Adaptive Responses of Large Yellow Croaker Larimichthys crocea to Ocean Acidification: Integrative Analysis of Gill and Kidney Transcriptomics and Antioxidant Enzyme Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection
2.5. Histological Observations
2.6. Oxidative Stress Enzyme Activities
2.7. RNA Extraction and Sequencing
2.8. Validation of Sequencing Data
2.9. Statistical Analysis
3. Results
3.1. pH Fluctuations in Water Under Acidic Stress
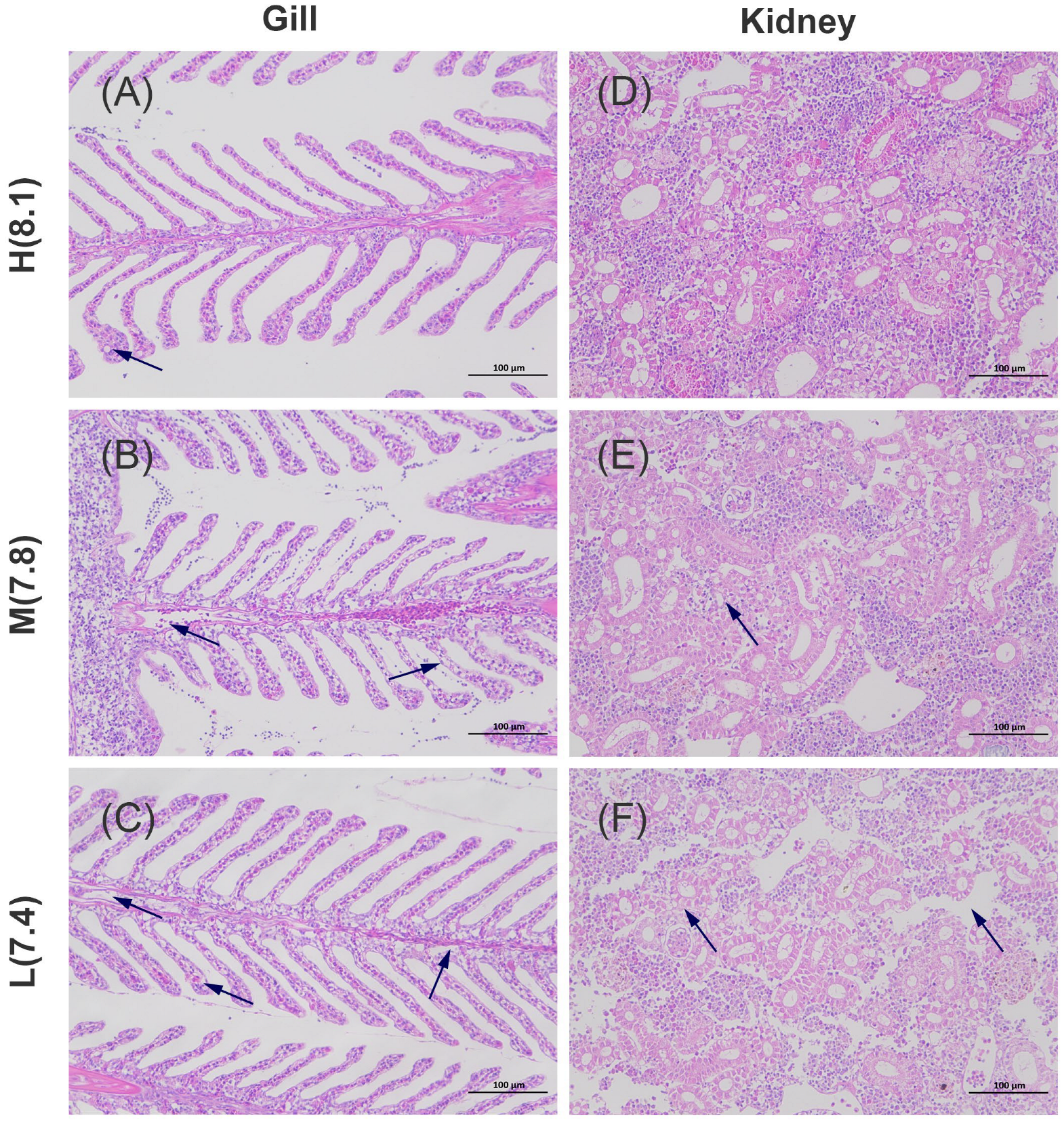
3.2. Histology Examination
3.3. Variation of Antioxidant Enzyme Activities
3.4. Sequencing Results and Differential Expression Analysis
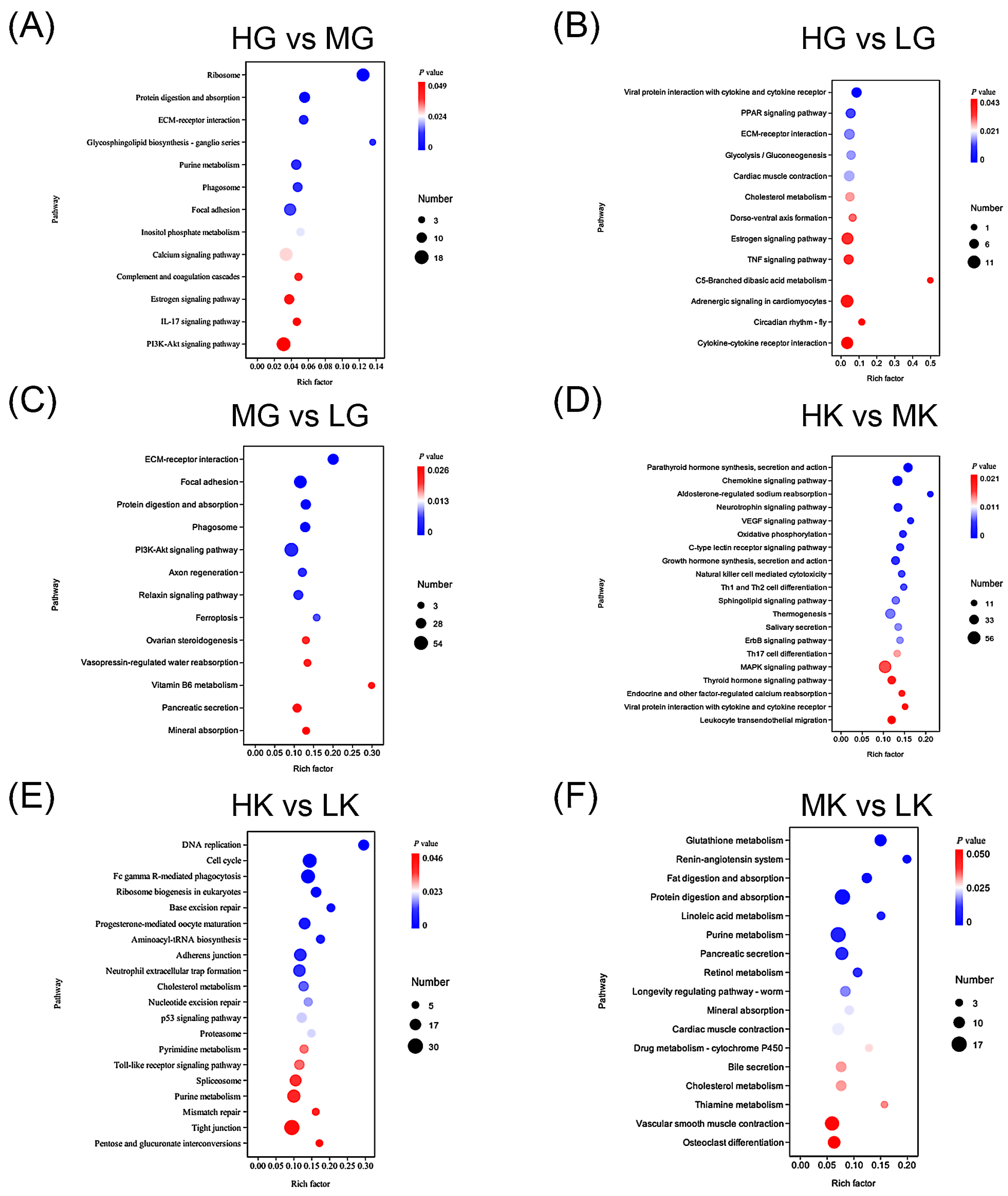
3.5. KEGG Pathway Enrichment Based on DEGs
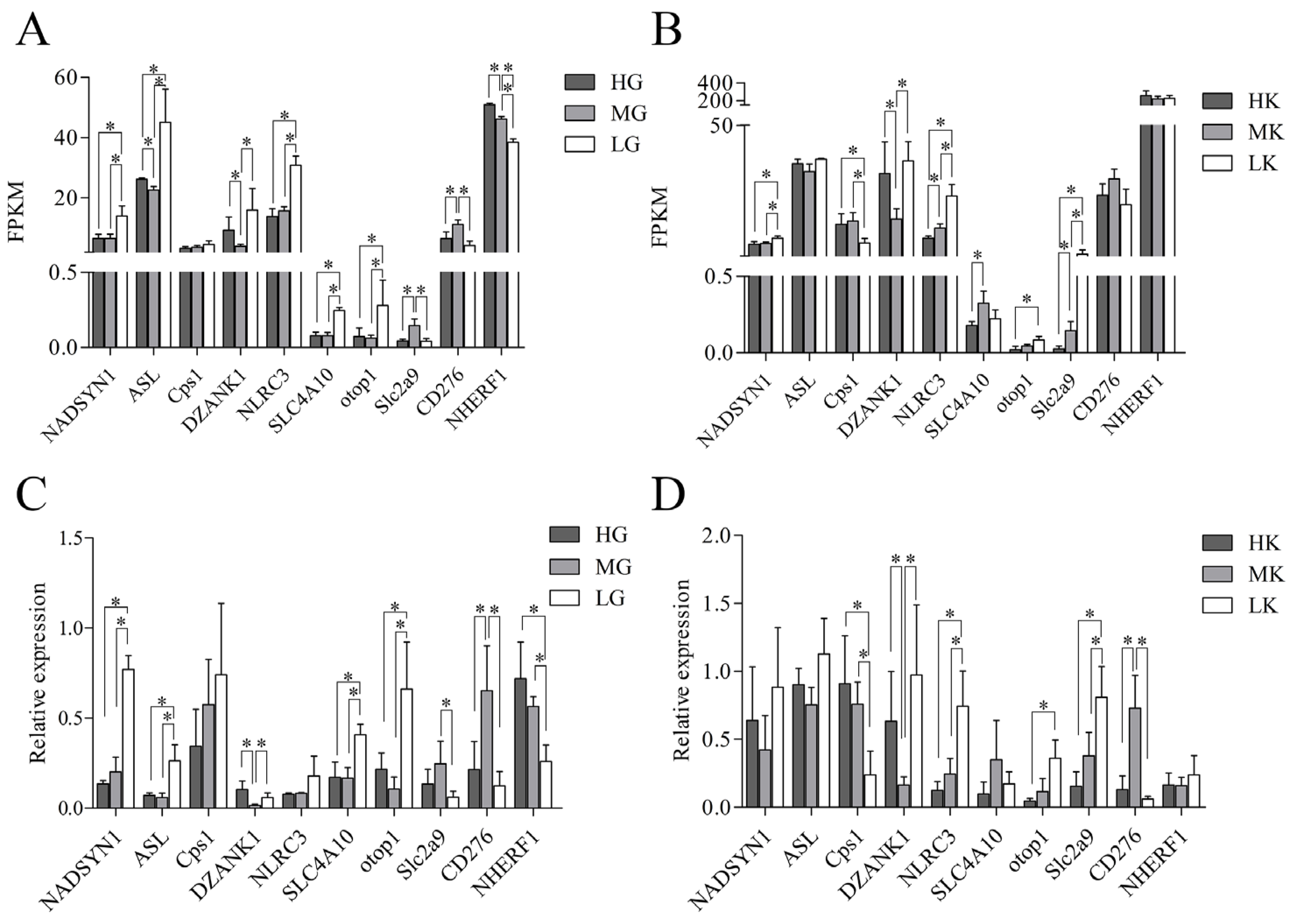
3.6. Sequencing Data Validation by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.R.; Jewett, E.B.; Reichert, J.; Robbins, L.; Shrestha, G.; Wieczorek, D.; Weisberg, S.B. Getting ocean acidification on decision makers’ to-do lists: Dissecting the process through case studies. Oceanography 2015, 28, 198–211. [Google Scholar] [CrossRef][Green Version]
- Lattuca, M.E.; Vanella, F.A.; Malanga, G.; Rubel, M.D.; Manríquez, P.H.; Torres, R.; Alter, K.; Marras, S.; Peck, M.A.; Domenici, P.; et al. Ocean acidification and seasonal temperature extremes combine to impair the thermal physiology of a sub-Antarctic fish. Sci. Total Environ. 2023, 856, 159284. [Google Scholar] [CrossRef] [PubMed]
- Marium, A.; Chatha, A.M.M.; Naz, S.; Khan, M.F.; Safdar, W.; Ashraf, I. Effect of temperature, pH, salinity and dissolved oxygen on fishes. J. Zool. System. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Servili, A.; Lévêque, E.; Mouchel, O.; Devergne, J.; Lebigre, C.; Roussel, S.; Mazurais, D.; Zambonino-Infante, J.L. Ocean acidification alters the acute stress response of a marine fish. Sci. Total Environ. 2023, 858, 159804. [Google Scholar] [CrossRef]
- Porteus, C.S.; Hubbard, P.C.; Uren Webster, T.M.; van Aerle, R.; Canário, A.V.M.; Santos, E.M.; Wilson, R.W. Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Clim. Change 2018, 8, 737–743. [Google Scholar] [CrossRef]
- Bignami, S.; Enochs, I.C.; Manzello, D.P.; Sponaugle, S.; Cowen, R.K. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc. Natl. Acad. Sci. USA 2013, 110, 7366–7370. [Google Scholar] [CrossRef]
- Coni, E.O.C.; Nagelkerken, I.; Ferreira, C.M.; Connell, S.D.; Booth, D.J. Ocean acidification may slow the pace of tropicalization of temperate fish communities. Nat. Clim. Change 2021, 11, 249–256. [Google Scholar] [CrossRef]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Olsen, S.H.; Elvevoll, E.O. pH-induced shift in hemoglobin spectra: A spectrophotometric comparison of Atlantic cod (Gadus morhua) and mammalian hemoglobin. J. Agric. Food Chem. 2011, 59, 1415–1422. [Google Scholar] [CrossRef]
- Strobel, A.; Bennecke, S.; Leo, E.; Mintenbeck, K.; Pörtner, H.O.; Mark, F.C. Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and pCO2. Front. Zool. 2012, 9, 28. [Google Scholar] [CrossRef]
- Gopi, N.; Rekha, R.; Vijayakumar, S.; Liu, G.X.; Monserrat, J.M.; Faggio, C.; Nor, S.A.M.; Vaseeharan, B. Interactive effects of freshwater acidification and selenium pollution on biochemical changes and neurotoxicity in Oreochromis mossambicus. Comp. Biochem. Phys. C Toxicol. Pharmacol. 2021, 250, 109161. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Shahsavarani, A.; Georgalis, T.; Bayaa, M.; Furimsky, M.; Thomas, S.L.Y. Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: Their role in ionic and acid-base regulation. J. Exp. Zool. 2003, 300, 53–62. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Yan, J.J.; Furukawa, F.; Hwang, P.P. Did acidic stress resistance in vertebrates evolve as Na+/H+ exchanger-mediated ammonia excretion in fish? BioEssays 2020, 42, 1900161. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Nagelkerken, I.; Connell, S.D.; Schunter, C. Natural CO2 seeps reveal adaptive potential to ocean acidification in fish. Evol. Appl. 2021, 14, 1794–1806. [Google Scholar] [CrossRef]
- Chuang, H.J.; Chiu, L.; Yan, J.J.; Chang, C.Y.; Tang, Y.H.; Chou, M.Y.; Yu, H.T.; Hwang, P.P. Responses of medaka (Oryzias latipes) ammonia production and excretion to overcome acidified environments. J. Hazard. Mater. 2023, 445, 130539. [Google Scholar] [CrossRef]
- Clark, T.D.; Raby, G.D.; Roche, D.G.; Binning, S.A.; Speers-Roesch, B.; Jutfelt, F.; Sundin, J. Ocean acidification does not impair the behaviour of coral reef fishes. Nature 2020, 577, 370–375. [Google Scholar] [CrossRef]
- Clements, J.C.; Sundin, J.; Clark, T.D.; Jutfelt, F. Meta-analysis reveals an extreme “decline effect” in the impacts of ocean acidification on fish behavior. PLoS Biol. 2022, 20, e3001511. [Google Scholar] [CrossRef]
- Sundin, J. The effects of ocean acidification on fishes—History and future outlook. J. Fish Biol. 2023, 103, 765–772. [Google Scholar] [CrossRef]
- Esbaugh, A.J. Physiological implications of ocean acidification for marine fish: Emerging patterns and new insights. J. Comp. Physiol. B 2018, 188, 1–13. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.Q.; Zhai, W.D.; Minella, M.; Vione, D.; Gao, K.; Minakata, D.; Arakaki, T.; Yoshioka, T.; Hayakawa, K.; et al. Reviews and syntheses: Ocean acidification and its potential impacts on marine ecosystems. Biogeosciences 2016, 13, 1767–1786. [Google Scholar] [CrossRef]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.I.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22, 8. [Google Scholar] [CrossRef]
- Cai, W.J.; Feely, R.A.; Testa, J.M.; Li, M.; Evans, W.; Alin, S.R.; Xu, Y.Y.; Pelletier, G.; Ahmed, A.; Greeley, D.J.; et al. Natural and anthropogenic drivers of acidification in large estuaries. Annu. Rev. Mar. Sci. 2021, 13, 23–55. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Hernandez-Ayon, J.M.; Ianson, D.; Hales, B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 2008, 320, 1490–1492. [Google Scholar] [CrossRef]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCulloch, M. Is ocean acidification an open ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar. Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef]
- Melzner, F.; Mark, F.C.; Seibel, B.A.; Tomanek, L. Ocean acidification and coastal marine invertebrates: Tracking CO2 effects from seawater to the cell. Ann. Rev. Mar. Sci. 2020, 12, 499–523. [Google Scholar] [CrossRef]
- Liu, M.; Mitcheson, Y. Profile of a fishery collapse: Why mariculture failed to save the large yellow croaker. Fish. Fish. 2008, 9, 219–242. [Google Scholar] [CrossRef]
- Liu, H.; Fan, S.J.; Xu, Q.L.; Wang, X.; Zhang, Y.L.; Chen, W.; Hu, Y.; Deng, X.Y.; Liu, H.Y.; Yang, C.Z.; et al. Germplasm innovation of large yellow croaker and its research progress. Reprod. Breed. 2025, 5, 44–53. [Google Scholar] [CrossRef]
- Tresguerres, M.; Clifford, A.M.; Harter, T.S.; Roa, J.N.; Thies, A.B.; Yee, D.P.; Brauner, C.J. Evolutionary links between intra-and extracellular acid-base regulation in fish and other aquatic animals. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 449–465. [Google Scholar] [CrossRef]
- Zimmer, A.M.; Perry, S.F. Physiology and aquaculture: A review of ion and acid-base regulation by the gills of fishes. Fish Fish. 2022, 23, 874–898. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Role of the kidneys in acid-base regulation and ammonia excretion in freshwater and seawater fish: Implications for nephrocalcinosis. Front. Physiol. 2023, 14, 1226068. [Google Scholar] [CrossRef]
- Claiborne, J.B.; Edwards, S.L.; Morrison-Shetlar, A.I. Acid-base regulation in fishes: Cellular and molecular mechanisms. J. Exp. Zool. 2002, 293, 302–319. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.B.; Wood, C.M. Kidney and urinary bladder responses of freshwater rainbow trout to isosmotic NaCl and NaHCO3 infusion. J. Exp. Biol. 1992, 173, 181–203. [Google Scholar] [CrossRef]
- Ivanis, G.; Braun, M.; Perry, S.F. Renal expression and localization of SLC9A3 sodium/hydrogen exchanger and its possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R971–R978. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.; Dichiera, A.M.; Jung, E.H.; Brauner, C.J. An atlas of plasma-accessible carbonic anhydrase availability in the model teleost, the rainbow trout. J. Comp. Physiol. B 2023, 193, 293–305. [Google Scholar] [CrossRef]
- Gracey, A.Y. Interpreting physiological responses to environmental change through gene expression profling. J. Exp. Biol. 2007, 210, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.C.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef]
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 2005, 110, C09S04. [Google Scholar] [CrossRef]
- Gattuso, J.P. Guide to Best Practices for Ocean Acidification Research and Data Reporting; Riebesell, U., Fabry, V., Hannson, L., Gattuso, J.P., Eds.; Publications Office of the European Union: Luxembourg, 2010; pp. 44–52. [Google Scholar]
- Weber, R.A.; Peleteiro, J.B.; García Martín, L.O.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Ye, T.; Tan, K.; Zhang, H.K.; Zheng, H.P. Potential causative factors of noble scallop Chlamys nobilis mass mortality in Nan’ao Island, Shantou, China in 2017. Sci. Total Environ. 2021, 751, 142268. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology, Oxygen Radicals in Biological Systems; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosenbraugh, N.; Farr, L.; Randall, R. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 182, 265–275. [Google Scholar] [CrossRef]
- Laverty, G.; Skadhauge, E. Adaptation of teleosts to very high salinity. Comp. Biochem. Physiol. A 2012, 163, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integ. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef]
- Tresguerres, M.; Hamilton, T.J. Acid-base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Blood biochemical variables, antioxidative status, and histological features of intestinal, gill, and liver tissues of African catfish (Clarias gariepinus) exposed to high salinity and high-temperature stress. Environ. Sci. Pollut. Res. 2022, 29, 56357–56369. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.; Longshaw, M.; Lyons, B.; Jones, G.; Green, M.; Feist, S. Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar. Environ. Res. 2003, 55, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Picos, R.A.; Sedeño-Díaz, J.E.; López-López, E. Histopathological indicators in fish for assessing environmental stress. In Environmental Indicators; Armon, R., Hänninen, O., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 663–675. [Google Scholar]
- Jadoon, S.; Malik, A. A review article on the formation, mechanism and biochemistry of MDA and MDA as a biomarker of oxidative stress. Int. J. Adv. Res. 2017, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Thophon, S.; Kruatrachue, M.; Upathan, E.S.; Pokethitiyook, P.; Sahaphong, S.; Jarikhuan, S. Histopathological alterations of white seabass, Lates calcarifer in acute and subchronic cadmium exposure. Environ. Pollut. 2003, 121, 307–320. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.J.; Wei, X.F.; Geng, C.Y.; Liu, W.Z.; Han, L.; Yuan, F.Y.; Wang, P.; Sun, Y.C. Effects of saline-alkaline stress on metabolome, biochemical parameters, and histopathology in the kidney of Crucian carp (Carassius auratus). Metabolites 2023, 13, 159. [Google Scholar] [CrossRef]
- Jing, Z.X.; Chen, Q.Y.; Yan, C.Z.; Zhang, C.Y.; Xu, Z.H.; Huang, X.L.; Wu, J.Y.; Li, Y.K.; Yang, S.Y. Effects of chronic heat stress on kidney damage, apoptosis, inflammation, and heat shock proteins of Siberian sturgeon (Acipenser baerii). Animals 2023, 13, 3733. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhou, S.J.; Wang, Y.G.; Sun, Y.Y.; Li, M.H.; Ma, Z.H. Effects of acidification stress on antioxidant and immunity in juvenile yellowfin tuna (Thunnus albacares). South China Fish. Sci. 2024, 20, 85–91. [Google Scholar]
- Damsgaard, C.; McGrath, M.; Wood, C.M.; Richards, J.G.; Brauner, C.J. Ion-regulation, acid/base-balance, kidney function, and effects of hypoxia in coho salmon, Oncorhynchus kisutch, after long-term acclimation to different salinities. Aquaculture 2020, 528, 735571. [Google Scholar] [CrossRef]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Chen, J.Q.; Cai, B.S.; Tian, C.X.; Jiang, D.N.; Shi, H.J.; Huang, Y.; Zhu, C.H.; Li, G.L.; Deng, S.P. RNA sequencing (RNA-Seq) analysis reveals liver lipid metabolism divergent adaptive response to low- and high-salinity stress in Spotted scat (Scatophagus argus). Animals 2023, 13, 1503. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid. Res. 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, R.; Ning, X.; You, L.; Mu, C.; Wang, C.; Wei, L.; Cong, M.; Wu, H.; Zhao, J. Effects of ocean acidification on immune responses of the Pacific oyster Crassostrea gigas. Fish Shellfish Immun. 2016, 49, 24–33. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Qin, J.G.; Ma, Z.H.; Yu, G. Acute acidification stress weakens the head kidney immune function of juvenile Lates calcarifer. Ecotox. Environ. Saf. 2021, 225, 112712. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zha, S.; Wang, Y.; Shi, W.; Xiao, G.; Chai, X.; Wu, H.; Liu, G. Benzo pyrene exposure under future ocean acidification scenarios weakens the immune responses of blood clam, Tegillarca granosa. Fish Shellfish Immun. 2017, 63, 465–470. [Google Scholar] [CrossRef]
- Machado, M.; Arenas, F.; Svendsen, J.C.; Azeredo, R.; Pfeifer, L.J.; Wilson, J.M.; Costas, B. Effects of water acidification on Senegalese sole Solea senegalensis health status and metabolic rate: Implications for immune responses and energy use. Front. Physiol. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Zimmermann, A.G.; Roberts, R.A.; Zhang, L.; Swanson, K.V.; Wen, H.; Davis, B.K.; Allen, I.C.; Holl, E.K.; Ye, Z.; et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 2012, 13, 823–831. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Files, D.B.; Liu, D.; Chen, L. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Campinho, M.A.; Mancera, J.M.; Martínez-Rodríguez, G.; Fuentes, J. Vasotocin and isotocin regulate aquaporin 1 function in the sea bream. J. Exp. Biol. 2015, 218, 684–693. [Google Scholar] [CrossRef]
- Tong, S.K.; Lee, H.L.; Lee, Y.C.; Wu, L.C.; Tsou, Y.L.; Lu, S.W.; Shih, S.W.; Hwang, P.P.; Chou, M.Y. Arginine vasopressin modulates ion and acid/base balance by regulating cell numbers of sodium chloride cotransporter and H+-ATPase rich ionocytes. Int. J. Mol. Sci. 2020, 21, 3957. [Google Scholar] [CrossRef]
- Chew, S.F.; Lp, Y.K. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar]
- Erez, A.; Nagamani, S.C.S.; Shchelochkov, O.A.; Premkumar, M.H.; Campeau, P.M.; Chen, Y.Q.; Garg, H.K.; Li, L.; Mian, A.; Bertin, T.K.; et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat. Med. 2011, 17, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.D.; Musa-Aziz, R.; Rojas, J.D.; Choi, I.; Daly, C.M.; Boron, W.F. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl−self-exchange activity. J. Biol. Chem. 2008, 283, 12777–12788. [Google Scholar] [CrossRef]
- Tu, Y.H.; Cooper, A.J.; Teng, B.; Chang, R.B.; Artiga, D.J.; Turner, H.N.; Mulhal, E.M.; Ye, W.; Smith, A.D.; Liman, E.R. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 2018, 359, 1047–1050. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Castro, A.; Ng, C.; Wang, Z.; Chaudhry, H.; Agbaje, Z.; Ulloa, G.A.; Yu, Y. The roles of two extracellular loops in proton sensing and permeation in human Otop1 proton channel. Commun. Biol. 2022, 5, 1110. [Google Scholar] [CrossRef]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014, 128, 26–34. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.C.; Sun, Y.L.; Chen, Y.; Chen, S.M.; Han, T.; Wang, J.T. Excessive substitution of fish meal with fermented soybean meal induces oxidative stress by impairing glutathione metabolism in Largemouth bass (Micropterus salmoides). Antioxidants 2023, 12, 2096. [Google Scholar] [CrossRef]
- Kumai, Y.; Bernier, N.J.; Perry, S.F. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J. Endocrinol. 2014, 220, 195–205. [Google Scholar] [CrossRef]
- Bader, M.; Ganten, D. Update on tissue renin-angiotensin systems. J. Mol. Med. 2008, 86, 615–621. [Google Scholar] [CrossRef]
- Tierney, M.L.; Luke, G.; Cramb, G.; Hazon, N. The role of the renin-angiotensin system in the control of blood pressure and drinking in the European eel, Anguilla anguilla. Gen. Comp. Endocrinol. 1995, 100, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hazon, N.; Tierney, M.L.; Takei, Y. Renin-angiotensin system in elasmobranch fish: A review. J. Exp. Zool. 1999, 284, 526–534. [Google Scholar] [CrossRef]
- Diez-Fernandez, C.; Hu, L.; Cervera, J.; Haeberle, J.; Rubio, V. Understanding carbamoyl phosphate synthetase (CPS1) deficiency by using the recombinantly purified human enzyme: Effects of CPS1 mutations that concentrate in a central domain of unknown function. Mol. Genet. Metab. 2014, 112, 123–132. [Google Scholar] [CrossRef] [PubMed]



| Tissues | Sample Name | Raw Reads | Clean Reads | Clean Data (bp) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|---|
| Gill | HG1 | 49,790,102 | 48,817,232 | 7.35 G | 98.40 | 95.77 | 46.05 |
| HG2 | 47,330,680 | 46,507,010 | 7.00 G | 98.37 | 95.63 | 44.52 | |
| HG3 | 59,354,782 | 58,282,594 | 8.78 G | 98.49 | 95.98 | 46.25 | |
| MG1 | 47,168,348 | 46,425,694 | 6.99 G | 98.45 | 95.76 | 46.91 | |
| MG2 | 56,646,552 | 55,621,006 | 8.38 G | 98.49 | 95.95 | 46.50 | |
| MG3 | 45,424,836 | 44,557,304 | 6.71 G | 98.45 | 95.87 | 46.13 | |
| LG1 | 62,603,754 | 61,397,376 | 9.23 G | 98.37 | 95.78 | 43.76 | |
| LG2 | 50,992,390 | 50,018,490 | 7.53 G | 98.39 | 95.76 | 44.85 | |
| LG3 | 36,776,506 | 36,168,694 | 5.44 G | 98.60 | 96.29 | 45.96 | |
| Kidney | HK1 | 44,555,002 | 43,673,624 | 6.57 G | 98.39 | 95.75 | 46.13 |
| HK2 | 48,738,750 | 47,954,210 | 7.22 G | 98.64 | 96.39 | 46.04 | |
| HK3 | 37,155,868 | 36,526,184 | 5.50 G | 98.57 | 96.17 | 46.39 | |
| MK1 | 49,127,630 | 48,366,024 | 7.29 G | 98.45 | 95.69 | 47.35 | |
| MK2 | 66,187,132 | 65,083,048 | 9.80 G | 98.56 | 96.07 | 47.36 | |
| MK3 | 54,160,660 | 53,111,080 | 8.00 G | 98.42 | 95.77 | 48.01 | |
| LK1 | 46,559,792 | 45,742,524 | 6.89 G | 98.52 | 96.06 | 46.38 | |
| LK2 | 56,608,916 | 55,531,990 | 8.36 G | 98.47 | 95.89 | 46.90 | |
| LK3 | 45,702,312 | 44,837,392 | 6.75 G | 98.45 | 95.90 | 45.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Zhang, X.; Liu, F.; Liang, X.; Guo, D.; Lou, B.; Xie, Z. Adaptive Responses of Large Yellow Croaker Larimichthys crocea to Ocean Acidification: Integrative Analysis of Gill and Kidney Transcriptomics and Antioxidant Enzyme Activities. Antioxidants 2025, 14, 872. https://doi.org/10.3390/antiox14070872
Ye T, Zhang X, Liu F, Liang X, Guo D, Lou B, Xie Z. Adaptive Responses of Large Yellow Croaker Larimichthys crocea to Ocean Acidification: Integrative Analysis of Gill and Kidney Transcriptomics and Antioxidant Enzyme Activities. Antioxidants. 2025; 14(7):872. https://doi.org/10.3390/antiox14070872
Chicago/Turabian StyleYe, Ting, Xiaoyan Zhang, Feng Liu, Xiao Liang, Dandan Guo, Bao Lou, and Zhigang Xie. 2025. "Adaptive Responses of Large Yellow Croaker Larimichthys crocea to Ocean Acidification: Integrative Analysis of Gill and Kidney Transcriptomics and Antioxidant Enzyme Activities" Antioxidants 14, no. 7: 872. https://doi.org/10.3390/antiox14070872
APA StyleYe, T., Zhang, X., Liu, F., Liang, X., Guo, D., Lou, B., & Xie, Z. (2025). Adaptive Responses of Large Yellow Croaker Larimichthys crocea to Ocean Acidification: Integrative Analysis of Gill and Kidney Transcriptomics and Antioxidant Enzyme Activities. Antioxidants, 14(7), 872. https://doi.org/10.3390/antiox14070872








