S.K.; Steinkamp, P.N.; et al. Vitrectomy Outcomes in Eyes with Diabetic Macular Edema and Vitreomacular Traction. Ophthalmology 2010, 117, 1087–1093.e3. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive Oxygen Species, Nox and Angiotensin II in Angiogenesis: Implications for Retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Coucha, M.; Elshaer, S.L.; Eldahshan, W.S.; Mysona, B.A.; El-Remessy, A.B. Molecular Mechanisms of Diabetic Retinopathy: Potential Therapeutic Targets. Middle East. Afr. J. Ophthalmol. 2015, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Guerra, M.; Singh, L.P.; Ibrahim, A.S. Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy. Antioxidants 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Sahajpal, N.S.; Goel, R.K.; Chaubey, A.; Aurora, R.; Jain, S.K. Pathological Perturbations in Diabetic Retinopathy: Hyperglycemia, AGEs, Oxidative Stress and Inflammatory Pathways. Curr. Protein Pept. Sci. 2019, 20, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Eshwaran, R.; Beck, S.C.; Hammes, H.-P.; Wieland, T.; Feng, Y. Contribution of the Hexosamine Biosynthetic Pathway in the Hyperglycemia-Dependent and -Independent Breakdown of the Retinal Neurovascular Unit. Mol. Metab. 2023, 73, 101736. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Arora, P.; Sandhir, R. Perturbed Biochemical Pathways and Associated Oxidative Stress Lead to Vascular Dysfunctions in Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2019, 2019, 8458472. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Buonfiglio, F.; Böhm, E.W.; Zhang, L.; Pfeiffer, N.; Korb, C.A.; Gericke, A. Diabetic Retinopathy: New Treatment Approaches Targeting Redox and Immune Mechanisms. Antioxidants 2024, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diabetes Rep. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.-T.; Zhang, J.-F. Inflammation in Diabetic Retinopathy: Possible Roles in Pathogenesis and Potential Implications for Therapy. Neural Regen. Res. 2022, 18, 976–982. [Google Scholar] [CrossRef]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef] [PubMed]
- Dagher, Z.; Park, Y.S.; Asnaghi, V.; Hoehn, T.; Gerhardinger, C.; Lorenzi, M. Studies of Rat and Human Retinas Predict a Role for the Polyol Pathway in Human Diabetic Retinopathy. Diabetes 2004, 53, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.C.; Nishimura, C.; Robison, W.G. Aldose Reductase and Polyol in Cultured Pericytes of Human Retinal Capillaries. Exp. Eye Res. 1989, 48, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Sima, A.A.; Nakajima, T.; Yagihashi, S.; Greene, D.A. Aldose Reductase in the BB Rat: Isolation, Immunological Identification and Localization in the Retina and Peripheral Nerve. Diabetologia 1987, 30, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Dănilă, A.-I.; Ghenciu, L.A.; Stoicescu, E.R.; Bolintineanu, S.L.; Iacob, R.; Săndesc, M.-A.; Faur, A.C. Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review. Biomedicines 2024, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Mathebula, S.D. Polyol Pathway: A Possible Mechanism of Diabetes Complications in the Eye. Afr. Vis. Eye Health 2015, 74, 5. [Google Scholar] [CrossRef]
- Mishra, B.; Swaroop, A.; Kandpal, R.P. Genetic Components in Diabetic Retinopathy. Indian J. Ophthalmol. 2016, 64, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J. Redox Imbalance Stress in Diabetes Mellitus: Role of the Polyol Pathway. Anim. Model. Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Silverman, S.M.; Liu, Y.; Wordinger, R.J.; Pang, I.-H.; Clark, A.F. In Vitro and In Vivo Neuroprotective Effects of cJun N-Terminal Kinase Inhibitors on Retinal Ganglion Cells. Mol. Neurodegener. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Gurel, Z.; Sheibani, N. O-Linked β-N-Acetylglucosamine (O-GlcNAc) Modification: A New Pathway to Decode Pathogenesis of Diabetic Retinopathy. Clin. Sci. 2018, 132, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Das Evcimen, N.; King, G.L. The Role of Protein Kinase C Activation and the Vascular Complications of Diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Sorenson, C.M.; Sheibani, N. Diabetes and Retinal Vascular Dysfunction. J. Ophthalmic Vis. Res. 2014, 9, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Ueda, S.; Matsui, T.; Nakamura, K.; Okuda, S. Role of Advanced Glycation End Products (AGEs) and Oxidative Stress in Diabetic Retinopathy. Curr. Pharm. Des. 2008, 14, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the Receptor for Advanced Glycation End Products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.-A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.-M.; et al. Local Low Dose Curcumin Treatment Improves Functional Recovery and Remyelination in a Rat Model of Sciatic Nerve Crush through Inhibition of Oxidative Stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Williamson, J.R. Hyperglycemic Cytosolic Reductive Stress “Pseudohypoxia”: Implications for Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1467–1470. [Google Scholar] [PubMed]
- Eichler, W.; Savković-Cvijić, H.; Bürger, S.; Beck, M.; Schmidt, M.; Wiedemann, P.; Reichenbach, A.; Unterlauft, J.D. Müller Cell-Derived PEDF Mediates Neuroprotection via STAT3 Activation. Cell. Physiol. Biochem. 2017, 44, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Simó-Servat, O.; Hernández, C.; Simó, R. Genetics in Diabetic Retinopathy: Current Concepts and New Insights. Curr. Genom. 2013, 14, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Zhong, Q.; Santos, J.M.; Kowluru, R.A. Mitochondria DNA Replication and DNA Methylation in the Metabolic Memory Associated with Continued Progression of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4881–4888. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Kowluru, R.A. Regulation of Matrix Metalloproteinase-9 by Epigenetic Modifications and the Development of Diabetic Retinopathy. Diabetes 2013, 62, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Santos, J.M.; Zhong, Q. Sirt1, a Negative Regulator of Matrix Metalloproteinase-9 in Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Lundstig, A.; Perfilyev, A.; Volkov, P.; Freiburghaus, T.; Lindholm, E.; Rönn, T.; Agardh, C.-D.; Ling, C. Genome-Wide Analysis of DNA Methylation in Subjects with Type 1 Diabetes Identifies Epigenetic Modifications Associated with Proliferative Diabetic Retinopathy. BMC Med. 2015, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 Gene Protects against the Development of Retinopathy in Diabetic Mice by Preventing Mitochondrial Damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Kowluru, R.A. DNA Methylation—A Potential Source of Mitochondria DNA Base Mismatch in the Development of Diabetic Retinopathy. Mol. Neurobiol. 2019, 56, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Abdelaziz, G.M.; Siddiquei, M.M.; Ahmad, A.; De Hertogh, G.; Abu El-Asrar, A.M. Cross-Talk between Sirtuin 1 and the Proinflammatory Mediator High-Mobility Group Box-1 in the Regulation of Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Curr. Eye Res. 2019, 44, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; Smith, S. Prediction of Diabetic Retinopathy: Role of Oxidative Stress and Relevance of Apoptotic Biomarkers. EPMA J. 2010, 1, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional Regulation of NF-κB by Reactive Oxygen Species: A Role of Unfolded Protein Response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid Peroxidation: Pathophysiological and Pharmacological Implications in the Eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhou, K.K.; Lee, K.; Gao, G.; Lyons, T.J.; Kowluru, R.; Ma, J. The Role of Lipid Peroxidation Products and Oxidative Stress in Activation of the Canonical Wingless-Type MMTV Integration Site (WNT) Pathway in a Rat Model of Diabetic Retinopathy. Diabetologia 2011, 54, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Krebs, M.P.; Reagan, A.M.; Howell, G.R. Vascular Inflammation Risk Factors in Retinal Disease. Annu. Rev. Vis. Sci. 2019, 5, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Aghadavod, E.; Mobini, M.; Heidari-Soureshjani, R.; Asemi, Z. Association between microRNAs Expression and Signaling Pathways of Inflammatory Markers in Diabetic Retinopathy. J. Cell. Physiol. 2019, 234, 7781–7787. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, A.S. Role of Oxidative Stress, Inflammation, Hypoxia and Angiogenesis in the Development of Diabetic Retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Kaur, H. The Role of Toll-Like Receptors in Diabetes-Induced Inflammation: Implications for Vascular Complications. Curr. Diab Rep. 2012, 12, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Capitão, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell. Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The Role of Inflammation in Immune System of Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic Retinopathy, a Vascular and Inflammatory Disease: Therapeutic Implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kotwani, A. Exploring the Various Aspects of the Pathological Role of Vascular Endothelial Growth Factor (VEGF) in Diabetic Retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin Restricts the Viability and Angiogenesis of Vascular Endothelial Cells by Suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the Eye and Anti-Inflammasome Therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Roh, K.-H.; Lim, N.-Y.; Park, S.J.; Park, S.; Kim, H.W. Role of the JAK/STAT Pathway in a Streptozotocin-Induced Diabetic Retinopathy Mouse Model. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Vanlandingham, P.A.; Nuno, D.J.; Quiambao, A.B.; Phelps, E.; Wassel, R.A.; Ma, J.-X.; Farjo, K.M.; Farjo, R.A. Inhibition of Stat3 by a Small Molecule Inhibitor Slows Vision Loss in a Rat Model of Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic Retinopathy Pathogenesis and the Ameliorating Effects of Melatonin; Involvement of Autophagy, Inflammation and Oxidative Stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic Macular Edema: Evidence-Based Management. Indian. J. Ophthalmol. 2018, 66, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T. Current Treatments for Diabetic Macular Edema. Int. J. Mol. Sci. 2023, 24, 9591. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Lois, N.; Medina, R.J.; Adamson, P.; Curtis, T.M. Advances in Our Understanding of Diabetic Retinopathy. Clin. Sci. 2013, 125, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yanyali, A.; Aytug, B.; Horozoglu, F.; Nohutcu, A.F. Bevacizumab (Avastin) for Diabetic Macular Edema in Previously Vitrectomized Eyes. Am. J. Ophthalmol. 2007, 144, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Classification of Oxidative Stress Based on Its Intensity. EXCLI J. 2014, 13, 922–937. [Google Scholar] [PubMed]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the Ocular Surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Are Major Contributors to Diabetes-Induced Oxidative Stress and Local Inflammation in the Retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.; Chan, P.-S.; Kern, T.S.; Kowluru, R.A. Oxidative Damage in the Retinal Mitochondria of Diabetic Mice: Possible Protection by Superoxide Dismutase. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Miller, C.M.; Kern, T.S. Hyperglycemia Increases Mitochondrial Superoxide in Retina and Retinal Cells. Free Radic. Biol. Med. 2003, 35, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Millán, I.; Desco, M.D.C.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Oliver, G.F.; Ashander, L.M.; Smith, J.R. Oxidative Stress and Its Regulation in Diabetic Retinopathy. Antioxidants 2023, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Atasi, L.; Ho, Y.-S. Role of Mitochondrial Superoxide Dismutase in the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.-S. Overexpression of Mitochondrial Superoxide Dismutase in Mice Protects the Retina from Diabetes-Induced Oxidative Stress. Free Radic. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Z.; Wang, W.; Liu, S.; Li, Y.; Sun, X.; Sutton, R.; Deng, L.; Liu, T.; Xia, Q.; et al. Resveratrol in Animal Models of Pancreatitis and Pancreatic Cancer: A Systematic Review with Machine Learning. Phytomedicine 2025, 139, 156538. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Momenai, R.; Karami-Mohajeri, S.; Ohadi, M.; Estabragh, M.A.R. Curcumin as a Natural Therapeutic Agent: A Rapid Review of Potential Clinical Uses and Mechanisms of Action. Iran. J. Pharm. Res. 2025, 24, e156983. [Google Scholar] [CrossRef]
- Ho, C.-H.; Fan, C.-K.; Chu, Y.-C.; Liu, S.-P.; Cheng, P.-C. Effects of Pterostilbene on Inducing Apoptosis in Normal Bladder and Bladder Cancer Cells. Tissue Cell 2025, 94, 102794. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sha, M.; Zhou, J.; Huang, Y. Quercetin Affects Apoptosis and Autophagy in Pediatric Acute Myeloid Leukaemia Cells by Inhibiting PI3K/AKT Signaling Pathway Activation through Regulation of miR-224-3p/PTEN Axis. BMC Cancer 2025, 25, 318. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of Epigallocatechin Gallate in Selected Malignant Neoplasms in Women. Nutrients 2025, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M. Resveratrol Supplementation and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4465–4480. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-E-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.D.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Aswar, U.; Vyas, N. Pterostilbene Ameliorates Type-2 Diabetes Mellitus—Induced Depressive-like Behavior by Mitigating Insulin Resistance, Inflammation and Ameliorating HPA Axis Dysfunction in Rat Brain. Brain Res. 2023, 1817, 148494. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An Effective Polyphenol in Alleviating Diabetes and Diabetic Complications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9163–9186. [Google Scholar] [CrossRef] [PubMed]
- Yurtseven, K.; Yücecan, S. Exploring the Potential of Epigallocatechin Gallate in Combating Insulin Resistance and Diabetes. Nutrients 2024, 16, 4360. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Nafady, M.H.; Islam, M.R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, M.H.-; Akhtar, M.F.; Perveen, A.; Md Ashraf, G.; et al. Resveratrol and Neuroprotection: An Insight into Prospective Therapeutic Approaches against Alzheimer’s Disease from Bench to Bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin Alleviates Alzheimer’s Disease by Inhibiting Inflammatory Response, Oxidative Stress and Activating the AMPK Pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Jia, L.; Zhou, Y.; Fu, Q.; Wang, Y.; Mu, D.; Wang, D.; Li, N.; Hou, Y. Pterostilbene Nanoemulsion Promotes Nrf2 Signaling Pathway to Downregulate Oxidative Stress for Treating Alzheimer’s Disease. Int. J. Pharm. 2024, 655, 124002. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Salazar, V.; Ruiz-Gabarre, D.; García-Escudero, V. Alzheimer’s Disease and Green Tea: Epigallocatechin-3-Gallate as a Modulator of Inflammation and Oxidative Stress. Antioxidants 2023, 12, 1460. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Ozawa, Y.; Kurihara, T.; Sasaki, M.; Yuki, K.; Miyake, S.; Noda, K.; Ishida, S.; Tsubota, K. Roles of AMP-Activated Protein Kinase in Diabetes-Induced Retinal Inflammation. Invest. Ophthalmol. Vis. Sci. 2011, 52, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, Y.; Huang, L.; Song, Y.; Yu, X.; Deng, B.; Zhou, X. Resveratrol Inhibits Neural Apoptosis and Regulates RAX/P-PKR Expression in Retina of Diabetic Rats. Nutr. Neurosci. 2022, 25, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, Y.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; et al. Resveratrol Inhibits Diabetic-Induced Müller Cells Apoptosis through MicroRNA-29b/Specificity Protein 1 Pathway. Mol. Neurobiol. 2017, 54, 4000–4014. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H.; Kittaneh, R.S.; Kilarkaje, N. Effects of Trans-Resveratrol on Type 1 Diabetes-Induced up-Regulation of Apoptosis and Mitogen-Activated Protein Kinase Signaling in Retinal Pigment Epithelium of Dark Agouti Rats. Eur. J. Pharmacol. 2021, 904, 174167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, S.-Y.; Song, Y.; Wang, Y.; Wan, Z.-W.; Sun, P.; Yu, X.-M.; Deng, B.; Zeng, K.-H. Resveratrol Protects Müller Cells Against Ferroptosis in the Early Stage of Diabetic Retinopathy by Regulating the Nrf2/GPx4/PTGS2 Pathway. Mol. Neurobiol. 2025, 62, 3412–3427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol Exhibits an Effect on Attenuating Retina Inflammatory Condition and Damage of Diabetic Retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri Soufi, F.; Arbabi-Aval, E.; Rezaei Kanavi, M.; Ahmadieh, H. Anti-Inflammatory Properties of Resveratrol in the Retinas of Type 2 Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-Resveratrol Inhibits Hyperglycemia-Induced Inflammation and Connexin Downregulation in Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Soufi, F.G.; Mohammad-nejad, D.; Ahmadieh, H. Resveratrol Improves Diabetic Retinopathy Possibly through Oxidative Stress—Nuclear Factor κB—Apoptosis Pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, J.; Wang, Z.; Yu, L.; Wang, J. Piceatannol Protects Human Retinal Pigment Epithelial Cells against Hydrogen Peroxide Induced Oxidative Stress and Apoptosis through Modulating PI3K/Akt Signaling Pathway. Nutrients 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Medoro, A.; Davinelli, S.; Scuderi, L.; Scuderi, G.; Scapagnini, G.; Fragiotta, S. Targeting Senescence, Oxidative Stress, and Inflammation: Quercetin-Based Strategies for Ocular Diseases in Older Adults. CIA 2025, 20, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, L.; Yao, G.-M.; Yan, H.-L.; Wang, L. Effects of Quercetin on Diabetic Retinopathy and Its Association with NLRP3 Inflammasome and Autophagy. Int. J. Ophthalmol. 2021, 14, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-R.; Liu, S.; Yang, H.-W.; Chen, X.-L. Quercetin Protects against Diabetic Retinopathy in Rats by Inducing Heme Oxygenase-1 Expression. Neural Regen. Res. 2021, 16, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; He, T.; Xing, Y.; Cao, T. Effects of Quercetin on the Expression of MCP-1, MMP-9 and VEGF in Rats with Diabetic Retinopathy. Exp. Ther. Med. 2017, 14, 6022–6026. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, L.; Yu, J. Effects of Blueberry Anthocyanins on Retinal Oxidative Stress and Inflammation in Diabetes through Nrf2/HO-1 Signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Du, S.; Ye, Z.; Yang, W.; Liu, Y. Blueberry Anthocyanin Extracts (BAEs) Protect Retinal and Retinal Pigment Epithelium Function from High-Glucose-Induced Apoptosis by Activating GLP-1R/Akt Signaling. J. Agric. Food Chem. 2025, 73, 5886–5898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green Tea Prevents Hyperglycemia-Induced Retinal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Ophthalmic Res. 2011, 47, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Green Tea Is Neuroprotective in Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-M.; Huang, J.-H.; Chiang, H.-S.; Wu, W.-B.; Lin, H.-H.; Hong, J.-Y.; Hung, C.-F. Effects of (-)-Epigallocatechin Gallate on RPE Cell Migration and Adhesion. Mol. Vis. 2010, 16, 586–595. [Google Scholar] [PubMed]
- Wang, L.; Sun, X.; Zhu, M.; Du, J.; Xu, J.; Qin, X.; Xu, X.; Song, E. Epigallocatechin-3-Gallate Stimulates Autophagy and Reduces Apoptosis Levels in Retinal Müller Cells under High-Glucose Conditions. Exp. Cell Res. 2019, 380, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, Z.; Lu, S.; Liu, A.; Liu, X. Naringin Attenuates Diabetic Retinopathy by Inhibiting Inflammation, Oxidative Stress and NF-κB Activation In Vivo and In Vitro. Iran. J. Basic. Med. Sci. 2017, 20, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, H.; Sun, H.; Zhang, Z.; Zheng, J.; Li, S.; Han, S. Eriodictyol Attenuates Cisplatin-Induced Kidney Injury by Inhibiting Oxidative Stress and Inflammation. Eur. J. Pharmacol. 2016, 772, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-F.; Guo, H.-J.; Huang, Y.; Wu, C.-T.; Zhang, X.-F. Eriodictyol, a Plant Flavonoid, Attenuates LPS-Induced Acute Lung Injury through Its Antioxidative and Anti-Inflammatory Activity. Exp. Ther. Med. 2015, 10, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol Prevents Early Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol Inhibits High Glucose-Induced Oxidative Stress and Inflammation in Retinal Ganglial Cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Protective Effect of Myricetin Derivatives from Syzygium Malaccense against Hydrogen Peroxide-Induced Stress in ARPE-19 Cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar] [PubMed]
- Kim, Y.S.; Kim, J.; Kim, K.M.; Jung, D.H.; Choi, S.; Kim, C.-S.; Kim, J.S. Myricetin Inhibits Advanced Glycation End Product (AGE)-Induced Migration of Retinal Pericytes through Phosphorylation of ERK1/2, FAK-1, and Paxillin In Vitro and In Vivo. Biochem. Pharmacol. 2015, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Mohamed, S.; Mustapha, N.M.; Fong, L.S.; Mohd Ishak, N.I. Gallic Acid and Myricetin-Rich Labisia Pumila Extract Mitigated Multiple Diabetic Eye Disorders in Rats. J. Food Biochem. 2021, 45, e13948. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Fang, J. Scutellarin Alleviates Diabetic Retinopathy via the Suppression of Nucleotide-Binding Oligomerization Domain (NOD)-Like Receptor Pyrin Domain Containing Protein 3 Inflammasome Activation. Curr. Eye Res. 2024, 49, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, X.-L.; Xu, M.; Chen, J.-L.; Wang, Y.-F.; Jie-Sun; Xiao, Y.-G.; Gao, A.-S.; Zhang, L.-C.; Liu, X.-Z.; et al. Network Pharmacology Mechanism of Scutellarin to Inhibit RGC Pyroptosis in Diabetic Retinopathy. Sci. Rep. 2023, 13, 6504. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Li, Y.; Yu, S.; Li, X.; Hu, Y.; Long, T.; Wang, L.; Li, W.; Ye, X.; Ke, Z.; et al. Scutellarin Prevents Angiogenesis in Diabetic Retinopathy by Downregulating VEGF/ERK/FAK/Src Pathway Signaling. J. Diabetes Res. 2019, 2019, 4875421. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zhang, T.; Ouyang, H.; Lu, B.; Wang, Z.; Ji, L. Scutellarin Alleviates Blood-Retina-Barrier Oxidative Stress Injury Initiated by Activated Microglia Cells during the Development of Diabetic Retinopathy. Biochem. Pharmacol. 2019, 159, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cheng, Y.; Dong, X.; Zhang, M.; Pei, C.; Zhang, M. Effects of Rhaponticin on Retinal Oxidative Stress and Inflammation in Diabetes through NRF2/HO-1/NF-κB Signalling. J. Biochem. Mol. Toxicol. 2020, 34, e22568. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; González-Reyes, S.; Pedraza-Chaverri, J. Natural Nrf2 Activators in Diabetes. Clin. Chim. Acta 2015, 448, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.-F.; Zhang, Q.; Liu, X.-Z. Protective Effects of Curcumin on Retinal Müller Cell in Early Diabetic Rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, J.; Ke, F.; Lan, M.; Li, D.; Tan, K.; Ling, J.; Wang, Y.; Wu, K.; Li, D. Curcumin Alleviates Diabetic Retinopathy in Experimental Diabetic Rats. Ophthalmic Res. 2018, 60, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Mrudula, T.; Suryanarayana, P.; Srinivas, P.N.B.S.; Reddy, G.B. Effect of Curcumin on Vascular Endothelial Growth Factor Expression in Diabetic Rat Retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2939. [Google Scholar]
- Khimmaktong, W.; Petpiboolthai, H.; Sriya, P.; Anupunpisit, V. Effects of Curcumin on Restoration and Improvement of Microvasculature Characteristic in Diabetic Rat’s Choroid of Eye. J. Med. Assoc. Thai 2014, 97 (Suppl. S2), S39–S46. [Google Scholar] [PubMed]
- Amini, S.; Dehghani, A.; Sahebkar, A.; Iraj, B.; Rezaeian-Ramsheh, A.; Askari, G.; Majeed, M.; Bagherniya, M. The Efficacy of Curcumin-Piperine Supplementation in Patients with Nonproliferative Diabetic Retinopathy: An Optical Coherence Tomography Angiography-Based Randomized Controlled Trial. J. Res. Med. Sci. 2024, 29, 64. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.M.; Bhuia, M.S.; Chowdhury, R.; Sheikh, S.; Ajmee, A.; Mollah, F.; Al Hasan, M.S.; Coutinho, H.D.M.; Islam, M.T. Potential Utilization of Ferulic Acid and Its Derivatives in the Management of Metabolic Diseases and Disorders: An Insight into Mechanisms. Cell Signal. 2024, 121, 111291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Y.; Wang, S.; He, Y.; Wang, W.; Li, Q.; Cao, X. Ferulic Acid Inhibits Advanced Glycation End Products (AGEs) Formation and Mitigates the AGEs-Induced Inflammatory Response in HUVEC Cells. J. Funct. Foods 2018, 48, 19–26. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Tan, H.; Zhang, Y.; Feng, Y. Protective Effect of a Chinese Medicine Formula He-Ying-Qing-Re Formula on Diabetic Retinopathy. J. Ethnopharmacol. 2015, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y.; Tan, H.-Y.; Li, S.; Wang, N.; Zhang, Y.; Feng, Y. Neuroprotective Effect of He-Ying-Qing-Re Formula on Retinal Ganglion Cell in Diabetic Retinopathy. J. Ethnopharmacol. 2018, 214, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zou, W.; Cao, X.; Xu, W.; Lu, Z.; Zhu, Y.; Hu, X.; Hu, J.; Zhu, Q. Ferulic Acid Attenuates High Glucose-Induced Apoptosis in Retinal Pigment Epithelium Cells and Protects Retina in Db/Db Mice. PeerJ 2022, 10, e13375. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Effect of Reinstitution of Good Glycemic Control on Retinal Oxidative Stress and Nitrative Stress in Diabetic Rats. Diabetes 2003, 52, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Nakamura, J.; Hamada, Y.; Naruse, K.; Nakashima, E.; Kato, K.; Kasuya, Y.; Yasuda, Y.; Kamiya, H.; Hotta, N. The Role of Polyol Pathway in Glucose-Induced Apoptosis of Cultured Retinal Pericytes. Diabetes Res. Clin. Pract. 2003, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Anderson, R. Dual Roles of Polyunsaturated Fatty Acids in Retinal Physiology and Pathophysiology Associated with Retinal Degeneration. Clin. Lipidol. 2009, 4, 821–827. [Google Scholar] [CrossRef]
- Liu, A.; Chang, J.; Lin, Y.; Shen, Z.; Bernstein, P.S. Long-Chain and Very Long-Chain Polyunsaturated Fatty Acids in Ocular Aging and Age-Related Macular Degeneration. J. Lipid Res. 2010, 51, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue Levels of Polyunsaturated Fatty Acids during Early Human Development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Terao, R.; Kaneko, H. Protective Effects and Molecular Signaling of N-3 Fatty Acids on Oxidative Stress and Inflammation in Retinal Diseases. Antioxidants 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Galano, J.-M.; Oger, C.; Leoncini, S.; Cortelazzo, A.; Ciccoli, L.; Durand, T.; Hayek, J.; Lee, J.C.-Y. Isoprostanoids in Clinical and Experimental Neurological Disease Models. Antioxidants 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, J.; Du, S.; Chen, Y.; Wang, S.; Wu, Q. ERK1/2/COX-2/PGE2 Signaling Pathway Mediates GPR91-Dependent VEGF Release in Streptozotocin-Induced Diabetes. Mol. Vis. 2014, 20, 1109–1121. [Google Scholar] [PubMed]
- Ayalasomayajula, S.P.; Kompella, U.B. Celecoxib, a Selective Cyclooxygenase-2 Inhibitor, Inhibits Retinal Vascular Endothelial Growth Factor Expression and Vascular Leakage in a Streptozotocin-Induced Diabetic Rat Model. Eur. J. Pharmacol. 2003, 458, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Millán, I.; Asensi, M.; Vento, M.; Oger, C.; Galano, J.-M.; Durand, T.; Ortega, Á.L. Analysis of Lipid Peroxidation by UPLC-MS/MS and Retinoprotective Effects of the Natural Polyphenol Pterostilbene. Antioxidants 2021, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, D.; Wang, J.; Huang, Q.; Zhang, H.; Liu, C.; Liu, X.; Ye, H. Study on the Mechanism of Plant Metabolites to Intervene Oxidative Stress in Diabetic Retinopathy. Front. Pharmacol. 2025, 16, 1517964. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf-Sánchez de Las Matas, R.; Torres-Cuevas, I.; Millán, I.; Desco, M.D.C.; Oblaré-Delgado, C.; Asensi, M.; Mena-Mollá, S.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Potential of Pterostilbene as an Antioxidant Therapy for Delaying Retinal Damage in Diabetic Retinopathy. Antioxidants 2025, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Namvar, E.; Attar, A.; Mortazavi, M. Progressing Nanotechnology to Improve Diagnosis and Targeted Therapy of Diabetic Retinopathy. Biomed. Pharmacother. 2025, 183, 117786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, N. Progress of Nanotechnology in Diabetic Retinopathy Treatment. Int. J. Nanomed. 2021, 16, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.; Kostyushev, D.; Zamyatnin, A.A.; Parodi, A. Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. Int. J. Transl. Med. 2021, 1, 306–322. [Google Scholar] [CrossRef]
- du Toit, L.C.; Choonara, Y.E.; Pillay, V. An Injectable Nano-Enabled Thermogel to Attain Controlled Delivery of P11 Peptide for the Potential Treatment of Ocular Angiogenic Disorders of the Posterior Segment. Pharmaceutics 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.E.-S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Nguyen, H.K.; Lee, J.E.; Suh, W. Therapeutic Effect of Apatinib-Loaded Nanoparticles on Diabetes-Induced Retinal Vascular Leakage. Int. J. Nanomed. 2016, 11, 3101–3109. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of Nanoencapsulation Using Poly (Lactide-Co-Glycolide) (PLGA) on Anti-Angiogenic Activity of Bevacizumab for Ocular Angiogenesis Therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Upadhya, A.; Tripathi, S.; Dighe, V.; Singh, K. Liposome-Polyethylenimine Complexes for the Effective Delivery of HuR siRNA in the Treatment of Diabetic Retinopathy. Drug Deliv. Transl. Res. 2023, 13, 1675–1698. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Makhmalzadeh, B.S.; Feghhi, M.; Rezai, A.; Bagheri, F. Evaluation of the Eff Ect of Naringenin Liposomal Formulation on Retinopathy in an Experimental Rabbit Model. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 469–479. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.; Liu, C.; Wang, C.; Zeng, X.; Yan, J.; Sun, Y. Efficiency Co-Delivery of Ellagic Acid and Oxygen by a Non-Invasive Liposome for Ameliorating Diabetic Retinopathy. Int. J. Pharm. 2023, 641, 122987. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Arora, M.; Dwivedi, S.; Chandrashekar, D.S.; Varambally, S.; Scott, E.M.; Kumar, M.N.V.R. Systemic Anti-Inflammatory Therapy Aided by Curcumin-Laden Double-Headed Nanoparticles Combined with Injectable Long-Acting Insulin in a Rodent Model of Diabetes Eye Disease. ACS Nano 2023, 17, 6857–6874. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Oliveira, D.; Cabral-Marques, H. Curcumin in Ophthalmology: Mechanisms, Challenges, and Emerging Opportunities. Molecules 2025, 30, 457. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, D.A.; Sreenivasa Reddy, S.; Bhanuprakash Reddy, G.; Katti, D.S. Simultaneous Amelioration of Diabetic Ocular Complications in Lens and Retinal Tissues Using a Non-Invasive Drug Delivery System. Int. J. Pharm. 2021, 608, 121045. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.A.; Escolar, M.; Perez, G.; Garcia-Quetglas, E.; Sadaba, B.; Azanza, J.R. Simultaneous Determination of Diosmin and Diosmetin in Human Plasma by Ion Trap Liquid Chromatography–Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry: Application to a Clinical Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2010, 51, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of Resveratrol Coated Gold Nanoparticles and Investigation of Their Effect on Diabetic Retinopathy in Streptozotocin Induced Diabetic Rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical Evaluation of Epigallocatechin Gallate-Loaded Cationic Lipid Nanoparticles (EGCG-LNs): In Vivo, In Vitro and Ex Vivo Studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Panda, S.P. Nexus of NFκB/VEGF/MMP9 Signaling in Diabetic Retinopathy-Linked Dementia: Management by Phenolic Acid-Enabled Nanotherapeutics. Life Sci. 2024, 358, 123123. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Geng, K.; Lu, X.; Shen, X.; Guo, Q. ROS-Responsive Nanoparticles with Antioxidative Effect for the Treatment of Diabetic Retinopathy. J. Biomater. Sci. Polym. Ed. 2025, 36, 440–461. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kushwaha, P.; Gupta, S. In Situ Forming Nanoemulgel for Diabetic Retinopathy: Development, Characterization, and in vitro Efficacy Assessment. Drug Res. 2025, 75, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Păpurică, A.-M.; Muntean, M.; Bungărdean, R.M.; Gheban, D.; Moldovan, B.; Katona, G.; David, L.; Filip, G.A. Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts. Metabolites 2023, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Tang, W.; Huang, Z.; Wang, X.; Gui, S.; Gao, X.; Xiao, D.; Tao, L.; Jiang, Z.; Wang, X. Ultrasmall Coordination Polymer Nanodots Fe-Quer Nanozymes for Preventing and Delaying the Development and Progression of Diabetic Retinopathy. Adv. Funct. Mater. 2023, 33, 2300261. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Z.; Chen, H.; Gu, C. Association of Socioeconomic Status with Diabetic Microvascular Complications: A UK Biobank Prospective Cohort Study. Diabetol. Metab. Syndr. 2025, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Low, J.R.; Gan, A.T.L.; Fenwick, E.K.; Gupta, P.; Wong, T.Y.; Teo, Z.L.; Thakur, S.; Tham, Y.C.; Sabanayagam, C.; Cheng, C.-Y.; et al. Role of Socio-Economic Factors in Visual Impairment and Progression of Diabetic Retinopathy. Br. J. Ophthalmol. 2021, 105, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Martinez, D.L.; Schlenker, M.B.; Ahmed, I.I.K. Socioeconomic Status and Vision Care Utilization in Canada: A Systematic Review. Can. J. Ophthalmol. 2025, 60, e541–e547. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Laddha, A.P.; Gaikwad, A.B.; Mulay, S.R.; Kulkarni, Y.A. Role of Dietary Modifications in the Management of Type 2 Diabetic Complications. Pharmacol. Res. 2021, 168, 105602. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Jiang, H. Association of Dietary Inflammatory Index with Ocular Diseases: A Population-Based Cross-Sectional Study. Eur. J. Med. Res. 2025, 30, 62. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Davenport, L. Barriers to Health Care Access for Low Income Families: A Review of Literature. J. Community Health Nurs. 2018, 35, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Y.; Rustam, Z.; Tran, D.; Han, D.; Bahrainian, M.; Channa, R.; Cai, C.X. Association of Neighborhood Socioeconomic Disadvantage with Proliferative Diabetic Retinopathy. Ophthalmol. Retin. 2025, 9, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Zaheer, H.A.; Clinger, O.; Liu, P.J.; Waxman, E.L.; McGinnis-Thomas, D.; Sahel, J.-A.; Williams, A.M. Characteristics Associated with Barriers to Eye Care: A Cross-Sectional Survey at a Free Vision Screening Event. Ophthalmic Res. 2023, 66, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Niyazmand, N.; Alam, K.; Wood, H.; Charng, J.; Gerritsma, S.; Niyazmand, H. Eye Health Literacy across the World and in Australia. Clin. Exp. Optom. 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.; Nikkhoo, B.; Miraki, P.; Rahmani, K. Investigating Metabolic Control and Complications in Type 2 Diabetic Patients with Low Income in Northwest of Iran, 2023. J. Health Popul. Nutr. 2025, 44, 38. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-P.; Buys, Y.M.; Hatch, W.; Trope, G.E. De-Insurance in Ontario Has Reduced Use of Eye Care Services by the Socially Disadvantaged. Can. J. Ophthalmol. 2012, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zooravar, D.; Soltani, P.; Khezri, S. Mediterranean Diet and Diabetic Microvascular Complications: A Systematic Review and Meta-Analysis. BMC Nutr. 2025, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is There Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, C.; Gong, Y.; Zhang, Y.; Jin, X.; Wei, S.; Zhang, M. Peripapillary Retinal Nerve Fiber Layer Changes in Preclinical Diabetic Retinopathy: A Meta-Analysis. PLoS ONE 2015, 10, e0125919. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.B.; Shin, Y.I.; Lee, M.W.; Park, G.S.; Kim, J.Y. Longitudinal Changes in the Peripapillary Retinal Nerve Fiber Layer Thickness of Patients With Type 2 Diabetes. JAMA Ophthalmol. 2019, 137, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, J.; Pollreisz, A.; Karst, S.; Hülsmann, M.; Hajdu, D.; Datlinger, F.; Egner, B.; Kriechbaum, K.; Pablik, E.; Schmidt-Erfurth, U.M. Longitudinal Analysis of Microvascular Perfusion and Neurodegenerative Changes in Early Type 2 Diabetic Retinal Disease. Br. J. Ophthalmol. 2022, 106, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J. A New View of Diabetic Retinopathy: A Neurodegenerative Disease of the Eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).