Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease
Abstract
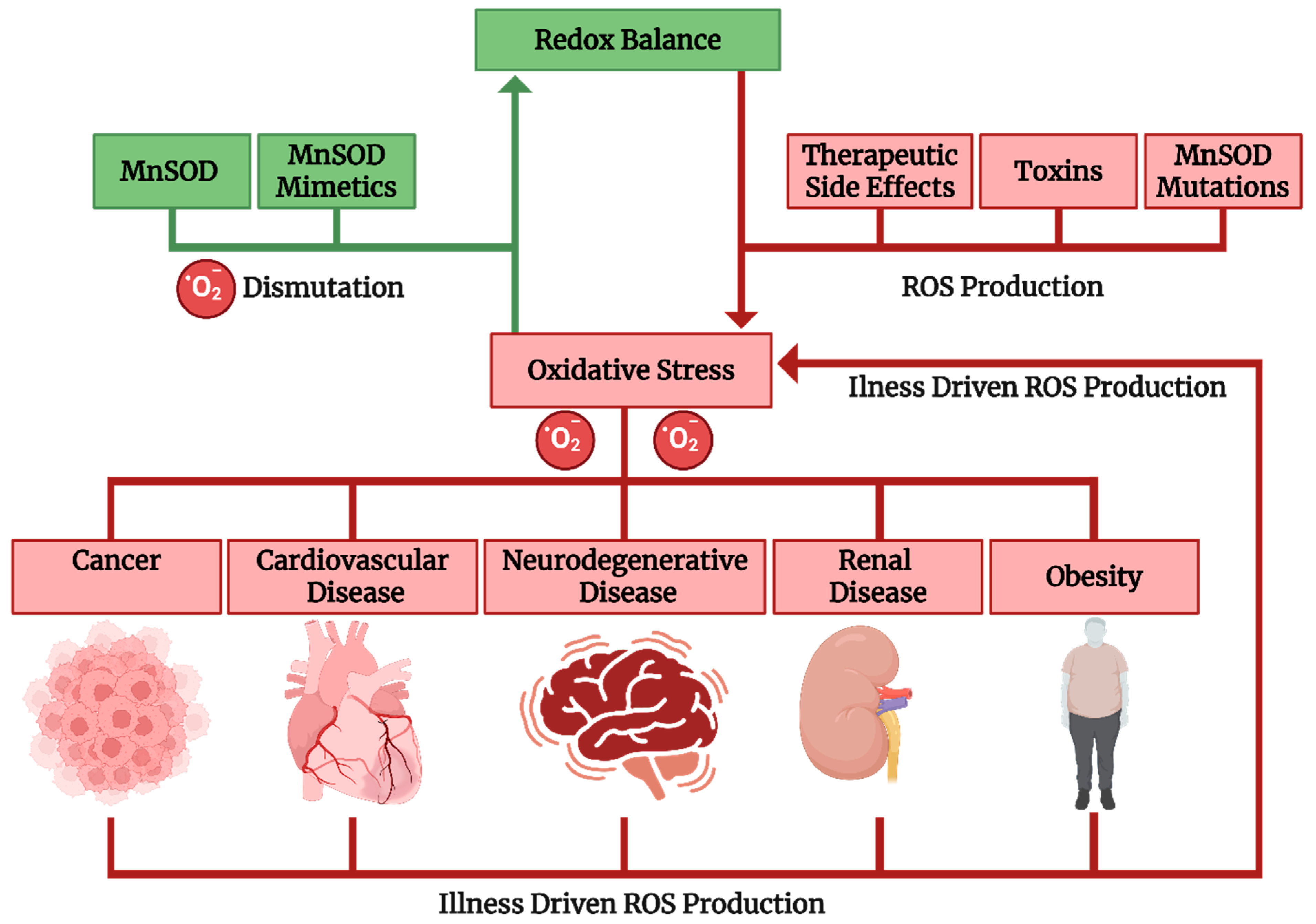
1. Introduction
2. MnSOD
2.1. Structure and Mechanism of Action
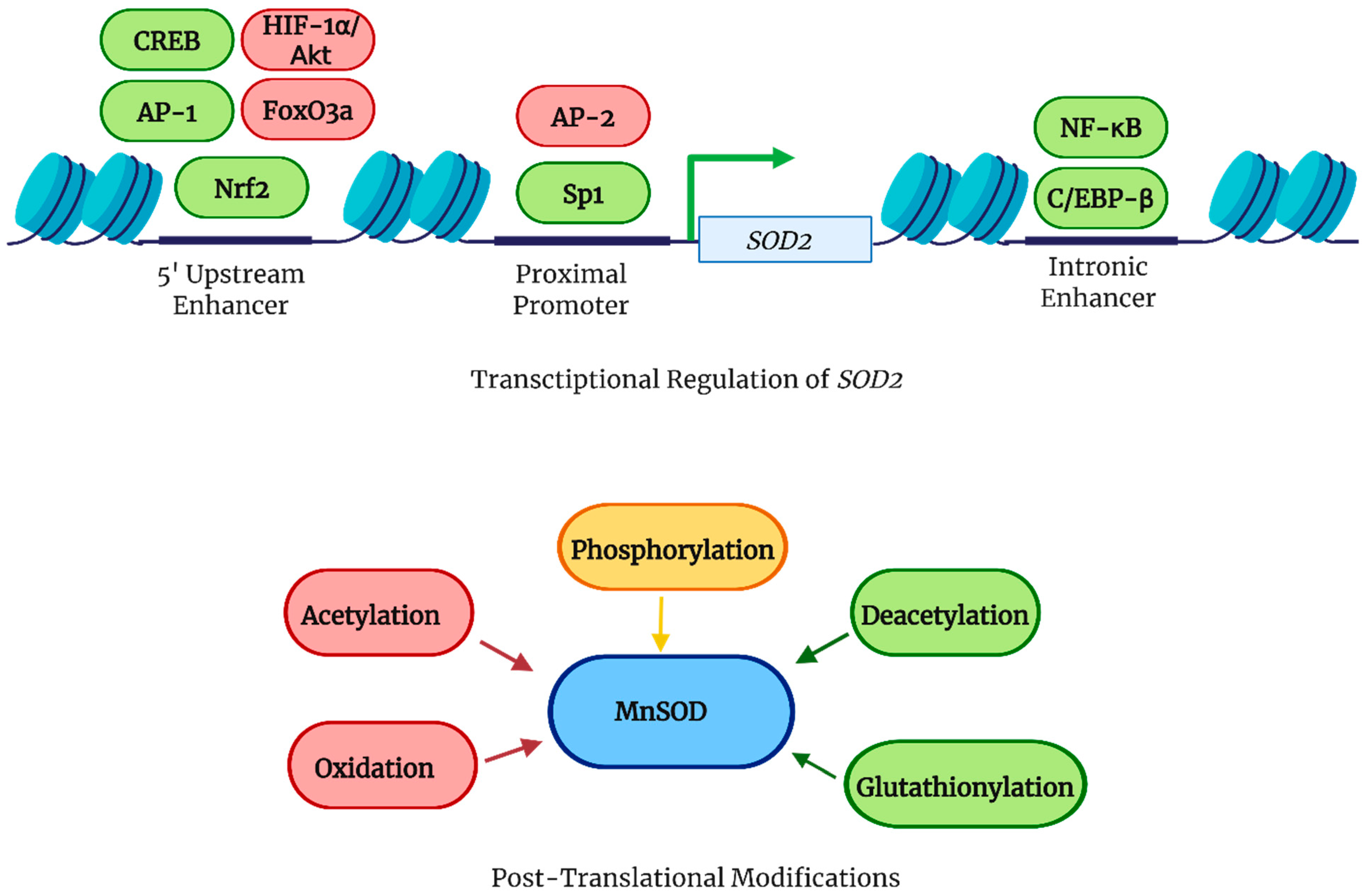
2.2. Transcriptional and Post-Translational Regulation of MnSOD
2.3. MnSOD as a Thermoreceptor
2.4. MnSOD and Obesity
2.5. MnSOD and Renal Disease
2.6. MnSOD in Cardiovascular Disease
2.7. MnSOD in Cancer
2.8. Implication in Neurodegenerative Diseases
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AF | Atrial fibrillation |
| AML | Acute myeloid leukemia |
| AMPK | AMP-activated protein kinase |
| AP-1 AP-2 | Activator protein 1 Activator protein 2 |
| ATF | Activating transcription factor |
| CPET | Concerted proton–electron transfer |
| CREB | cAMP response element-binding protein |
| DJ-1 | the multifunctional redox-sensitive protein DJ-1 (PARK7) |
| DMF | Dimethyl fumarate |
| ERK | Extracellular signal-regulated kinase |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| IL | Interleukin |
| MS | Multiple sclerosis |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Reduced nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor kappa B |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ONOO | Peroxynitrite |
| PD | Parkinson’s disease |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKC | Protein kinase C |
| PPHN | Persistent pulmonary hypertension of the newborn |
| ROS | Reactive oxygen species |
| SIRT3 | Sirtuin 3 |
| STZ | Streptozotocin |
| TCA | Tricarboxylic acid |
| WAT1 | First-shell water ligand bound to manganese ion |
References
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Borgstahl, G.E.; Parge, H.E.; Hickey, M.J.; Beyer, W.F., Jr.; Hallewell, R.A.; Tainer, J.A. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell 1992, 71, 107–118. [Google Scholar] [CrossRef]
- Karnati, S.; Lüers, G.; Pfreimer, S.; Baumgart-Vogt, E. Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes. Histochem. Cell Biol. 2013, 140, 105–117. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968, 243, 5753–5760. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.B.; McCord, J.; Fridovich, I. Superoxide dismutase from Escherichia coli B: A new manganese-containing enzyme. J. Biol. Chem. 1970, 245, 6176–6181. [Google Scholar] [CrossRef] [PubMed]
- Creagan, R.; Tischfield, J.; Ricciuti, F.; Ruddle, F.H. Chromosome assignments of genes in man using mouse-human somatic cell hybrids: Mitochondrial superoxide dismutase (indophenol oxidase-B, tetrameric) to chromosome 6. Humangenetik 1973, 20, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Barra, D.; Schinina, M.E.; Simmaco, M.; Bannister, J.V.; Bannister, W.H.; Rotilio, G.; Bossa, F. The primary structure of human liver manganese superoxide dismutase. J. Biol. Chem. 1984, 259, 12595–12601. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide dismutase and neurological disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Ninić, A.; Sopić, M.; Munjas, J.; Spasojević-Kalimanovska, V.; Kotur-Stevuljević, J.; Bogavac-Stanojević, N.; Ivanišević, J.; Simić-Ogrizović, S.; Kravljača, M.; Jelić-Ivanović, Z. Association Between Superoxide Dismutase Isoenzyme Gene Expression and Total Antioxidant Status in Patients with an End-Stage Renal Disease. Balkan. Med. J. 2018, 35, 431–436. [Google Scholar] [CrossRef]
- Case, A.J. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants 2017, 6, 82. [Google Scholar] [CrossRef]
- Quint, P.S.; Domsic, J.F.; Cabelli, D.E.; McKenna, R.; Silverman, D.N. Role of a glutamate bridge spanning the dimeric interface of human manganese superoxide dismutase. Biochemistry 2008, 47, 4621–4628. [Google Scholar] [CrossRef]
- Bonetta Valentino, R. The structure-function relationships and physiological roles of MnSOD mutants. Biosci. Rep. 2022, 42, BSR20220202. [Google Scholar] [CrossRef]
- Sheng, Y.; Butler Gralla, E.; Schumacher, M.; Cascio, D.; Cabelli, D.E.; Valentine, J.S. Six-coordinate manganese(3+) in catalysis by yeast manganese superoxide dismutase. Proc. Natl. Acad. Sci. USA 2012, 109, 14314–14319. [Google Scholar] [CrossRef] [PubMed]
- Hearn, A.S.; Stroupe, M.E.; Cabelli, D.E.; Lepock, J.R.; Tainer, J.A.; Nick, H.S.; Silverman, D.N. Kinetic Analysis of Product Inhibition in Human Manganese Superoxide Dismutase. Biochemistry 2001, 40, 12051–12058. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Trickel, S.R.; Borgstahl, G.E.O. Substrate-analog binding and electrostatic surfaces of human manganese superoxide dismutase. J. Struct. Biol. 2017, 199, 68–75. [Google Scholar] [CrossRef]
- Greenleaf, W.B.; Perry, J.J.; Hearn, A.S.; Cabelli, D.E.; Lepock, J.R.; Stroupe, M.E.; Tainer, J.A.; Nick, H.S.; Silverman, D.N. Role of hydrogen bonding in the active site of human manganese superoxide dismutase. Biochemistry 2004, 43, 7038–7045. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Borgstahl, G.E.O. Direct detection of coupled proton and electron transfers in human manganese superoxide dismutase. Nat. Commun. 2021, 12, 2079. [Google Scholar] [CrossRef] [PubMed]
- McAdam, M.E.; Fox, R.A.; Lavelle, F.; Fielden, E.M. A pulse-radiolysis study of the manganese-containing superoxide dismutase from Bacillus stearothermophilus. A kinetic model for the enzyme action. Biochem. J. 1977, 165, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.A.; Rodriguez, J.A.; Cabelli, D.E. Theoretical studies of manganese and iron superoxide dismutases: Superoxide binding and superoxide oxidation. J. Phys. Chem. B 2005, 109, 24502–24509. [Google Scholar] [CrossRef]
- Perry, J.J.; Hearn, A.S.; Cabelli, D.E.; Nick, H.S.; Tainer, J.A.; Silverman, D.N. Contribution of human manganese superoxide dismutase tyrosine 34 to structure and catalysis. Biochemistry 2009, 48, 3417–3424. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Slobodnik, K.; Struble, L.R.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Myles, D.A.A.; Kroll, T.; Borgstahl, G.E.O. Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition. Nat. Commun. 2024, 15, 5973. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Zhang, D. MnSOD serves as the central molecule in adaptive thermogenesis (MnSOD functions as a thermoreceptor). Adv. Redox Res. 2021, 3, 100027. [Google Scholar] [CrossRef]
- Xu, Y.; Porntadavity, S.; St Clair, D.K. Transcriptional regulation of the human manganese superoxide dismutase gene: The role of specificity protein 1 (Sp1) and activating protein-2 (AP-2). Biochem. J. 2002, 362, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.D.; Suliman, H.B.; Bartz, R.R.; Piantadosi, C.A. Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J. Biol. Chem. 2014, 289, 41–52. [Google Scholar] [CrossRef]
- Jones, P.L.; Ping, D.; Boss, J.M. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol. Cell Biol. 1997, 17, 6970–6981. [Google Scholar] [CrossRef]
- Ennen, M.; Minig, V.; Grandemange, S.; Touche, N.; Merlin, J.L.; Besancenot, V.; Brunner, E.; Domenjoud, L.; Becuwe, P. Regulation of the high basal expression of the manganese superoxide dismutase gene in aggressive breast cancer cells. Free Radic. Biol. Med. 2011, 50, 1771–1779. [Google Scholar] [CrossRef]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; Hayes McDonald, W.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 2010, 40, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed]
- MacMillan-Crow, L.A.; Crow, J.P.; Thompson, J.A. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 1998, 37, 1613–1622. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Hsieh, Y.; Tu, C.; O’Connor, D.; Nick, H.S.; Silverman, D.N. Catalytic Properties of Human Manganese Superoxide Dismutase*. J. Biol. Chem. 1996, 271, 17687–17691. [Google Scholar] [CrossRef]
- Buettner, G.R.; Ng, C.F.; Wang, M.; Rodgers, V.G.J.; Schafer, F.Q. A New Paradigm: Manganese Superoxide Dismutase Influences the Production of H2O2 in Cells and Thereby Their Biological State. Free Radic. Biol. Med. 2006, 41, 1338–1350. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 532, 112–116. [Google Scholar] [CrossRef]
- Ježek, J.; Dlasková, A.; Zelenka, J.; Jabůrek, M.; Ježek, P. H2O2-Activated Mitochondrial Phospholipase iPLA2γ Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein–Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic β-Cells. Antioxid. Redox Signal. 2015, 23, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Jabůrek, M.; Ježek, J.; Zelenka, J.; Ježek, P. Antioxidant activity by a synergy of redox-sensitive mitochondrial phospholipase A2 and uncoupling protein-2 in lung and spleen. Int. J. Biochem. Cell Biol. 2013, 45, 816–825. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S. Significance of TRP channels in oxidative stress. Eur. J. Pharmacol. 2016, 793, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Buffolo, M.; Pires, K.M.; Pei, S.; Scherer, P.E.; Boudina, S. Adipocyte-Specific Deletion of Manganese Superoxide Dismutase Protects From Diet-Induced Obesity Through Increased Mitochondrial Uncoupling and Biogenesis. Diabetes 2016, 65, 2639–2651. [Google Scholar] [CrossRef]
- Montano, M.A.E.; Barrio Lera, J.P.; Gottlieb, M.G.V.; Schwanke, C.H.A.; da Rocha, M.I.U.M.; Manica-Cattani, M.F.; dos Santos, G.F.; da Cruz, I.B.M. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and elderly obesity. Mol. Cell. Biochem. 2009, 328, 33–40. [Google Scholar] [CrossRef]
- Garcia-Irigoyen, O.; Bovenga, F.; Piglionica, M.; Piccinin, E.; Cariello, M.; Arconzo, M.; Peres, C.; Corsetto, P.A.; Rizzo, A.M.; Ballanti, M.; et al. Enterocyte superoxide dismutase 2 deletion drives obesity. iScience 2022, 25, 103707. [Google Scholar] [CrossRef]
- Boyle, K.E.; Newsom, S.A.; Janssen, R.C.; Lappas, M.; Friedman, J.E. Skeletal Muscle MnSOD, Mitochondrial Complex II, and SIRT3 Enzyme Activities Are Decreased in Maternal Obesity During Human Pregnancy and Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E1601–E1609. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Sepassi, L.; Quiroz, Y.; Ni, Z.; Wallace, D.C.; Vaziri, N.D. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl. Physiol. 2007, 102, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Noiri, E.; Nakao, A.; Uchida, K.; Tsukahara, H.; Ohno, M.; Fujita, T.; Brodsky, S.; Goligorsky, M.S. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal. Physiol. 2001, 281, F948–F957. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zeng, Z.; Fang, H.; Li, F.; Zhang, X.; Tan, W. SIRT3 Inactivation Promotes Acute Kidney Injury Through Elevated Acetylation of SOD2 and p53. J. Surg. Res. 2019, 233, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Marine, A.; Simmons, S.; Saba, H.; Mitchell, T.; Shimizu, T.; Shirasawa, T.; Macmillan-Crow, L.A. Generation and characterization of a novel kidney-specific manganese superoxide dismutase knockout mouse. Free Radic. Biol. Med. 2011, 51, 406–416. [Google Scholar] [CrossRef]
- MacMillan-Crow, L.A.; Crow, J.P.; Kerby, J.D.; Beckman, J.S.; Thompson, J.A. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. USA 1996, 93, 11853–11858. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondrial Dysfunction in the Diabetic Kidney. Adv. Exp. Med. Biol. 2017, 982, 553–562. [Google Scholar] [CrossRef]
- Xu, J.; Kitada, M.; Koya, D. The impact of mitochondrial quality control by Sirtuins on the treatment of type 2 diabetes and diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165756. [Google Scholar] [CrossRef]
- Ma, S.X.; Li, X.J.; Duan, T.T.; Pei, M.; Zou, L.; Yu, X.Y.; Zhao, Y.Y. Moshen granule ameliorates membranous nephropathy by regulating NF-ƙB/Nrf2 pathways via aryl hydrocarbon receptor signalling. Heliyon 2023, 9, e20019. [Google Scholar] [CrossRef]
- Trambas, I.A.; Bowen, L.; Thallas-Bonke, V.; Snelson, M.; Sourris, K.C.; Laskowski, A.; Tauc, M.; Rubera, I.; Zheng, G.; Harris, D.C.H.; et al. Proximal tubular deletion of superoxide dismutase-2 reveals disparate effects on kidney function in diabetes. Redox. Biol. 2025, 82, 103601. [Google Scholar] [CrossRef]
- Shimoda-Matsubayashi, S.; Matsumine, H.; Kobayashi, T.; Nakagawa-Hattori, Y.; Shimizu, Y.; Mizuno, Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem. Biophys. Res. Commun. 1996, 226, 561–565. [Google Scholar] [CrossRef]
- Möllsten, A.; Marklund, S.L.; Wessman, M.; Svensson, M.; Forsblom, C.; Parkkonen, M.; Brismar, K.; Groop, P.H.; Dahlquist, G. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes 2007, 56, 265–269. [Google Scholar] [CrossRef]
- Nomiyama, T.; Tanaka, Y.; Piao, L.; Nagasaka, K.; Sakai, K.; Ogihara, T.; Nakajima, K.; Watada, H.; Kawamori, R. The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J. Hum. Genet. 2003, 48, 138–141. [Google Scholar] [CrossRef]
- Houldsworth, A.; Hodgkinson, A.; Shaw, S.; Millward, A.; Demaine, A.G. Polymorphic differences in the SOD-2 gene may affect the pathogenesis of nephropathy in patients with diabetes and diabetic complications. Gene 2015, 569, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, M.G.; Kim, D.S.; Kim, T.W. Manganese superoxide dismutase gene polymorphism (V16A) is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism 2006, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, T.; Wang, N.; Wang, F.; Li, M.; Jiang, J.; Zhao, R.; Li, L.; Zhao, W.; Zhu, Q.; et al. The manganese superoxide dismutase Val16Ala polymorphism is associated with decreased risk of diabetic nephropathy in Chinese patients with type 2 diabetes. Mol. Cell Biochem. 2009, 322, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Ascencio-Montiel Ide, J.; Parra, E.J.; Valladares-Salgado, A.; Gómez-Zamudio, J.H.; Kumate-Rodriguez, J.; Escobedo-de-la-Peña, J.; Cruz, M. SOD2 gene Val16Ala polymorphism is associated with macroalbuminuria in Mexican type 2 diabetes patients: A comparative study and meta-analysis. BMC Med. Genet. 2013, 14, 110. [Google Scholar] [CrossRef]
- Tian, C.; Fang, S.; Du, X.; Jia, C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: A meta-analysis. Diabetologia 2011, 54, 803–811. [Google Scholar] [CrossRef]
- Sutton, A.; Khoury, H.; Prip-Buus, C.; Cepanec, C.; Pessayre, D.; Degoul, F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 2003, 13, 145–157. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the β cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Hu, H.; Yang, D.; Yang, F. Effects of β-carotene on glucose metabolism dysfunction in humans and type 2 diabetic rats. Acta Mater. Medica 2022, 1, 138–153. [Google Scholar] [CrossRef]
- Suri, S.; Mitra, P.; Bankul, A.; Saxena, I.; Garg, M.K.; Bohra, G.K.; Sharma, P. Altered expression of specific antioxidant (SOD1 and SOD2) and DNA repair (XRCC1 and OGG1) genes in patients with newly diagnosed type-2 diabetes mellitus. Minerva Endocrinol 2021, 49, 398–405. [Google Scholar] [CrossRef]
- Eddaikra, A.; Amroun, H.; Raache, R.; Galleze, A.; Abdallah-Elhadj, N.; Azzouz, M.; Meçabih, F.; Mechti, B.; Abbadi, M.C.; Touil-Boukoffa, C.; et al. Clinical variables and ethnicity may influenced by polymorphism of CAT -262C/T and MnSOD 47C/T antioxidant enzymes in Algerian type1 diabetes without complications. Gene 2018, 670, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Epstein, P.N. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes 2005, 54, 1437–1446. [Google Scholar] [CrossRef]
- Bertera, S.; Crawford, M.L.; Alexander, A.M.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Trucco, M. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes 2003, 52, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Ivanović-Matić, S.; Mihailović, M.; Dinić, S.; Martinović, V.; Bogojević, D.; Grigorov, I.; Poznanović, G. The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J. Physiol. Sci. 2010, 60, 259–266. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, S.; Metreveli, N.S.; Epstein, P.N. Protection of Cardiac Mitochondria by Overexpression of MnSOD Reduces Diabetic Cardiomyopathy. Diabetes 2006, 55, 798–805. [Google Scholar] [CrossRef]
- Elrashidy, R.A.; Kavran, M.; Asker, M.E.; Mohamed, H.E.; Daneshgari, F.; Liu, G. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am. J. Physiol. Renal. Physiol. 2019, 317, F906–F912. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Zhong, Q.; Mohammad, G.; Ho, Y.S.; Kowluru, R.A. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic. Res. 2010, 44, 313–321. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Ma, B.; Sun, X.; Chen, B. DJ-1 regulates mitochondrial function and promotes retinal ganglion cell survival under high glucose-induced oxidative stress. Front. Pharmacol. 2024, 15, 1455439. [Google Scholar] [CrossRef]
- Lv, T.; Lu, Y.; Liu, Y.; Feng, H.; Li, C.; Sheng, W.; Cui, Z.; Zhu, S.; Gu, X.; Yang, Z.; et al. General Control of Amino Acid Synthesis 5-Like 1-Mediated Acetylation of Manganese Superoxide Dismutase Regulates Oxidative Stress in Diabetic Kidney Disease. Oxid. Med. Cell Longev. 2021, 2021, 6691226. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Schaffer, J.E. Metabolic stress in the myocardium: Adaptations of gene expression. J. Mol. Cell. Cardiol. 2013, 55, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Romuk, E.; Jacheć, W.; Kozielska-Nowalany, E.; Birkner, E.; Zemła-Woszek, A.; Wojciechowska, C. Superoxide dismutase activity as a predictor of adverse outcomes in patients with nonischemic dilated cardiomyopathy. Cell Stress Chaperones 2019, 24, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Case, A.J.; Tian, J.; Zimmerman, M.C. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox. Biol. 2017, 11, 82–90. [Google Scholar] [CrossRef]
- Chan, S.H.; Tai, M.H.; Li, C.Y.; Chan, J.Y. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006, 40, 2028–2039. [Google Scholar] [CrossRef]
- Li, L.; Crockett, E.; Wang, D.H.; Galligan, J.J.; Fink, G.D.; Chen, A.F. Gene transfer of endothelial NO synthase and manganese superoxide dismutase on arterial vascular cell adhesion molecule-1 expression and superoxide production in deoxycorticosterone acetate-salt hypertension. Arter. Thromb. Vasc. Biol. 2002, 22, 249–255. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Eis, A.; Teng, R.J.; Bakhutashvili, I.; Kaul, S.; Davis, J.M.; Konduri, G.G. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L870–L879. [Google Scholar] [CrossRef]
- Schultz, A.; Olorundami, O.A.; Teng, R.J.; Jarzembowski, J.; Shi, Z.Z.; Kumar, S.N.; Pritchard, K., Jr.; Konduri, G.G.; Afolayan, A.J. Decreased OLA1 (Obg-Like ATPase-1) Expression Drives Ubiquitin-Proteasome Pathways to Downregulate Mitochondrial SOD2 (Superoxide Dismutase) in Persistent Pulmonary Hypertension of the Newborn. Hypertension 2019, 74, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.L.; Kuo, L.T.; Sung, F.C.; Yeh, C.C. Association between Polymorphisms of Antioxidant Gene (MnSOD, CAT, and GPx1) and Risk of Coronary Artery Disease. Biomed. Res. Int. 2018, 2018, 5086869. [Google Scholar] [CrossRef] [PubMed]
- Souiden, Y.; Mallouli, H.; Meskhi, S.; Chaabouni, Y.; Rebai, A.; Chéour, F.; Mahdouani, K. MnSOD and GPx1 polymorphism relationship with coronary heart disease risk and severity. Biol. Res. 2016, 49, 22. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Loch, T.; Vakhrusheva, O.; Piotrowska, I.; Ziolkowski, W.; Ebelt, H.; Braun, T.; Bober, E. Different extent of cardiac malfunction and resistance to oxidative stress in heterozygous and homozygous manganese-dependent superoxide dismutase-mutant mice. Cardiovasc. Res. 2009, 82, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Roos, C.M.; Zhang, B.; Hagler, M.A.; Verzosa, G.C.; Huang, R.; Oehler, E.A.; Arghami, A.; Miller, J.D. Effects of Altering Mitochondrial Antioxidant Capacity on Molecular and Phenotypic Drivers of Fibrocalcific Aortic Valve Stenosis. Front. Cardiovasc. Med. 2021, 8, 694881. [Google Scholar] [CrossRef]
- Ohashi, M.; Runge, M.S.; Faraci, F.M.; Heistad, D.D. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arter. Thromb. Vasc. Biol. 2006, 26, 2331–2336. [Google Scholar] [CrossRef]
- Ballinger, S.W.; Patterson, C.; Knight-Lozano, C.A.; Burow, D.L.; Conklin, C.A.; Hu, Z.; Reuf, J.; Horaist, C.; Lebovitz, R.; Hunter, G.C.; et al. Mitochondrial integrity and function in atherogenesis. Circulation 2002, 106, 544–549. [Google Scholar] [CrossRef]
- Harrison, C.M.; Pompilius, M.; Pinkerton, K.E.; Ballinger, S.W. Mitochondrial oxidative stress significantly influences atherogenic risk and cytokine-induced oxidant production. Environ. Health Perspect. 2011, 119, 676–681. [Google Scholar] [CrossRef]
- Daosukho, C.; Ittarat, W.; Lin, S.-m.; Sawyer, D.B.; Kiningham, K.; Lien, Y.-C.; Clair, D.K.S. Induction of manganese superoxide dismutase (MnSOD) mediates cardioprotective effect of tamoxifen (TAM). J. Mol. Cell. Cardiol. 2005, 39, 792–803. [Google Scholar] [CrossRef]
- Jain, K.; Gu, S.X.; Hwa, J. SOD2 in platelets: With age comes responsibility. J. Thromb. Haemost. 2023, 21, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Liu, D.; Li, Z.; Fu, Y.; Tse, G.; Li, G.; Liu, T.; Xu, G. Manganese Superoxide Dismutase as a Novel Oxidative Stress Biomarker for Predicting Paroxysmal Atrial Fibrillation. J. Clin. Med. 2022, 11, 5131. [Google Scholar] [CrossRef]
- Gong, J.; Sun, P.; Li, L.; Zou, Z.; Wu, Q.; Sun, L.; Li, H.; Gu, Z.; Su, L. Heat stress suppresses MnSOD expression via p53-Sp1 interaction and induces oxidative stress damage in endothelial cells: Protective effects of MitoQ10 and Pifithrin-α. Heliyon 2023, 9, e22805. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, Y.; Park, S.H.; Liu, G.; O’Brien, J.; Jiang, H.; Gius, D. SIRT3-Mediated Dimerization of IDH2 Directs Cancer Cell Metabolism and Tumor Growth. Cancer Res. 2017, 77, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Pollard, P.J.; Brière, J.J.; Alam, N.A.; Barwell, J.; Barclay, E.; Wortham, N.C.; Hunt, T.; Mitchell, M.; Olpin, S.; Moat, S.J.; et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 2005, 14, 2231–2239. [Google Scholar] [CrossRef]
- Hao, H.X.; Khalimonchuk, O.; Schraders, M.; Dephoure, N.; Bayley, J.P.; Kunst, H.; Devilee, P.; Cremers, C.W.; Schiffman, J.D.; Bentz, B.G.; et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009, 325, 1139–1142. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox. Signal 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Dhar, S.K.; Tangpong, J.; Chaiswing, L.; Oberley, T.D.; St Clair, D.K. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011, 71, 6684–6695. [Google Scholar] [CrossRef]
- Zhao, Y.; Oberley, T.D.; Chaiswing, L.; Lin, S.M.; Epstein, C.J.; Huang, T.T.; St Clair, D. Manganese superoxide dismutase deficiency enhances cell turnover via tumor promoter-induced alterations in AP-1 and p53-mediated pathways in a skin cancer model. Oncogene 2002, 21, 3836–3846. [Google Scholar] [CrossRef] [PubMed]
- Darby Weydert, C.J.; Smith, B.B.; Xu, L.; Kregel, K.C.; Ritchie, J.M.; Davis, C.S.; Oberley, L.W. Inhibition of oral cancer cell growth by adenovirusMnSOD plus BCNU treatment. Free Radic. Biol. Med. 2003, 34, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Ough, M.; Lewis, A.; Zhang, Y.; Hinkhouse, M.M.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic. Res. 2004, 38, 1223–1233. [Google Scholar] [CrossRef]
- Zhong, W.; Oberley, L.W.; Oberley, T.D.; Yan, T.; Domann, F.E.; St Clair, D.K. Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Differ. 1996, 7, 1175–1186. [Google Scholar]
- Zhong, W.; Yan, T.; Webber, M.M.; Oberley, T.D. Alteration of cellular phenotype and responses to oxidative stress by manganese superoxide dismutase and a superoxide dismutase mimic in RWPE-2 human prostate adenocarcinoma cells. Antioxid. Redox. Signal 2004, 6, 513–522. [Google Scholar] [CrossRef]
- Paku, M.; Haraguchi, N.; Takeda, M.; Fujino, S.; Ogino, T.; Takahashi, H.; Miyoshi, N.; Uemura, M.; Mizushima, T.; Yamamoto, H.; et al. SIRT3-Mediated SOD2 and PGC-1α Contribute to Chemoresistance in Colorectal Cancer Cells. Ann. Surg. Oncol. 2021, 28, 4720–4732. [Google Scholar] [CrossRef]
- Hsueh, W.T.; Chen, S.H.; Chien, C.H.; Chou, S.W.; Chi, P.I.; Chu, J.M.; Chang, K.Y. SOD2 Enhancement by Long-Term Inhibition of the PI3K Pathway Confers Multi-Drug Resistance and Enhanced Tumor-Initiating Features in Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 11260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Ding, J.; Tan, C.; Hu, W.; Li, Y.; Huang, W.; Xu, Y. HZ08 suppresses RelB-activated MnSOD expression and enhances Radiosensitivity of prostate Cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 174. [Google Scholar] [CrossRef]
- Noh, J.K.; Woo, S.R.; Yun, M.; Lee, M.K.; Kong, M.; Min, S.; Kim, S.I.; Lee, Y.C.; Eun, Y.G.; Ko, S.G. SOD2- and NRF2-associated Gene Signature to Predict Radioresistance in Head and Neck Cancer. Cancer Genom. Proteom. 2021, 18, 675–684. [Google Scholar] [CrossRef]
- Ma, C.S.; Lv, Q.M.; Zhang, K.R.; Tang, Y.B.; Zhang, Y.F.; Shen, Y.; Lei, H.M.; Zhu, L. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2021, 42, 613–623. [Google Scholar] [CrossRef]
- Ollila, E.; Ahtikoski, A.; Puistola, U.; Karihtala, P.; Urpilainen, E. Redox-state-regulating enzymes have prognostic value in diabetic endometrial cancer patients: Impact of statin use? Front. Oncol. 2024, 14, 1393103. [Google Scholar] [CrossRef]
- Piecuch, J.Z.; Kucharzewski, M.; Wyrobiec, G.; Brzozowa-Zasada, M. Immunohistochemical detection of MnSOD in colon adenocarcinoma patients—Clinical application. Prz. Gastroenterol. 2024, 19, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Shi, Z.; Shi, M.; Li, H.; Qian, X.; Peng, C.; Ding, X.; Zhang, S.; Lv, Y.; Wang, L.; et al. PPARγ/SOD2 Protects Against Mitochondrial ROS-Dependent Apoptosis via Inhibiting ATG4D-Mediated Mitophagy to Promote Pancreatic Cancer Proliferation. Front. Cell Dev. Biol. 2021, 9, 745554. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta Vallur, P.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.J.; Palmer, J.M.; Walters, M.K.; Nancarrow, D.J.; Parsons, P.G.; Hayward, N.K. Simple tandem repeat allelic deletions confirm the preferential loss of distal chromosome 6q in melanoma. Int. J. Cancer 1994, 58, 203–206. [Google Scholar] [CrossRef]
- Bravard, A.; Hoffschir, F.; Sabatier, L.; Ricoul, M.; Pinton, A.; Cassingena, R.; Estrade, S.; Luccioni, C.; Dutrillaux, B. Early superoxide dismutase alterations during SV40-transformation of human fibroblasts. Int. J. Cancer 1992, 52, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, F.; Dhar, S.K.; Bosch, A.; St Clair, W.H.; Kasarskis, E.J.; St Clair, D.K. Mutations in the SOD2 promoter reveal a molecular basis for an activating protein 2-dependent dysregulation of manganese superoxide dismutase expression in cancer cells. Mol. Cancer Res. 2008, 6, 1881–1893. [Google Scholar] [CrossRef]
- Douglas, D.B.; Akiyama, Y.; Carraway, H.; Belinsky, S.A.; Esteller, M.; Gabrielson, E.; Weitzman, S.; Williams, T.; Herman, J.G.; Baylin, S.B. Hypermethylation of a small CpGuanine-rich region correlates with loss of activator protein-2alpha expression during progression of breast cancer. Cancer Res. 2004, 64, 1611–1620. [Google Scholar] [CrossRef]
- Xu, Y.; Krishnan, A.; Wan, X.S.; Majima, H.; Yeh, C.C.; Ludewig, G.; Kasarskis, E.J.; St Clair, D.K. Mutations in the promoter reveal a cause for the reduced expression of the human manganese superoxide dismutase gene in cancer cells. Oncogene 1999, 18, 93–102. [Google Scholar] [CrossRef]
- Minig, V.; Kattan, Z.; van Beeumen, J.; Brunner, E.; Becuwe, P. Identification of DDB2 protein as a transcriptional regulator of constitutive SOD2 gene expression in human breast cancer cells. J. Biol. Chem. 2009, 284, 14165–14176. [Google Scholar] [CrossRef]
- Hitchler, M.J.; Wikainapakul, K.; Yu, L.; Powers, K.; Attatippaholkun, W.; Domann, F.E. Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics 2006, 1, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Peng, B.; Pompeia, C.; Thomas, S.; Cho, E.; Clausen, P.A.; Marquez, V.E.; Farrar, W.L. Epigenetic silencing of manganese superoxide dismutase (SOD-2) in KAS 6/1 human multiple myeloma cells increases cell proliferation. Cancer Biol. Ther. 2005, 4, 585–592. [Google Scholar] [CrossRef]
- Hurt, E.M.; Thomas, S.B.; Peng, B.; Farrar, W.L. Integrated molecular profiling of SOD2 expression in multiple myeloma. Blood 2007, 109, 3953–3962. [Google Scholar] [CrossRef]
- Hurt, E.M.; Thomas, S.B.; Peng, B.; Farrar, W.L. Molecular consequences of SOD2 expression in epigenetically silenced pancreatic carcinoma cell lines. Br. J. Cancer 2007, 97, 1116–1123. [Google Scholar] [CrossRef]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Drane, P.; Bravard, A.; Bouvard, V.; May, E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene 2001, 20, 430–439. [Google Scholar] [CrossRef]
- Pani, G.; Bedogni, B.; Anzevino, R.; Colavitti, R.; Palazzotti, B.; Borrello, S.; Galeotti, T. Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Res. 2000, 60, 4654–4660. [Google Scholar] [PubMed]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef]
- Dhar, S.K.; Xu, Y.; Chen, Y.; St Clair, D.K. Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. J. Biol. Chem. 2006, 281, 21698–21709. [Google Scholar] [CrossRef]
- Zhao, Y.; Chaiswing, L.; Velez, J.M.; Batinic-Haberle, I.; Colburn, N.H.; Oberley, T.D.; St Clair, D.K. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005, 65, 3745–3750. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Li, Z.; Kelley, N.; Ouyang, M.; Wu, J.W.; Meng, F.; Ou, W.B. Anti-proliferative and anti-invasive effects of exogenous thermostable MnSOD in gastric cancer associated with p53 and ZEB1 expression. J. Cancer 2025, 16, 2062–2074. [Google Scholar] [CrossRef]
- Xu, Y.; Kiningham, K.K.; Devalaraja, M.N.; Yeh, C.C.; Majima, H.; Kasarskis, E.J.; St Clair, D.K. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999, 18, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; St Clair, D.K.; Fang, F.; Warren, G.W.; Rangnekar, V.M.; Crooks, P.A.; St Clair, W.H. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-kappaB inhibition and enhanced by the presence of PTEN. Mol. Cancer Ther. 2007, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Kamarajugadda, S.; Cai, Q.; Chen, H.; Nayak, S.; Zhu, J.; He, M.; Jin, Y.; Zhang, Y.; Ai, L.; Martin, S.S.; et al. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013, 4, e504. [Google Scholar] [CrossRef] [PubMed]
- Delhalle, S.; Deregowski, V.; Benoit, V.; Merville, M.P.; Bours, V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene 2002, 21, 3917–3924. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Spentzos, D.; Fountzilas, E.; Francoeur, N.; Sanisetty, S.; Grammatikos, A.P.; Hecht, J.L.; Cannistra, S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011, 71, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Acquaah-Mensah, G.; Singhal, M.; Malhotra, D.; Biswal, S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput. Biol. 2008, 4, e1000166. [Google Scholar] [CrossRef]
- Solis, L.M.; Behrens, C.; Dong, W.; Suraokar, M.; Ozburn, N.C.; Moran, C.A.; Corvalan, A.H.; Biswal, S.; Swisher, S.G.; Bekele, B.N.; et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin. Cancer Res. 2010, 16, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Hanada, N.; Takahata, T.; Zhou, Q.; Ye, X.; Sun, R.; Itoh, J.; Ishiguro, A.; Kijima, H.; Mimura, J.; Itoh, K.; et al. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC Cancer 2012, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef]
- Li, M.; Chiu, J.F.; Mossman, B.T.; Fukagawa, N.K. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J. Biol. Chem. 2006, 281, 40429–40439. [Google Scholar] [CrossRef]
- Kato, M.; Yuan, H.; Xu, Z.G.; Lanting, L.; Li, S.L.; Wang, M.; Hu, M.C.; Reddy, M.A.; Natarajan, R. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 3325–3335. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Pennington, J.D.; Bisht, K.S.; Aykin-Burns, N.; Kim, H.S.; Mishra, M.; Sun, L.; Nguyen, P.; Ahn, B.H.; Leclerc, J.; et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 2008, 4, 291–299. [Google Scholar] [CrossRef]
- Lim, S.W.; Chen, W.C.; Ko, H.J.; Su, Y.F.; Wu, C.H.; Huang, F.L.; Li, C.F.; Tsai, C.Y. 6-Gingerol Induced Apoptosis and Cell Cycle Arrest in Glioma Cells via MnSOD and ERK Phosphorylation Modulation. Biomol. Ther. 2025, 33, 129–142. [Google Scholar] [CrossRef]
- Basilotta, R.; Lanza, M.; Filippone, A.; Casili, G.; Mannino, D.; De Gaetano, F.; Chisari, G.; Colarossi, L.; Motta, G.; Campolo, M.; et al. Therapeutic Potential of Dimethyl Fumarate in Counteract Oral Squamous Cell Carcinoma Progression by Modulating Apoptosis, Oxidative Stress and Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2023, 24, 2777. [Google Scholar] [CrossRef]
- Kang, B.G.; Shende, M.; Inci, G.; Park, S.H.; Jung, J.S.; Kim, S.B.; Kim, J.H.; Mo, Y.W.; Seo, J.H.; Feng, J.H.; et al. Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification. Cancer Biol. Ther. 2023, 24, 20–32. [Google Scholar] [CrossRef]
- Gao, Y.H.; Li, C.X.; Shen, S.M.; Li, H.; Chen, G.Q.; Wei, Q.; Wang, L.S. Hypoxia-inducible factor 1α mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem. Biophys. Res. Commun. 2013, 435, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Gonzalez, J.; Zhang, T.; Kamel-Reid, S.; Wells, R.A. The aryl hydrocarbon receptor nuclear translocator (ARNT) modulates the antioxidant response in AML cells. Leuk Res. 2013, 37, 1750–1756. [Google Scholar] [CrossRef]
- Solari, C.; Vázquez Echegaray, C.; Cosentino, M.S.; Petrone, M.V.; Waisman, A.; Luzzani, C.; Francia, M.; Villodre, E.; Lenz, G.; Miriuka, S.; et al. Manganese Superoxide Dismutase Gene Expression Is Induced by Nanog and Oct4, Essential Pluripotent Stem Cells’ Transcription Factors. PLoS ONE 2015, 10, e0144336. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Bartolome, R.; Patel, V.; Cotrim, A.; Leelahavanichkul, K.; Molinolo, A.A.; Mitchell, J.B.; Gutkind, J.S. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem. Cell 2012, 11, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Bray, J.A.; Esocobar, P.; Bigbee, W.L.; Watkins, S.; Greenberger, J.S. Overexpression of the human manganese superoxide dismutase (MnSOD) transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat. Oncol. Investig. 1999, 7, 331–342. [Google Scholar] [CrossRef]
- Deng, X.; Ewton, D.Z.; Friedman, E. Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by reducing levels of reactive oxygen species. Cancer Res. 2009, 69, 3317–3324. [Google Scholar] [CrossRef]
- Stuart, J.J.; Egry, L.A.; Wong, G.H.; Kaspar, R.L. The 3′ UTR of human MnSOD mRNA hybridizes to a small cytoplasmic RNA and inhibits gene expression. Biochem. Biophys. Res. Commun. 2000, 274, 641–648. [Google Scholar] [CrossRef]
- Pandit, H.; Zhang, W.; Li, Y.; Agle, S.; Li, X.; Li, S.P.; Cui, G.; Li, Y.; Martin, R.C. Manganese superoxide dismutase expression is negatively associated with microRNA-301a in human pancreatic ductal adenocarcinoma. Cancer Gene Ther. 2015, 22, 481–486. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, F.; Zhang, J.; Josson, S.; St Clair, W.H.; St Clair, D.K. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS ONE 2010, 5, e14356. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Jiang, L.; Wang, A.; Shi, F.; Ye, H.; Zhou, X. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genom. Proteom. 2009, 6, 131–139. [Google Scholar]
- Kriegel, A.J.; Fang, Y.; Liu, Y.; Tian, Z.; Mladinov, D.; Matus, I.R.; Ding, X.; Greene, A.S.; Liang, M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: A novel role of miR-382. Nucleic Acids Res. 2010, 38, 8338–8347. [Google Scholar] [CrossRef]
- Cui, Y.; She, K.; Tian, D.; Zhang, P.; Xin, X. miR-146a Inhibits Proliferation and Enhances Chemosensitivity in Epithelial Ovarian Cancer via Reduction of SOD2. Oncol. Res. 2016, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; McCord, J.M. Paradoxical effects of thiol reagents on Jurkat cells and a new thiol-sensitive mutant form of human mitochondrial superoxide dismutase. Cancer Res. 2003, 63, 159–163. [Google Scholar]
- Zhang, H.J.; Yan, T.; Oberley, T.D.; Oberley, L.W. Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype. Cancer Res. 1999, 59, 6276–6283. [Google Scholar] [PubMed]
- Liu, G.; Zhou, W.; Park, S.; Wang, L.I.; Miller, D.P.; Wain, J.C.; Lynch, T.J.; Su, L.; Christiani, D.C. The SOD2 Val/Val genotype enhances the risk of nonsmall cell lung carcinoma by p53 and XRCC1 polymorphisms. Cancer 2004, 101, 2802–2808. [Google Scholar] [CrossRef]
- Wheatley-Price, P.; Asomaning, K.; Reid, A.; Zhai, R.; Su, L.; Zhou, W.; Zhu, A.; Ryan, D.P.; Christiani, D.C.; Liu, G. Myeloperoxidase and superoxide dismutase polymorphisms are associated with an increased risk of developing pancreatic adenocarcinoma. Cancer 2008, 112, 1037–1042. [Google Scholar] [CrossRef]
- Becuwe, P.; Ennen, M.; Klotz, R.; Barbieux, C.; Grandemange, S. Manganese superoxide dismutase in breast cancer: From molecular mechanisms of gene regulation to biological and clinical significance. Free Radic. Biol. Med. 2014, 77, 139–151. [Google Scholar] [CrossRef]
- Bag, A.; Bag, N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: A review. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3298–3305. [Google Scholar] [CrossRef]
- Moradabadi, A.; Fekri-Soofiabadi, M.; Soltani, A.; Dabiri, S. An investigation into the manganese superoxide dismutase (MnSOD Val-9Ala) gene polymorphisms employing high-resolution melting in patients with gastric cancer: A preliminary study. Cancer Treat. Res. Commun. 2025, 44, 100942. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, X.; Dean, A.E.; Brien, J.O.; Gao, Y.; Tran, E.L.; Park, S.H.; Liu, G.; Kieffer, M.B.; Jiang, H.; et al. Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter. Nat. Commun. 2019, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Danes, J.M.; Hart, P.C.; Zhu, Y.; Huang, Y.; de Abreu, A.L.; O’Brien, J.; Mathison, A.J.; Tang, B.; Frasor, J.M.; et al. SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 23534–23541. [Google Scholar] [CrossRef]
- Dhar, S.K.; St Clair, D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012, 52, 2209–2222. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lyu, L.H.; Miao, H.K.; Bahr, T.; Zhang, Q.Y.; Liang, T.; Zhou, H.B.; Chen, G.R.; Bai, Y. Redox regulation by SOD2 modulates colorectal cancer tumorigenesis through AMPK-mediated energy metabolism. Mol. Carcinog. 2020, 59, 545–556. [Google Scholar] [CrossRef]
- Hart, P.C.; Mao, M.; de Abreu, A.L.; Ansenberger-Fricano, K.; Ekoue, D.N.; Ganini, D.; Kajdacsy-Balla, A.; Diamond, A.M.; Minshall, R.D.; Consolaro, M.E.; et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015, 6, 6053. [Google Scholar] [CrossRef]
- Quiros-Gonzalez, I.; Gonzalez-Menendez, P.; Mayo, J.C.; Hevia, D.; Artime-Naveda, F.; Fernandez-Vega, S.; Fernandez-Fernandez, M.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Sainz, R.M. Androgen-Dependent Prostate Cancer Cells Reprogram Their Metabolic Signature upon GLUT1 Upregulation by Manganese Superoxide Dismutase. Antioxidants 2022, 11, 313. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yu, L.; Ying, L.; Balic, J.; Gao, H.; Deng, N.T.; West, A.; Yan, F.; Ji, C.B.; Gough, D.; et al. Toll-like receptor 2 regulates metabolic reprogramming in gastric cancer via superoxide dismutase 2. Int. J. Cancer 2019, 144, 3056–3069. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Schreek, S.; Cinar, B.; Stasche, A.S.; Lee, S.H.; Zeug, A.; Dolgner, T.; Niessen, J.; Ponimaskin, E.; Shcherbata, H.; et al. SOD2 is a regulator of proteasomal degradation promoting an adaptive cellular starvation response. Cell Rep. 2025, 44, 115434. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Cai, T.; Zhang, S.; Yan, X.; Zhou, L.; He, Z.; Xue, P.; Li, J.; Zheng, M.; Yang, X.; et al. Identification of hub genes associated with neutrophils infiltration in colorectal cancer. J. Cell Mol. Med. 2021, 25, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, X.; Wang, X.; Guo, W.; Wen, Y.; Meng, W.; Peng, D.; Lv, P.; Zhang, X.; Shen, H. TNF-α-dependent lung inflammation upregulates superoxide dismutase-2 to promote tumor cell proliferation in lung adenocarcinoma. Mol. Carcinog. 2020, 59, 1088–1099. [Google Scholar] [CrossRef]
- Al Haq, A.T.; Tseng, H.Y.; Chen, L.M.; Wang, C.C.; Hsu, H.L. Targeting prooxidant MnSOD effect inhibits triple-negative breast cancer (TNBC) progression and M2 macrophage functions under the oncogenic stress. Cell Death Dis. 2022, 13, 49. [Google Scholar] [CrossRef]
- Lou, J.; Dong, J.; Xu, R.; Zeng, H.; Fang, L.; Wu, Y.; Liu, Y.; Wang, S. Remodeling of the tumor microenvironment using an engineered oncolytic vaccinia virus improves PD-L1 inhibition outcomes. Biosci. Rep. 2021, 41, BSR20204186. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef]
- Pandey, M.K.; Mittra, P.; Maheshwari, P. The lipid peroxidation product as a marker of oxidative stress in epilepsy. J. Clin. Diagn. Res. 2012, 6, 590–592. [Google Scholar]
- Pearson, J.N.; Rowley, S.; Liang, L.P.; White, A.M.; Day, B.J.; Patel, M. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol. Dis. 2015, 82, 289–297. [Google Scholar] [CrossRef]
- Kegler, A.; Caprara, A.L.F.; Pascotini, E.T.; Arend, J.; Gabbi, P.; Duarte, M.; Furian, A.F.; Oliveira, M.S.; Royes, L.F.F.; Fighera, M.R. Apoptotic Markers Are Increased in Epilepsy Patients: A Relation with Manganese Superoxide Dismutase Ala16Val Polymorphism and Seizure Type through IL-1β and IL-6 Pathways. Biomed. Res. Int. 2020, 2020, 6250429. [Google Scholar] [CrossRef]
- Andreassen, O.A.; Ferrante, R.J.; Dedeoglu, A.; Albers, D.W.; Klivenyi, P.; Carlson, E.J.; Epstein, C.J.; Beal, M.F. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 2001, 167, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nagai, Y.; Wada, K.; Koike, T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem. Biophys. Res. Commun. 2012, 429, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, O.O.; Maznychenko, A.V.; Klyuchko, O.M.; Mankovska, I.M.; Butowska, K.; Borowik, A.; Piosik, J.; Sokolowska, I. C(60) Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins. Int. J. Mol. Sci. 2021, 22, 5444. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Corniola, R.; Zou, Y.; Leu, D.; Fike, J.R.; Huang, T.T. Paradoxical relationship between Mn superoxide dismutase deficiency and radiation-induced cognitive defects. PLoS ONE 2012, 7, e49367. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Ke, J.; Wang, Y.; Wu, H. Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxid. Med. Cell Longev. 2021, 2021, 1697070. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Loreno, E.G.; Miriyala, S.; Panchatcharam, M.; Lu, X.; Sun, H. Chronic Low-Dose Alcohol Consumption Attenuates Post-Ischemic Inflammation via PPARγ in Mice. Int. J. Mol. Sci. 2021, 22, 5121. [Google Scholar] [CrossRef]
- Guo, S.; Li, F.; Wills, M.; Yip, J.; Wehbe, A.; Peng, C.; Geng, X.; Ding, Y. Chlorpromazine and Promethazine (C+P) Reduce Brain Injury after Ischemic Stroke through the PKC-δ/NOX/MnSOD Pathway. Mediat. Inflamm. 2022, 2022, 6886752. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Zhou, P.; Xu, H.; Meng, X.; Ding, T.; Nan, F.; Sun, G.; Sun, X. Notoginseng leaf triterpenes ameliorates mitochondrial oxidative injury via the NAMPT-SIRT1/2/3 signaling pathways in cerebral ischemic model rats. J. Ginseng. Res. 2023, 47, 199–209. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Zhou, P.; Xu, H.; Meng, X.; Ding, T.; Nan, F.; Sun, G.; Sun, X. Notoginseng Leaf Triterpenes Ameliorates OGD/R-Induced Neuronal Injury via SIRT1/2/3-Foxo3a-MnSOD/PGC-1α Signaling Pathways Mediated by the NAMPT-NAD Pathway. Oxid. Med. Cell Longev. 2020, 2020, 7308386. [Google Scholar] [CrossRef]
- Przedborski, S.; Kostic, V.; Jackson-Lewis, V.; Naini, A.B.; Simonetti, S.; Fahn, S.; Carlson, E.; Epstein, C.J.; Cadet, J.L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 1992, 12, 1658–1667. [Google Scholar] [CrossRef]
- Filograna, R.; Godena, V.K.; Sanchez-Martinez, A.; Ferrari, E.; Casella, L.; Beltramini, M.; Bubacco, L.; Whitworth, A.J.; Bisaglia, M. Superoxide Dismutase (SOD)-mimetic M40403 Is Protective in Cell and Fly Models of Paraquat Toxicity: IMPLICATIONS FOR PARKINSON DISEASE. J. Biol. Chem. 2016, 291, 9257–9267. [Google Scholar] [CrossRef]
- Shi, H.; Deng, H.X.; Gius, D.; Schumacker, P.T.; Surmeier, D.J.; Ma, Y.C. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum. Mol. Genet. 2017, 26, 1915–1926. [Google Scholar] [CrossRef]
- Kim, D.Y.; Leem, Y.H.; Kim, H.S. MLKL Inhibitor Reduces Oxidative Stress, Inflammation, and Dopaminergic Neuronal Cell Death in MPTP-Induced Parkinson’s Disease Mouse Model. Biomol. Ther. 2025, 33, 429–437. [Google Scholar] [CrossRef]
- Massaad, C.A.; Washington, T.M.; Pautler, R.G.; Klann, E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13576–13581. [Google Scholar] [CrossRef]
- Murakami, K.; Shimizu, T.; Irie, K. Formation of the 42-mer Amyloid β Radical and the Therapeutic Role of Superoxide Dismutase in Alzheimer’s Disease. J. Amino. Acids 2011, 2011, 654207. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Zhang, Z.; Hongpaisan, J. PKCε activator protects hippocampal microvascular disruption and memory defect in 3×Tg-Alzheimer’s disease mice with cerebral microinfarcts. Front. Aging Neurosci. 2023, 15, 1272361. [Google Scholar] [CrossRef]
- Millien, G.; Wang, H.; Zhang, Z.; Alkon, D.L.; Hongpaisan, J. PKCε Activation Restores Loss of PKCε, Manganese Superoxide Dismutase, Vascular Endothelial Growth Factor, and Microvessels in Aged and Alzheimer’s Disease Hippocampus. Front. Aging Neurosci. 2022, 14, 836634. [Google Scholar] [CrossRef]
- Lanza, M.; Basilotta, R.; Cuzzocrea, S.; Bulzomì, M.; Oddo, S.; Casili, G.; Esposito, E. An RNAi-Mediated Reduction in Transcription Factor Nrf-2 Blocks the Positive Effects of Dimethyl Fumarate on Metabolic Stress in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 11303. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Cao, X.; Zhao, H.; Gao, L.; Xia, P.; Pei, G. A Newly Synthesized Rhamnoside Derivative Alleviates Alzheimer’s Amyloid-β-Induced Oxidative Stress, Mitochondrial Dysfunction, and Cell Senescence through Upregulating SIRT3. Oxid. Med. Cell Longev. 2020, 2020, 7698560. [Google Scholar] [CrossRef]
- Patrick, S.; Corrigan, R.; Grizzanti, J.; Mey, M.; Blair, J.; Pallas, M.; Camins, A.; Lee, H.G.; Casadesus, G. Neuroprotective Effects of the Amylin Analog, Pramlintide, on Alzheimer’s Disease Are Associated with Oxidative Stress Regulation Mechanisms. J. Alzheimers Dis. 2019, 69, 157–168. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Sittirattanayeunyong, S.; Hongpaisan, J. Association of Apolipoprotein E4-related Microvascular Disease in the Alzheimer’s Disease Hippocampal CA1 Stratum Radiatum. Neuroscience 2023, 526, 204–222. [Google Scholar] [CrossRef]
- Villasana, L.E.; Akinyeke, T.; Weber, S.; Raber, J. Paradoxical effects of (137)Cs irradiation on pharmacological stimulation of reactive oxygen species in hippocampal slices from apoE2 and apoE4 mice. Oncotarget 2017, 8, 76587–76605. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Ahmad, L.; Marchesi, N.; Greco, G.; Boschi, F.; Masi, F.; Mallucci, G.; Bergamaschi, R.; Colombo, E.; Pascale, A. Disclosing the Novel Protective Mechanisms of Ocrelizumab in Multiple Sclerosis: The Role of PKC Beta and Its Down-Stream Targets. Int. J. Mol. Sci. 2024, 25, 8923. [Google Scholar] [CrossRef]
- Lis, M.; Niedziela, N.; Adamczyk-Zostawa, J.; Zalejska-Fiolka, J.; Szczygieł, J.; Sowa, A.; Świętek, A.; Adamczyk-Sowa, M. Comparative Effects of Vitamin D Supplementation on Oxidative Stress in Relapsing-Remitting Multiple Sclerosis. Curr. Issues Mol. Biol. 2024, 46, 14119–14131. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Kumar, G.; Thadathil, N.; Piekarz, K.M.; Mohammed, S.; Lopez, S.D.; Qaisar, R.; Walton, D.; Brown, J.L.; Murphy, A.; et al. Neuronal deletion of MnSOD in mice leads to demyelination, inflammation and progressive paralysis that mimics phenotypes associated with progressive multiple sclerosis. Redox. Biol. 2023, 59, 102550. [Google Scholar] [CrossRef]
- Singh, I.; Samuvel, D.J.; Choi, S.; Saxena, N.; Singh, A.K.; Won, J. Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model. Immunology 2018, 154, 434–451. [Google Scholar] [CrossRef]
- Grujicic, J.; Allen, A.R. MnSOD Mimetics in Therapy: Exploring Their Role in Combating Oxidative Stress-Related Diseases. Antioxidants 2024, 13, 1444. [Google Scholar] [CrossRef]
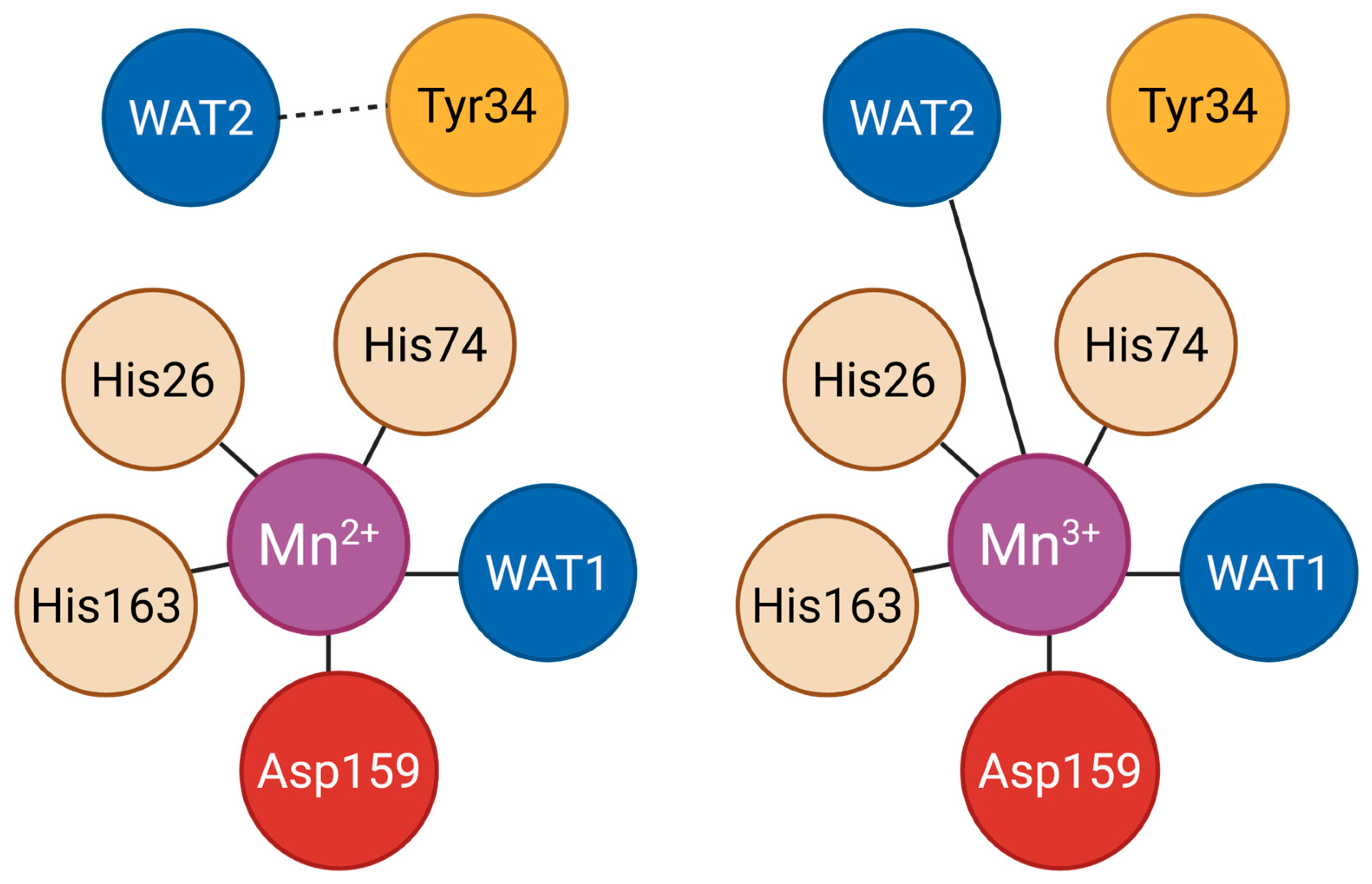
| Step | Pathway | Reaction Description | Key Residues and Features | Rate Constant | References |
|---|---|---|---|---|---|
| 1 | Both | O2•− reduces Mn3+ to Mn2+; hydroxide ligand is protonated to H2O by Gln143 | Inner sphere: His26, His74, His163, Asp159, WAT1; Gln143 donates proton | k1 | McAdam et al., 1977 [26]; Abreu et al., 2005 [27] |
| 2 | Fast | Second O2•− oxidizes Mn2+ to Mn3+; produces H2O2 via reaction with Tyr34 and water ligand | Outer sphere: Tyr34, WAT1; enzyme is regenerated | k2 | McAdam et al., 1977 [26]; Perry et al., 2009 [28]; Bonetta Valentino et al., 2022 [20] |
| 3 | Slow | Second O2•− binds Mn2+ to form a reversible peroxide adduct that inhibits activity | Product-inhibited complex formation | k3 | McAdam et al., 1977 [26]; Bonetta Valentino et al., 2022 [20] |
| 4 | Slow | Two protons regenerate Mn3+ and release H2O2, restoring activity | Proton donors: water ligand and outer sphere residues | k4 | McAdam et al., 1977 [26]; Bonetta Valentino et al., 2022 [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujicic, J.; Allen, A.R. Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease. Antioxidants 2025, 14, 848. https://doi.org/10.3390/antiox14070848
Grujicic J, Allen AR. Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease. Antioxidants. 2025; 14(7):848. https://doi.org/10.3390/antiox14070848
Chicago/Turabian StyleGrujicic, Jovan, and Antiño R. Allen. 2025. "Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease" Antioxidants 14, no. 7: 848. https://doi.org/10.3390/antiox14070848
APA StyleGrujicic, J., & Allen, A. R. (2025). Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease. Antioxidants, 14(7), 848. https://doi.org/10.3390/antiox14070848






