Resolvin D2 Reduces UVB Skin Pathology by Targeting Cytokines, Oxidative Stress, and NF-κB Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Groups
- (1)
- Group that was non-irradiated and saline-treated.
- (2)
- Group that was irradiated and saline-treated control group.
- (3)
- Group that was irradiated and treated group at a 0.3 ng/mouse dose of RvD2.
- (4)
- Group that was irradiated and treated group at a 1.0 ng/mouse dose of RvD2.
- (5)
- Group that was irradiated and treated group a 3.0 ng/mouse dose of RvD2.
2.3. Model of Induction of Skin Lesion by UVB Irradiation
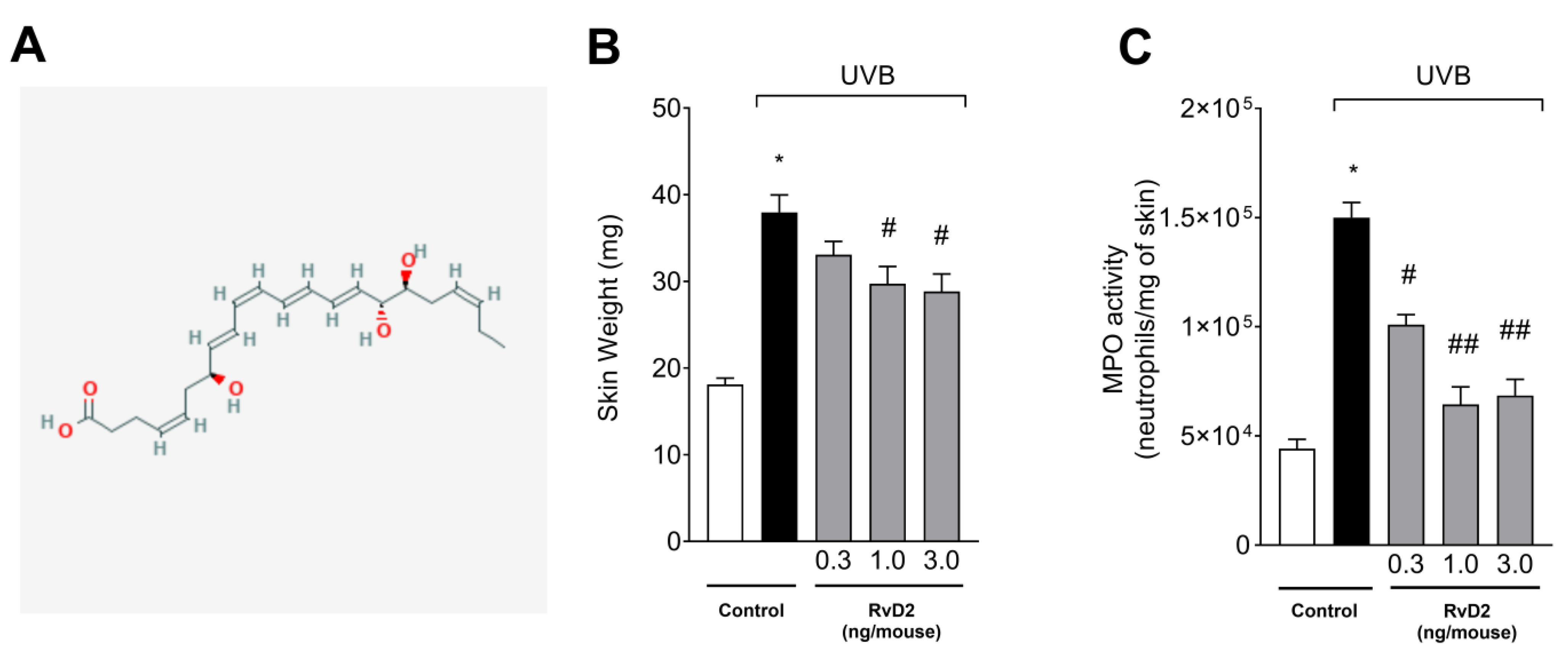
2.4. Skin Edema
2.5. Myeloperoxidase Activity (MPO)
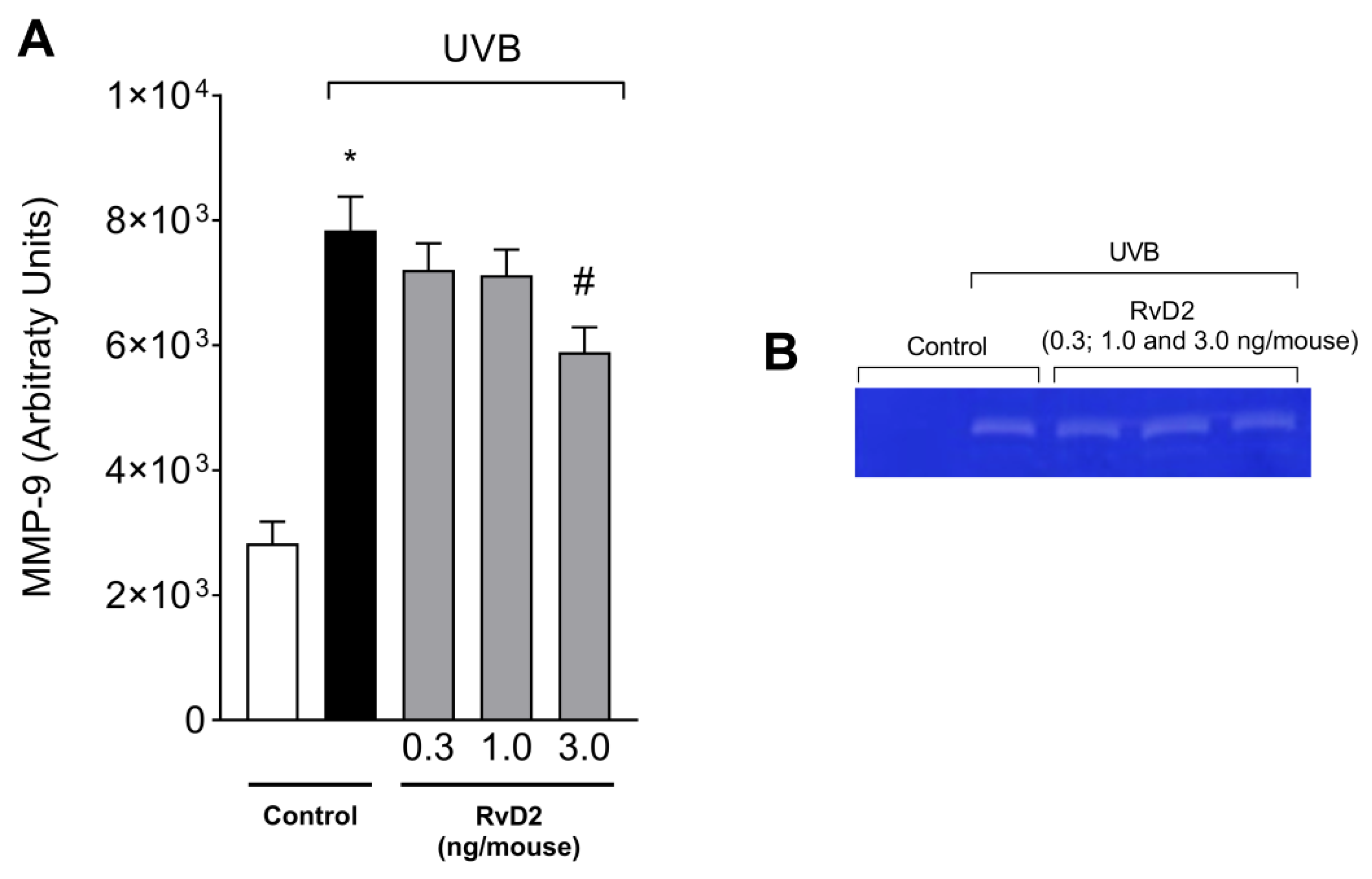
2.6. Determination of the Activity/Secretion of Metalloproteinase-9 (MMP-9)
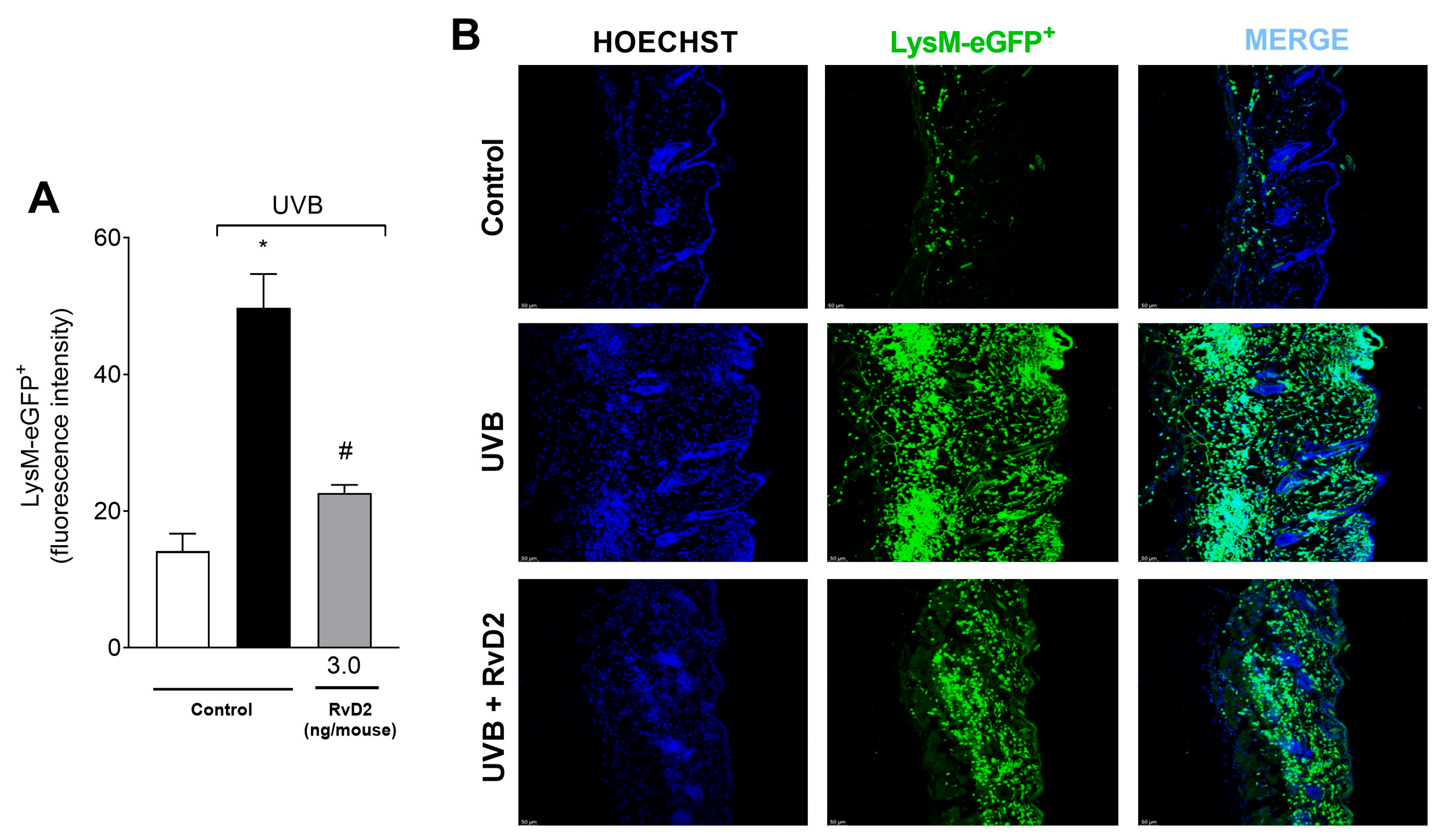
2.7. Fluorescence Detection of LysM-GFP+ Cells
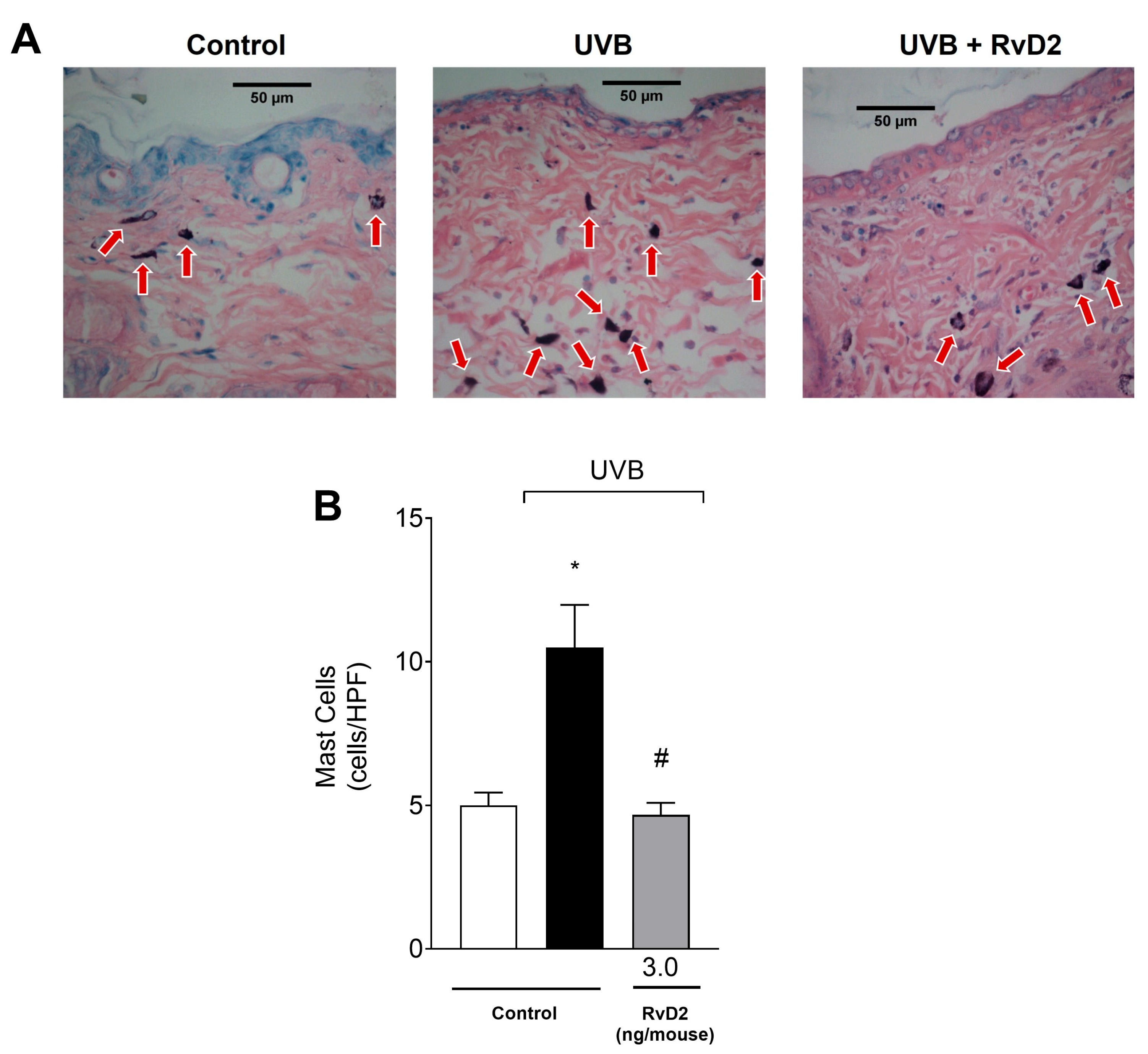
2.8. Histopathological Evaluation by Optical Microscopy
2.9. Dosage of Proinflammatory Cytokines TNF-α, IL-33, and IL-1β and Anti-Inflammatory Cytokines TGF-β and IL-10
2.10. Evaluation of Ferric Reducing Antioxidant Power (FRAP)
2.11. Evaluation of the Antioxidant Power by ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid)) Radical Sequestration Test
2.12. Evaluation of the Level of Endogenous Antioxidant: Reduced Glutathione (GSH)
2.13. Assay for Catalase Activity (CAT)
2.14. Evaluation of the Superoxide Anion Production
2.15. Lipid Hydroperoxide (LOOH) Assay
2.16. Fluorescent Western Blot
2.17. mRNA Expression of Cybb (gp91phox) and Cox2 by RT-qPCR
2.18. Statistical Analysis
3. Results
3.1. RvD2 Reduces UVB-Irradiation-Induced Skin Edema and Myeloperoxidase (MPO) Activity
3.2. RvD2 Inhibits UVB Irradiation-Triggered MMP-9 Enzymatic Activity
3.3. RvD2 Decreases LysM-eGFP+ Cells Recruitment Triggered by UVB Irradiation
3.4. RvD2 Reduces UVB-Irradiation-Induced Mast Cell Counts
3.5. RvD2 Reduces UVB-Irradiation-Induced Inflammatory Parameters by Decreasing the Epidermal Thickness and Sunburn Cell Formation
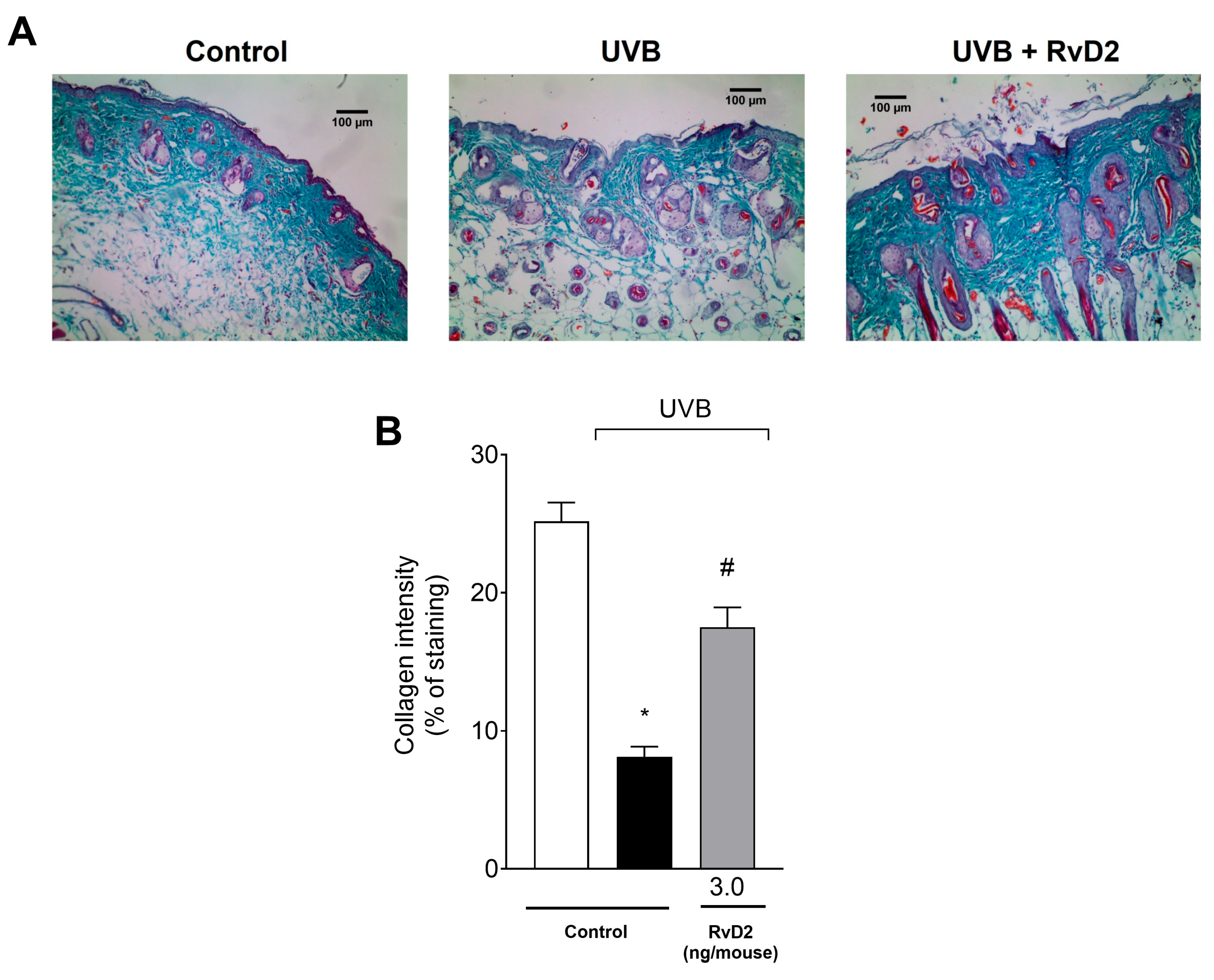
3.6. RvD2 Reduces UVB-Irradiation-Induced Damage to Collagen Fibers
3.7. RvD2 Inhibits Skin Inflammation Reducing Cytokine Production Caused by UVB Irradiation
3.8. RvD2 Optimizes the Skin’s Antioxidant Profile Modification Caused by UVB Irradiation
3.9. RvD2 Inhibits NF-κB Pathway Activation and Downstream It Targets mRNA Expression Caused by UVB Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of Ultraviolet B Signaling and Photocarcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. A Sunlight Damage to Cellular DNA: Focus on Oxidatively Generated Lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Hoyk, Z.; Tóth, M.E.; Lénárt, N.; Nagy, D.; Dukay, B.; Csefová, A.; Zvara, Á.; Seprényi, G.; Kincses, A.; Walter, F.R.; et al. Cerebrovascular Pathology in Hypertriglyceridemic APOB-100 Transgenic Mice. Front. Cell. Neurosci. 2018, 12, 380. [Google Scholar] [CrossRef]
- Hattori, H.; Subramanian, K.K.; Sakai, J.; Jia, Y.; Li, Y.; Porter, T.F.; Loison, F.; Sarraj, B.; Kasorn, A.; Jo, H.; et al. Small-Molecule Screen Identifies Reactive Oxygen Species as Key Regulators of Neutrophil Chemotaxis. Proc. Natl. Acad. Sci. USA 2010, 107, 3546–3551. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-ΚB Regulates Phagocytic NADPH Oxidase by Inducing the Expression of Gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Ogando, D.G.; Kim, E.T.; Li, S.; Bonanno, J.A. Corneal Edema in Inducible Slc4a11 Knockout Is Initiated by Mitochondrial Superoxide Induced Src Kinase Activation. Cells 2023, 12, 1528. [Google Scholar] [CrossRef]
- Beissert, S.; Loser, K. Molecular and Cellular Mechanisms of Photocarcinogenesis. Photochem. Photobiol. 2008, 84, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ivan, A.L.M.; Campanini, M.Z.; Martinez, R.M.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.T.M.C.; Vilela, F.M.P.; Martins, F.S.; Zarpelon, A.C.; Cunha, T.M.; et al. Pyrrolidine Dithiocarbamate Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Hairless Mice and Exhibits Antioxidant Activity in Vitro. J. Photochem. Photobiol. B 2014, 138, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Melo, C.B.P.; Borghi, S.M.; Pinto, I.C.; Bussmann, A.J.C.; Baracat, M.M.; Georgetti, S.R.; Jr, W.A.V.; et al. Lipoxin A4 Inhibits UV Radiation-Induced Skin Inflammation and Oxidative Stress in Mice. J. Dermatol. Sci. 2018, 91, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospíšil, P.; Kzhyshkowska, J. ROS Signaling in Innate Immunity via Oxidative Protein Modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ross, M.S.; Parrett, M.L.; Oberyszyn, T.M. Topical Application of a Selective Cyclooxygenase Inhibitor Suppresses UVB Mediated Cutaneous Inflammation. Prostaglandins Other Lipid Mediat. 2000, 62, 367–384. [Google Scholar] [CrossRef]
- Salvemini, D.; Mazzon, E.; Dugo, L.; Riley, D.P.; Serraino, I.; Caputi, A.P.; Cuzzocrea, S. Pharmacological Manipulation of the Inflammatory Cascade by the Superoxide Dismutase Mimetic, M40403. Br. J. Pharmacol. 2001, 132, 815–827. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.-Q.; Zweier, J.L.; Samouilov, A.; Macarthur, H.; Misko, T.P.; Currie, M.G.; Cuzzocrea, S.; Sikorski, J.A.; Riley, D.P. A Nonpeptidyl Mimic of Superoxide Dismutase with Therapeutic Activity in Rats. Science 1999, 286, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St. Clair, D.; Batinic-Haberle, I. Manganese Superoxide Dismutase, MnSOD and Its Mimics. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear Factor-KB: Its Role in Health and Disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Song, J.; Zhang, W.; Wang, J.; Yang, H.; Zhao, X.; Zhou, Q.; Wang, H.; Li, L.; Du, G. Activation of Nrf2 Signaling by Salvianolic Acid C Attenuates NF-κB Mediated Inflammatory Response Both in Vivo and in Vitro. Int. Immunopharmacol. 2018, 63, 299–310. [Google Scholar] [CrossRef]
- Seki, H.; Sasaki, T.; Ueda, T.; Arita, M. Resolvins as Regulators of the Immune System. Sci. World J. 2010, 10, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Yang, L.; Gao, X.; Tian, D.; Yang, W.; Xue, S.; Cao, Z.; Sun, T. Resolvin D2 Activates Anti-Inflammatory Microglia via Restoring Autophagy Flux and Alleviate Neuropathic Pain Following Spinal Cord Injury in Rats. Exp. Neurol. 2023, 370, 114573. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.; Bonin, J.L.; Khan, S.; Eid, M.; Sadhu, S.; Rahtes, A.; Lipscomb, M.; Biswas, N.; Decker, C.; Nabage, M.; et al. Resolvin D2–G-Protein Coupled Receptor 18 Enhances Bone Marrow Function and Limits Steatosis and Hepatic Collagen Accumulation in Aging. Am. J. Pathol. 2023, 193, 1953–1968. [Google Scholar] [CrossRef]
- Brüggemann, T.R.; Peh, H.Y.; Tavares, L.P.; Nijmeh, J.; Shay, A.E.; Rezende, R.M.; Lanser, T.B.; Serhan, C.N.; Levy, B.D. Eosinophil Phenotypes Are Functionally Regulated by Resolvin D2 during Allergic Lung Inflammation. Am. J. Respir. Cell Mol. Biol. 2023, 69, 666–677. [Google Scholar] [CrossRef]
- Botten, N.; Hodges, R.R.; Bair, J.; Utheim, T.P.; Serhan, C.N.; Yang, M.; Dartt, D.A. Resolvin D2 Uses Multiple Ca2+-Dependent Signaling Pathways to Stimulate Mucin Secretion in Rat and Human Conjunctival Goblet Cells. J. Cell. Physiol. 2022, 237, 3816–3833. [Google Scholar] [CrossRef]
- Tang, X.; Liu, L.; Miao, Z.; Zhang, J.; Cai, X.; Zhao, B.Q.; Chen, G.; Schultzberg, M.; Zhao, Y.; Wang, X. Resolution of Inflammation Is Disturbed in Acute Ischemic Stroke with Diabetes Mellitus and Rescued by Resolvin D2 Treatment. Free Radic. Biol. Med. 2022, 188, 194–205. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, G.; Zhang, H. GPR18 Agonist Resolvin D2 Reduces Early Brain Injury in a Rat Model of Subarachnoid Hemorrhage by Multiple Protective Mechanisms. Cell. Mol. Neurobiol. 2022, 42, 2379–2392. [Google Scholar] [CrossRef]
- Zuo, G.; Zhang, D.; Mu, R.; Shen, H.; Li, X.; Wang, Z.; Li, H.; Chen, G. Resolvin D2 Protects against Cerebral Ischemia/Reperfusion Injury in Rats. Mol. Brain 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Liu, Y.M.; Otawara, M.; Chico Calero, I.; Stephanie Nam, A.; Yu, Y.M.; Chang, P.; Butler, K.L.; Nazarian, R.M.; Goverman, J.; et al. Resolvin D2 Limits Secondary Tissue Necrosis after Burn Wounds in Rats. J. Burn Care Res. 2018, 39, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Yu, Y.M.; Kurihara, T.; Vasilyev, A.; Ibrahim, A.; Oklu, R.; Zhao, G.; Nair, A.V.; Brown, D.; Fischman, A.J.; et al. Kidney and Liver Injuries after Major Burns in Rats Are Prevented by Resolvin D2. Crit. Care Med. 2016, 44, e241–e252. [Google Scholar] [CrossRef]
- Kurihara, T.; Jones, C.N.; Yu, Y.; Fischman, A.J.; Watada, S.; Tompkins, R.G.; Fagan, S.P.; Irimia, D. Resolvin D2 Restores Neutrophil Directionality and Improves Survival after Burns. FASEB J. 2013, 27, 2270–2281. [Google Scholar] [CrossRef] [PubMed]
- Bohr, S.; Patel, S.J.; Sarin, D.; Irimia, D.; Yarmush, M.L.; Berthiaume, F. Resolvin D2 Prevents Secondary Thrombosis and Necrosis in a Mouse Burn Wound Model. Wound Repair Regen. 2013, 21, 35–43. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 Recovers Neural Injury by Suppressing Inflammatory Mediators Expression in Lipopolysaccharide-Induced Parkinson’s Disease Rat Model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef]
- Chen, J.-P.; Xu, H.-Y.; Liao, L.; Zhang, Z. Resolvin D2 Prevents Inflammation and Oxidative Stress in the Retina of Streptozocin-Induced Diabetic Mice. Int. J. Clin. Exp. Pathol. 2020, 13, 1986–1994. [Google Scholar]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 Is a Potent Regulator of Leukocytes and Controls Microbial Sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef]
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.N.; Langevin, H.M. Stretching Impacts Inflammation Resolution in Connective Tissue. J. Cell. Physiol. 2016, 231, 1621–1627. [Google Scholar] [CrossRef]
- Campanini, M.Z.; Pinho-Ribeiro, F.A.; Ivan, A.L.M.; Ferreira, V.S.; Vilela, F.M.P.; Vicentini, F.T.M.C.; Martineza, R.M.; Zarpelon, A.C.; Fonseca, M.J.V.; Faria, T.J.; et al. Efficacy of Topical Formulations Containing Pimenta Pseudocaryophyllus Extract against UVB-Induced Oxidative Stress and Inflammation in Hairless Mice. J. Photochem. Photobiol. B 2013, 127, 153–160. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-ribeiro, F.A.; Ste, V.S.; Caviglione, C.V.; Barbosa, S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced In Fl Ammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Kajiya, K.; Hirakawa, S.; Detmar, M. Vascular Endothelial Growth Factor-A Mediates Ultraviolet B-Induced Impairment of Lymphatic Vessel Function. Am. J. Pathol. 2006, 169, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tanaka, M.; Misawa, E.; Yao, R.; Nabeshima, K.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Oral Administration of Aloe Vera Gel Powder Prevents Uvb-Induced Decrease in Skin Elasticity via Suppression of Overexpression of Mmps in Hairless Mice. Biosci. Biotechnol. Biochem. 2016, 80, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Cuelho, C.H.F.; Alves, G.d.A.D.; Lovatto, M.O.; Bonilha, I.F.; Barbisan, F.; da Cruz, I.B.M.; Oliveira, S.M.; Fachinetto, R.; do Canto, G.S.; Manfron, M.P. Topical Formulation Containing Ilex Paraguariensis Extract Increases Metalloproteinases and Myeloperoxidase Activities in Mice Exposed to UVB Radiation. J. Photochem. Photobiol. B 2018, 189, 95–103. [Google Scholar] [CrossRef]
- Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; de Matos, R.L.N.; Vale, D.L.; Melo, C.P.B.; Fattori, V.; Saraiva-Santos, T.; Mendes-Pierotti, S.; Bertozzi, M.M.; et al. Resolvin D5 Protects Female Hairless Mouse Skin from Pathological Alterations Caused by UVB Irradiation. Antioxidants 2024, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ediriwickrema, A.; Yang, F.; Lewis, J.; Girardi, M.; Saltzman, W.M. A Sunblock Based on Bioadhesive Nanoparticles. Nat. Mater. 2015, 14, 1278–1285. [Google Scholar] [CrossRef]
- Schwarz, A.; Bhardwaj, R.; Aragane, Y.; Mahnke, K.; Riemann, H.; Metze, D.; Luger, T.A.; Schwarz, T. Ultraviolet-B-Induced Apoptosis of Keratinocytes: Evidence for Partial Involvement of Tumor Necrosis Factor-α in the Formation of Sunburn Cells. J. Investig. Dermatol. 1995, 104, 922–927. [Google Scholar] [CrossRef]
- Song, J.H.; Piao, M.J.; Han, X.; Ah Kang, K.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S.; et al. Anti-Wrinkle Effects of Sargassum Muticum Ethyl Acetate Fraction on Ultraviolet B-Irradiated Hairless Mouse Skin and Mechanistic Evaluation in the Human HaCaT Keratinocyte Cell Line. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef]
- Kim, C.-S.; Cha, J.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.-M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. Ser. A 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender Differences in Antioxidant Capacity of Rat Tissues Determined by 2,2′-Azinobis (3-Ethylbenzothiazoline 6-Sulfonate; ABTS) and Ferric Reducing Antioxidant Power (FRAP) Assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 47–52. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sabitha, K.E.; Shyamaladevi, C.S. Attenuation of 4-Nitroquinoline 1-Oxide Induced in Vitro Lipid Peroxidation by Green Tea Polyphenols. Life Sci. 2007, 80, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Ferreira, A.L.A.; Matsubara, L.S. Radicais Livres: Conceitos, Doenças Relacionadas, Sistema de Defesa e Estresse Oxidativo. Rev. Ass. Med. Brasil. 1997, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Kobayashi, H.; Yamate, Y.; Ishii, M.; Sato, E.F. Intercellular Pathway through Hyaluronic Acid in UVB-Induced Inflammation. Exp. Dermatol. 2012, 21, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Flecha, B.; Llesuy, S.; Boveris, A. Hydroperoxide-Iniated Chemiluminescence: An Assay for Oxidative Stress in Biopsies of Heart, Liver, and Muscle. Free Radic. Biol. Med. 1991, 10, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO−cGMP−PKG−KATPChannel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- PubChem National Library of Medicine Compound Summary: Resolvin D2. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Resolvin-D2 (accessed on 26 June 2025).
- Zhang, L.; Gu, W.; Liu, T.; Pei, H.; Ma, Y.; Zhao, Y.; Huang, S.; Chen, M. NDRG2 Deficiency Exacerbates UVB-Induced Skin Inflammation and Oxidative Stress Damage. Inflammation 2024. [Google Scholar] [CrossRef]
- Baker, B.S.; Bokth, S.; Garioch, J.J.; Powles, A.V.; Lewis, H.; Valdimarsson, H.; Fry, L. Decreased T Cell Reactivity to Trypsinized Group A, Type M22 Streptococci in Psoriasis. Acta Derm. Venereol. 1994, 74, 276–278. [Google Scholar] [CrossRef]
- Ganz, T. Antimicrobial Polypeptides. J. Leukoc. Biol. 2004, 75, 34–38. [Google Scholar] [CrossRef]
- Hart, P.H.; Grimbaldeston, M.A.; Finlay-Jones, J.J. Mast Cells in UV-B-Induced Immunosuppression. J. Photochem. Photobiol. B Biol. 2000, 55, 81–87. [Google Scholar] [CrossRef]
- Ikai, K.; Danno, K.; Horio, T.; Narumiya, S. Effect of Ultraviolet Irradiation on Mast Cell-Deficient W/Wv Mice. J. Investig. Dermatol. 1985, 85, 82–84. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Anbar, T.S.; Yehia, M.; Abdel-Aziz, R.T.A. The Use of Intralesional Corticosteroid Combined with Narrowband Ultraviolet B in Vitiligo Treatment: Clinical, Histopathologic, and Histometric Evaluation. Int. J. Dermatol. 2022, 61, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B Regulation of Transcription Factor Families: Roles of Nuclear Factor-Kappa B (NF-ΚB) and Activator Protein-1 (AP-1) in UVB-Induced Skin Carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Chang, E.J.; Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Ultraviolet B Radiation Activates NF-ΚB and Induces INOS Expression in HR-1 Hairless Mouse Skin: Role of IκB Kinase-β. Mol. Carcinog. 2011, 50, 310–317. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Beak, S.M.; Lee, Y.S.; Kim, J.A. NADPH Oxidase and Cyclooxygenase Mediate the Ultraviolet B-Induced Generation of Reactive Oxygen Species and Activation of Nuclear Factor-ΚB in HaCaT Human Keratinocytes. Biochimie 2004, 86, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Aram, G.; Potter, J.J.; Liu, X.; Wang, L.; Torbenson, M.S.; Mezey, E. Deficiency of Nicotinamide Adenine Dinucleotide Phosphate, Reduced Form Oxidase Enhances Hepatocellular Injury but Attenuates Fibrosis after Chronic Carbon Tetrachloride Administration. Hepatology 2009, 49, 911–919. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef][Green Version]
- Halliday, G.M. Inflammation, Gene Mutation and Photoimmunosuppression in Response to UVR-Induced Oxidative Damage Contributes to Photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Hanson, K.M.; Gratton, E.; Bardeen, C.J. Sunscreen Enhancement of UV-Induced Reactive Oxygen Species in the Skin. Free Radic. Biol. Med. 2006, 41, 1205–1212. [Google Scholar] [CrossRef]
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Adverse Reactions to Sunscreen Agents: Epidemiology, Responsible Irritants and Allergens, Clinical Characteristics, and Management. Dermatitis 2014, 25, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Albers, R.; Antoine, J.; Blum, S.; Ferns, G.A.; Folkerts, G. Inflammatory Disease Processes and Interactions with Nutrition. Br. J. Nutr. 2009, 101, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N. Controlling the Resolution of Acute Inflammation: A New Genus of Dual Anti-Inflammatory and Proresolving Mediators. J. Periodontol. 2008, 79, 1520–1526. [Google Scholar] [CrossRef]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-Associated Functions of IL-33: The New Kid in the IL-1 Family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward Rheumatoid Arthritis Therapy; the Old and the New. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef]
- Contassot, E.; Beer, H.; French, L. Interleukin-1, Inflammasomes, Autoinflammation and the Skin. Swiss Med. Wkly. 2012, 142, w13590. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Singampalli, K.L.; Balaji, S.; Wang, X.; Parikh, U.M.; Kaul, A.; Gilley, J.; Birla, R.K.; Bollyky, P.L.; Keswani, S.G. The Role of an IL-10/Hyaluronan Axis in Dermal Wound Healing. Front. Cell Dev. Biol. 2020, 8, 636. [Google Scholar] [CrossRef]
- Bento, A.F.; Claudino, R.F.; Dutra, R.C.; Marcon, R.; Calixto, J.B. Omega-3 Fatty Acid-Derived Mediators 17(R)-Hydroxy Docosahexaenoic Acid, Aspirin-Triggered Resolvin D1 and Resolvin D2 Prevent Experimental Colitis in Mice. J. Immunol. 2011, 187, 1957–1969. [Google Scholar] [CrossRef]
- Navarro, V.P.; Nelson-Filho, P.; Silva, L.A.B.; Freitas, A.C. A Participação Das Metaloproteinases Da Matriz Nos Processos Fisiológicos Da Cavidade Bucal. Rev. Odontol. UNESP 2006, 35, 233–248. [Google Scholar]
- Michalak, K.M.; Biedermann, T.; Reichmann, E.; Meuli, M.; Klar, A.S. Induction of Angiogenic and Inflammation-Associated Dermal Biomarkers Following Acute UVB Exposure on Bio-Engineered Pigmented Dermo-Epidermal Skin Substitutes in Vivo. Pediatr. Surg. Int. 2018, 35, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Alam, S.; Pal, A.; Kumar, M.; Singh, D.; Ansari, K.M. UVB Exposure Enhanced Benzanthrone-Induced in Fl Ammatory Responses in SKH-1 Mouse Skin by Activating the Expression of COX-2 and INOS through MAP Kinases/NF-kB/AP-1 Signalling Pathways. Food Chem. Toxicol. 2016, 96, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Parrett, M.L.; Ross, M.S.; Tober, K.L.; Robertson, F.M.; Oberyszyn, T.M. Inhibition of Ultraviolet Light B-Induced Cutaneous Inflammation by A Specific Cyclooxygenase-2 Inhibitor. Adv. Exp. Med. Biol. 2002, 5, 85–92. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, B.H.; Lee, H.; Jeon, B.; Lee, Y.S.; Kwon, M.-J.; Kim, T.-Y. Regulation of Skin Inflammation and Angiogenesis by EC-SOD via HIF-1α and NF-ΚB Pathways. Free Radic. Biol. Med. 2011, 51, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.d.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Scott, T.L.; Christian, P.A.; Kesler, M.V.; Donohue, K.M.; Shelton, B.; Wakamatsu, K.; Shosuke, I.; D’Orazio, J. Pigment-Independent CAMP-Mediated Epidermal Thickening Protects against Cutaneous UV Injury by Keratinocyte Proliferation. Exp. Dermatol. 2012, 21, 771–777. [Google Scholar] [CrossRef]
- Bayerl, C.; Taake, S.; Moll, I.; Jung, E.G. Characterization of Sunburn Cells after Exposure to Ultraviolet Light. Photodermatol. Photoimmunol. Photomed. 1995, 11, 149–154. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. The UV Response of the Skin: A Review of the MAPK, NFκB and TNFα Signal Transduction Pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef]
- Godar, D.E. UVA1 Radiation Triggers Two Different Final Apoptotic Pathways. J. Investig. Dermatol. 1999, 112, 3–12. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Vale, D.L.; Steffen, V.S.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Trans-Chalcone Added in Topical Formulation Inhibits Skin Inflammation and Oxidative Stress in a Model of Ultraviolet B Radiation Skin Damage in Hairless Mice. J. Photochem. Photobiol. B 2017, 171, 139–146. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Benabdoune, H.; Rondon, E.P.; Shi, Q.; Fernandes, J.; Ranger, P.; Fahmi, H.; Benderdour, M. The Role of Resolvin D1 in the Regulation of Inflammatory and Catabolic Mediators in Osteoarthritis. Inflamm. Res. 2016, 65, 635–645. [Google Scholar] [CrossRef]
- Croasdell, A.; Thatcher, T.H.; Kottmann, R.M.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Sime, P.J.; Phipps, R.P. Resolvins Attenuate Inflammation and Promote Resolution in Cigarette Smokeexposed Human Macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L888–L901. [Google Scholar] [CrossRef]
- Akagi, D.; Chen, M.; Toy, R.; Chatterjee, A.; Conte, M.S. Systemic Delivery of Proresolving Lipid Mediators Resolvin D2 and Maresin 1 Attenuates Intimal Hyperplasia in Mice. FASEB J. 2015, 29, 2504–2513. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.; Na, K.M.; Surh, Y.J.; Kim, T.Y. [6]-Gingerol Prevents UVB-Induced ROS Production and COX-2 Expression in Vitro and in Vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S. COX-2 Expression Is Induced by UVB Exposure in Human Skin: Implications for the Development of Skin Cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kundu, J.K.; Shin, J.W.; Na, H.K.; Surh, Y.J. Docosahexaenoic Acid Inhibits UVB-Induced Activation of NF-ΚB and Expression of COX-2 and NOX-4 in HR-1 Hairless Mouse Skin by Blocking MSK1 Signaling. PLoS ONE 2011, 6, e28065. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nakano, H.; Yamazaki, T.; Tamai, K.; Hanada, K.; Takahashi, G.; Nakano, H. Enhancement of Ultraviolet-Induced Apoptosis by NF-JB Decoy Oligonucleotides. Br. J. Dermatol. 2005, 153, 47–51. [Google Scholar] [CrossRef]
- Melo, C.P.B.; Saito, P.; Martinez, R.M.; Staurengo-Ferrari, L.; Pinto, I.C.; Rodrigues, C.C.A.; Badaro-Garcia, S.; Vignoli, J.A.; Baracat, M.M.; Bussmann, A.J.C.; et al. Aspirin-Triggered Resolvin D1 (AT-RvD1) Protects Mouse Skin against UVB-Induced Inflammation and Oxidative Stress. Molecules 2023, 28, 2417. [Google Scholar] [CrossRef]
- Martinez, R.M.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Fattori, V.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Zaninelli, T.H.; Saraiva-Santos, T.; et al. Pretreatment with Acetylsalicylic Acid Alleviates UVB Irradiation-Induced Skin Pathology in Hairless Mice and BOC-2 (an ALX/FPR2 Receptor Antagonist) Reduces Its Activity. Pharmacol. Res. Rep. 2025, 3, 100024. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; Silva, M.D.V.d.; Martelossi-Cebinelli, G.; Fattori, V.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections. Molecules 2023, 28, 5032. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of Resolvin D2 Receptor Mediating Resolution of Infections and Organ Protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Alnouri, M.W.; Roquid, K.A.; Bonnavion, R.; Cho, H.; Heering, J.; Kwon, J.; Jäger, Y.; Wang, S.P.; Günther, S.; Wettschureck, N.; et al. SPMs Exert Anti-Inflammatory and pro-Resolving Effects through Positive Allosteric Modulation of the Prostaglandin EP4 Receptor. Proc. Natl. Acad. Sci. USA 2024, 121, e2407130121. [Google Scholar] [CrossRef]
- Costa, V.V.; Resende, F.; Melo, E.M.; Teixeira, M.M. Resolution Pharmacology and the Treatment of Infectious Diseases. Br. J. Pharmacol. 2024, 181, 917–937. [Google Scholar] [CrossRef]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef]




| Antibodies Used for Fluorescent Western Blot | ||||||
|---|---|---|---|---|---|---|
| Target | Primary Antibody | Dilution | Supplier/Cat No. | Secondary Antibody | Dilution | Supplier/Cat No. |
| IκB-α | IκBα (L35A5) Mouse mAb (Amino-terminal Antigen) | 1:1000 | Cell Signaling/4814 | Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | 1:1000 | Invitrogen/A11001 |
| β-actin | β-Actin (D6A8) Rabbit mAb | 1:1000 | Cell Signaling/8457 | Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 790 | 1:1000 | Invitrogen/A113667 |
| Primer sequences for RT-qPCR | ||||||
| Gene (Target) | Forward sequence (5′ to 3′) | Reverse sequence (5′ to 3′) | ||||
| Cybb (Gp91phox) | AGC TAT GAG GTG GTG ATG TTA GTG G | CAC AAT ATT TGT ACC AGA CAG ACT TGA G | ||||
| Cox2 (COX-2) | GTG GAA AAA CCT CGT CCA GA | GCT CGG CTT CCA GTA TTG AG | ||||
| Actb (β-actin) | AGC TGC GTT TTA CAC CCT TT | AAG CCA TGC CAA TGT TGT CT | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, I.C.; Saito, P.; Rodrigues, C.C.A.; Martinez, R.M.; Melo, C.P.B.; Piva, M.; Kumagai, C.M.; Vale, D.L.; Saraiva-Santos, T.; Bussmann, A.J.C.; et al. Resolvin D2 Reduces UVB Skin Pathology by Targeting Cytokines, Oxidative Stress, and NF-κB Activation. Antioxidants 2025, 14, 830. https://doi.org/10.3390/antiox14070830
Pinto IC, Saito P, Rodrigues CCA, Martinez RM, Melo CPB, Piva M, Kumagai CM, Vale DL, Saraiva-Santos T, Bussmann AJC, et al. Resolvin D2 Reduces UVB Skin Pathology by Targeting Cytokines, Oxidative Stress, and NF-κB Activation. Antioxidants. 2025; 14(7):830. https://doi.org/10.3390/antiox14070830
Chicago/Turabian StylePinto, Ingrid C., Priscila Saito, Camilla C. A. Rodrigues, Renata M. Martinez, Cristina P. B. Melo, Maiara Piva, Clovis M. Kumagai, David L. Vale, Telma Saraiva-Santos, Allan J. C. Bussmann, and et al. 2025. "Resolvin D2 Reduces UVB Skin Pathology by Targeting Cytokines, Oxidative Stress, and NF-κB Activation" Antioxidants 14, no. 7: 830. https://doi.org/10.3390/antiox14070830
APA StylePinto, I. C., Saito, P., Rodrigues, C. C. A., Martinez, R. M., Melo, C. P. B., Piva, M., Kumagai, C. M., Vale, D. L., Saraiva-Santos, T., Bussmann, A. J. C., Baracat, M. M., Georgetti, S. R., Vicentini, F. T. M. C., Verri, W. A., & Casagrande, R. (2025). Resolvin D2 Reduces UVB Skin Pathology by Targeting Cytokines, Oxidative Stress, and NF-κB Activation. Antioxidants, 14(7), 830. https://doi.org/10.3390/antiox14070830







