Hydnocarpin, a Natural Flavonolignan, Induces the ROS-Mediated Apoptosis of Ovarian Cancer Cells and Reprograms Tumor-Associated Immune Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Annexin V/PI
2.6. Western Blot
2.7. Mitochondrial Membrane Potential Assay
2.8. DCFH-DA Assay
2.9. Reverse Transcriptase PCR
2.10. Phagocytosis Assay
2.11. Statistical Analysis
3. Results
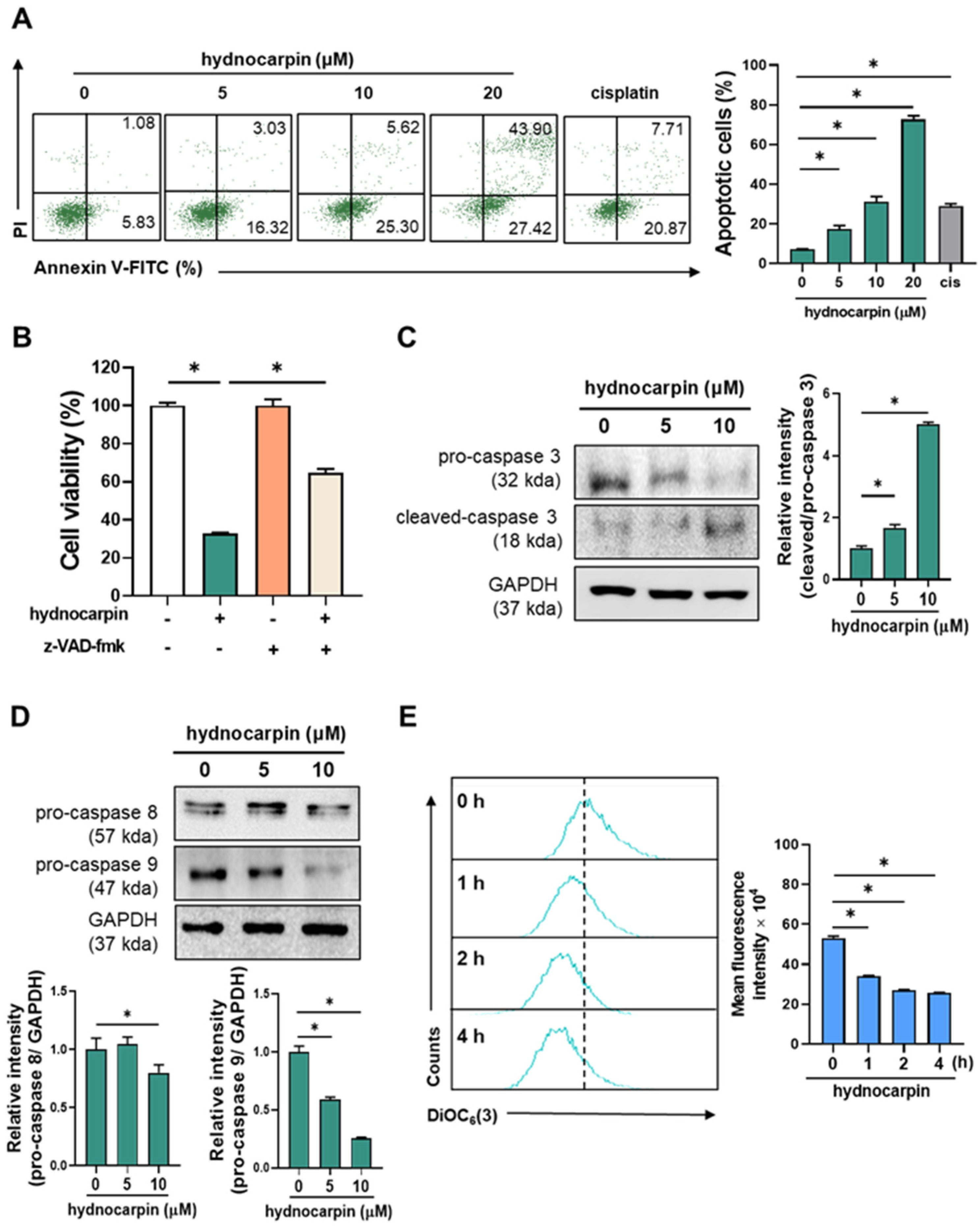
3.1. Hydnocarpin Induces the Caspase-Dependent Apoptosis of Human Ovarian Cancer Cells
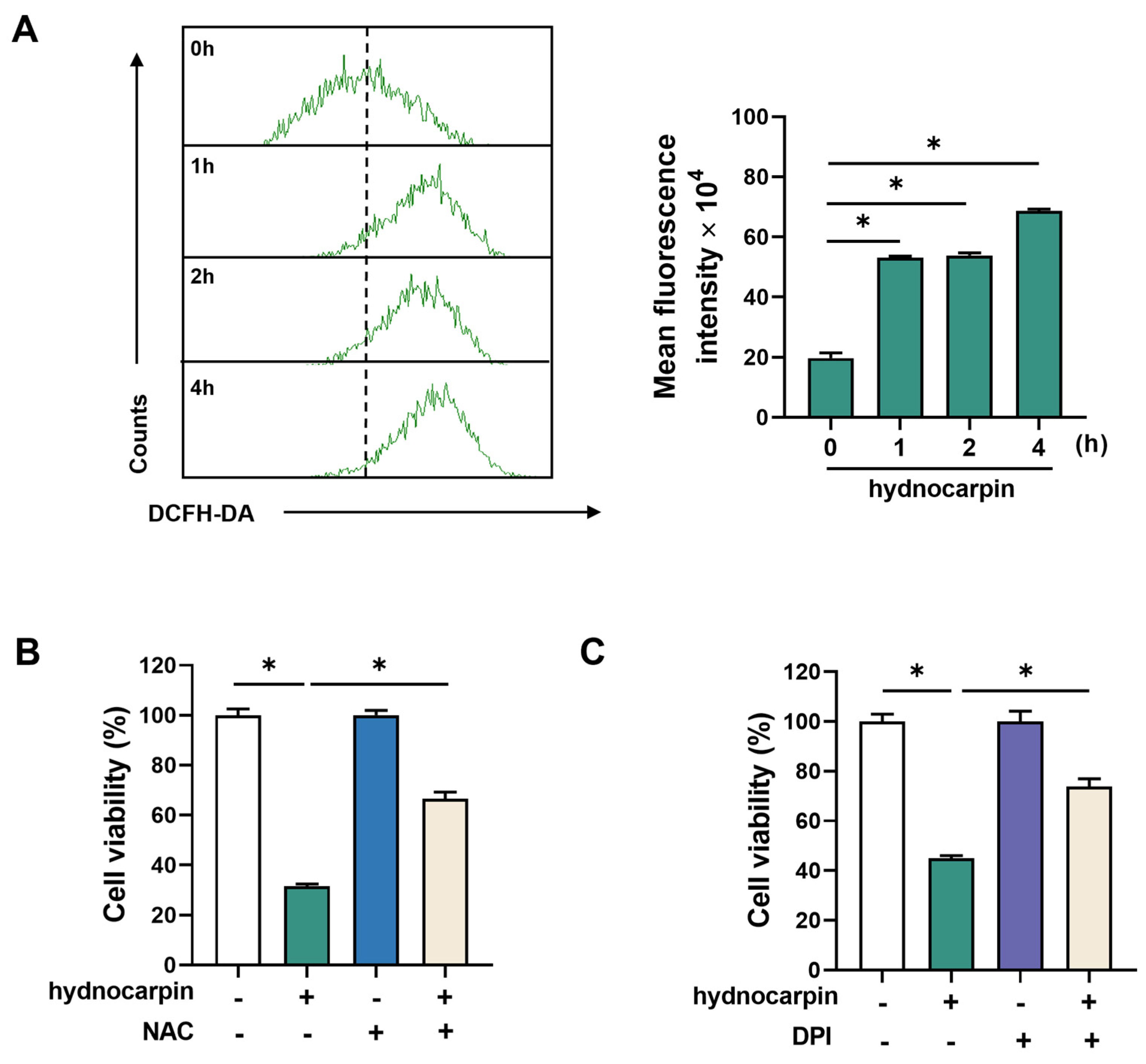
3.2. Hydnocarpin-Induced Ovarian Cancer Cell Apoptosis Involves Reactive Oxygen Species (ROS) Production and NADPH Oxidase (NOX) Activation
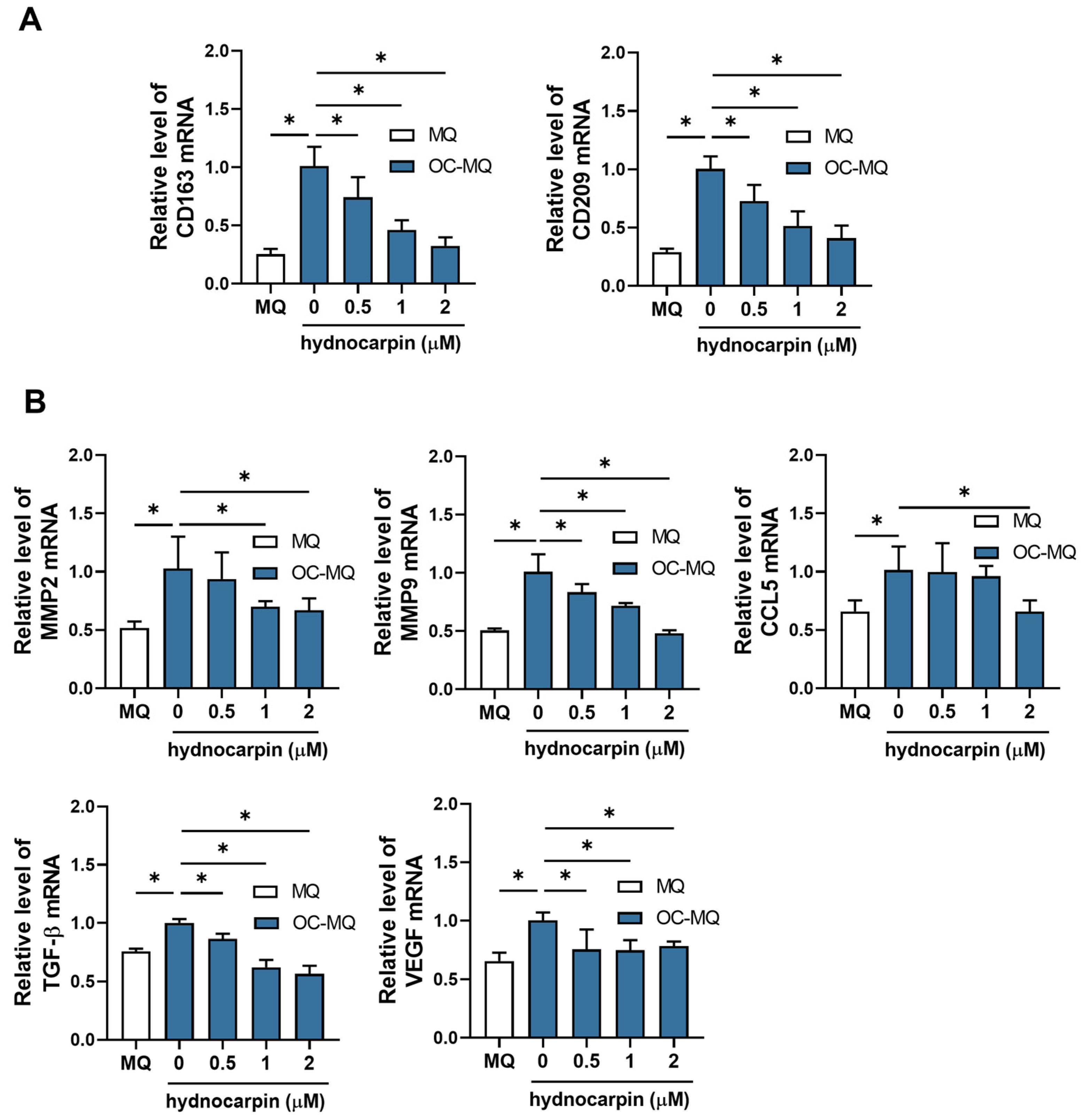
3.3. Hydnocarpin Reprograms the Ovarian Cancer-Stimulated MQs (OC-MQs) by Suppressing the Tumor-Promoting Genes and Enhancing the Phagocytic Activity
3.4. Hydnocarpin Modulates the Immune Evasion-Related Genes in the Tumor-Associated Immune Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| PF | PuerariaFlos |
| TAMs | Tumor-associated macrophages |
| ROS | Reactive oxygen species |
| CM | Conditioned media |
| MQs | Macrophages |
| OC-MQs | Ovarian cancer-stimulated macrophages |
| OC-TCs | Ovarian cancer-stimulated T cells |
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef]
- Lim, H.J.; Ledger, W. Targeted therapy in ovarian cancer. Womens Health 2016, 12, 363–378. [Google Scholar] [CrossRef]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent Pat. Anticancer Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Konishi, I. Immune checkpoint inhibition in ovarian cancer. Int. Immunol. 2016, 28, 339–348. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Zhou, S. Targeting tumor microenvironment in ovarian cancer: Premise and promise. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188361. [Google Scholar] [CrossRef]
- Kinjo, J.-E.; Takeshita, T.; Abe, Y.; Terada, N.; Yamashita, H.; Yamasaki, M.; Takeuchi, K.; Murakami, K.; Tomimatsu, T.; Nohara, T. Studies on the Constituents of Pueraria lobata. IV.: Chemical Constituents in the Flowers and the Leaves. Chem. Pharm. Bull. 1988, 36, 1174–1179. [Google Scholar] [CrossRef]
- Lau, C.S.; Carrier, D.J.; Beitle, R.R.; Howard, L.R.; Lay, J.O.; Liyanage, R.; Clausen, E.C. A glycoside flavonoid in kudzu (Pueraria lobata) identification, quantification, and determination of antioxidant activity. In Proceedings of the Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals, Chattanooga, TN, USA, 9 May–12 May 2004; pp. 783–794. [Google Scholar]
- Yuan, D.; Xie, Y.Y.; Bai, X.; Wu, X.; Yang, J.Y.; Wu, C.F. Inhibitory activity of isoflavones of Pueraria flowers on nitric oxide production from lipopolysaccharide-activated primary rat microglia. J. Asian Nat. Prod. Res. 2009, 11, 471–481. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Li, R.; Zhang, H.; Sun, Y.; Jiang, L.; Wang, X.; Xiong, Y. Flos Puerariae-Semen Hoveniae medicinal pair extract ameliorates DSS-induced inflammatory bowel disease through regulating MAPK signaling and modulating gut microbiota composition. Front. Pharmacol. 2022, 13, 1034031. [Google Scholar] [CrossRef]
- Lertpatipanpong, P.; Janpaijit, S.; Park, E.Y.; Kim, C.T.; Baek, S.J. Potential Anti-Diabetic Activity of Pueraria lobata Flower (Flos Puerariae) Extracts. Molecules 2020, 25, 3970. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Son, S.R.; Kim, J.Y.; Choi, J.H.; Jang, D.S. Chemical Constituents of the Flowers of Pueraria lobata and Their Cytotoxic Properties. Plants 2022, 11, 1651. [Google Scholar] [CrossRef] [PubMed]
- Vimberg, V.; Kuzma, M.; Stodůlková, E.; Novák, P.; Bednárová, L.; Šulc, M.; Gažák, R. Hydnocarpin-Type Flavonolignans: Semisynthesis and Inhibitory Effects on Staphylococcus aureus Biofilm Formation. J. Nat. Prod. 2015, 78, 2095–2103. [Google Scholar] [CrossRef]
- Sharma, D.K.; Hall, I.H. Hypolipidemic, anti-inflammatory, and antineoplastic activity and cytotoxicity of flavonolignans isolated from Hydnocarpus wightiana seeds. J. Nat. Prod. 1991, 54, 1298–1302. [Google Scholar] [CrossRef]
- Hong, H.; Lou, S.; Zheng, F.; Gao, H.; Wang, N.; Tian, S.; Huang, G.; Zhao, H. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway. Phytomedicine 2022, 101, 154143. [Google Scholar] [CrossRef]
- Lou, S.; Hong, H.; Maihesuti, L.; Gao, H.; Zhu, Z.; Xu, L.; Tian, S.; Kai, G.; Huang, G.; Zhao, H. Inhibitory effect of hydnocarpin D on T-cell acute lymphoblastic leukemia via induction of autophagy-dependent ferroptosis. Exp. Biol. Med. 2021, 246, 1541–1553. [Google Scholar] [CrossRef]
- Bultum, L.E.; Tolossa, G.B.; Lee, D. Combining empirical knowledge, in silico molecular docking and ADMET profiling to identify therapeutic phytochemicals from Brucea antidysentrica for acute myeloid leukemia. PLoS ONE 2022, 17, e0270050. [Google Scholar] [CrossRef]
- Lee, M.A.; Kim, W.K.; Park, H.J.; Kang, S.S.; Lee, S.K. Anti-proliferative activity of hydnocarpin, a natural lignan, is associated with the suppression of Wnt/β-catenin signaling pathway in colon cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 5511–5514. [Google Scholar] [CrossRef]
- Ou, H.L.; Wu, H.; Ren, Y.L.; Si, Y.; Duan, Z.Q.; Liu, X.W. Hydnocarpin inhibits malignant progression of triple negative breast cancer via CNOT4-mediated ubiquitination and degradation of YAP. China J. Chin. Mater. Medica. 2023, 48, 4483–4492. [Google Scholar] [CrossRef]
- Youmbi, L.M.; Makong, Y.S.D.; Mbaveng, A.T.; Tankeo, S.B.; Fotso, G.W.; Ndjakou, B.L.; Wansi, J.D.; Beng, V.P.; Sewald, N.; Ngadjui, B.T.; et al. Cytotoxicity of the methanol extracts and compounds of Brucea antidysenterica (Simaroubaceae) towards multifactorial drug-resistant human cancer cell lines. BMC Complement. Med. Ther. 2023, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Kalbacova, M.; Vrbacky, M.; Drahota, Z.; Melkova, Z. Comparison of the effect of mitochondrial inhibitors on mitochondrial membrane potential in two different cell lines using flow cytometry and spectrofluorometry. Cytom. A 2003, 52, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, H.; Wu, S. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim. Biophys. Acta 1998, 1404, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Chen, M.; Guerrero, A.D.; Huang, L.; Shabier, Z.; Pan, M.; Tan, T.H.; Wang, J. Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members. J. Biol. Chem. 2007, 282, 33888–33895. [Google Scholar] [CrossRef]
- Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta (BBA) Bioenerg. 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef]
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.J. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front. Immunol. 2019, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L.; O’Fallon, S.; Somoza, C.; Phillips, J.H.; Linsley, P.S.; Okumura, K.; Ito, D.; Azuma, M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 1995, 154, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mulati, K.; Hamanishi, J.; Matsumura, N.; Chamoto, K.; Mise, N.; Abiko, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Murakami, R.; et al. VISTA expressed in tumour cells regulates T cell function. Br. J. Cancer 2019, 120, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Sohn, I.C.; Kim, Y.K.; Choi, J.H.; Choi, J.W.; Park, H.J.; Itoh, Y.; Miyamoto, K. Tectorigenin, an isoflavone of Pueraria thunbergiana Benth., induces differentiation and apoptosis in human promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2001, 24, 1117–1121. [Google Scholar] [CrossRef]
- Arya, J.S.; Joseph, M.M.; Sherin, D.R.; Nair, J.B.; Manojkumar, T.K.; Maiti, K.K. Exploring Mitochondria-Mediated Intrinsic Apoptosis by New Phytochemical Entities: An Explicit Observation of Cytochrome c Dynamics on Lung and Melanoma Cancer Cells. J. Med. Chem. 2019, 62, 8311–8329. [Google Scholar] [CrossRef]
- Yang, W.H.; Huang, Z.; Wu, J.; Ding, C.C.; Murphy, S.K.; Chi, J.T. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol. Cancer Res. 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef]
- Liu, W.J.; Huang, Y.X.; Wang, W.; Zhang, Y.; Liu, B.J.; Qiu, J.G.; Jiang, B.H.; Liu, L.Z. NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells. Cells 2021, 10, 1647. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Dürr, P.; et al. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Motozawa, K.; Omagari, D.; Gojoubori, T.; Ikeda, T.; Asano, M.; Gionhaku, N. Comparison of MMP2 and MMP9 expression levels between primary and metastatic regions of oral squamous cell carcinoma. J. Oral Sci. 2016, 58, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef]
- Li, W.; Wu, F.; Zhao, S.; Shi, P.; Wang, S.; Cui, D. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 2022, 67, 49–57. [Google Scholar] [CrossRef]
- Sung, N.Y.; Yang, M.S.; Song, D.S.; Byun, E.B.; Kim, J.K.; Park, J.H.; Song, B.S.; Lee, J.W.; Park, S.H.; Park, H.J.; et al. The procyanidin trimer C1 induces macrophage activation via NF-κB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur. J. Pharmacol. 2013, 714, 218–228. [Google Scholar] [CrossRef]
- Altaf, H.; Revell, P.A. Evidence for active antigen presentation by monocyte/macrophages in response to stimulation with particles: The expression of NFκB transcription factors and costimulatory molecules. Inflammopharmacology 2013, 21, 279–290. [Google Scholar] [CrossRef]
- Jin, S.; Liu, W.; He, X.; Zhang, Y.; Chen, W.; Wu, Y.; Liu, J. VISTA deficiency exerts anti-tumor effects in breast cancer through regulating macrophage polarization. Int. Immunopharmacol. 2024, 136, 112365. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Jeong, R.L.; Lee, D.Y.; Cho, J.G. A New flavonolignan from the aerial Parts of Oryza sativa L. inhibits nitric oxide production in RAW 264.7 macrophage cells. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 865–870. [Google Scholar] [CrossRef]
- Ma, Q.G.; Wei, R.R.; Shang, D.L.; Sang, Z.P.; Dong, J.H. Structurally Diverse Flavonolignans with Immunosuppressive and Neuroprotective Activities from the Fruits of Hippophae rhamnoides L. J. Agric. Food Chem. 2020, 68, 6564–6575. [Google Scholar] [CrossRef]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Bueno Pérez, L.; Pan, L.; Sass, E.; Gupta, S.V.; Lehman, A.; Kinghorn, A.D.; Lucas, D.M. Potentiating effect of the flavonolignan (-)-hydnocarpin in combination with vincristine in a sensitive and P-gp-expressing acute lymphoblastic leukemia cell line. Phytother. Res. 2013, 27, 1735–1738. [Google Scholar] [CrossRef]
- Shrivastav, A.; Singh, D.; Chaudhary, D.S.; Verma, N.; Mishra, A.K. Assessment of the Dermal Toxicity of Hydnocarpus wightiana Seeds Extract in Experimental Rats. Biosci. Biotechnol. Res. Asia 2024, 21, 1029–1036. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39 (Suppl. S2), W270–W277. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kim, Y.; Woo, S.-Y.; Kim, J.-O.; Kim, H.; Son, S.-R.; Jang, D.S.; Choi, J.-H. Hydnocarpin, a Natural Flavonolignan, Induces the ROS-Mediated Apoptosis of Ovarian Cancer Cells and Reprograms Tumor-Associated Immune Cells. Antioxidants 2025, 14, 846. https://doi.org/10.3390/antiox14070846
Kim J-Y, Kim Y, Woo S-Y, Kim J-O, Kim H, Son S-R, Jang DS, Choi J-H. Hydnocarpin, a Natural Flavonolignan, Induces the ROS-Mediated Apoptosis of Ovarian Cancer Cells and Reprograms Tumor-Associated Immune Cells. Antioxidants. 2025; 14(7):846. https://doi.org/10.3390/antiox14070846
Chicago/Turabian StyleKim, Jae-Yoon, Yejin Kim, Soo-Yeon Woo, Jin-Ok Kim, Hyunsoo Kim, So-Ri Son, Dae Sik Jang, and Jung-Hye Choi. 2025. "Hydnocarpin, a Natural Flavonolignan, Induces the ROS-Mediated Apoptosis of Ovarian Cancer Cells and Reprograms Tumor-Associated Immune Cells" Antioxidants 14, no. 7: 846. https://doi.org/10.3390/antiox14070846
APA StyleKim, J.-Y., Kim, Y., Woo, S.-Y., Kim, J.-O., Kim, H., Son, S.-R., Jang, D. S., & Choi, J.-H. (2025). Hydnocarpin, a Natural Flavonolignan, Induces the ROS-Mediated Apoptosis of Ovarian Cancer Cells and Reprograms Tumor-Associated Immune Cells. Antioxidants, 14(7), 846. https://doi.org/10.3390/antiox14070846





