Abstract
This study aimed to characterize the time-dependent effects of methane (CH4) inhalation, initiated at defined intervals following sepsis onset, on organ function, systemic oxygen utilization, and mitochondrial respiration in a rodent model. Adult rats were subjected to abdominal sepsis or sham operation. Septic animals were assigned to groups receiving 2.2% CH4 in normoxic air at specific post-insult phases (early: 3–6 h; intermediate: 16–19 h; late: 19–22 h), while a control group remained untreated. At 24 h, organ function was evaluated using a Rat-Specific Organ Failure Assessment (ROFA) score, along with measurements of plasma myeloperoxidase (MPO) activity, Complex I–II-linked oxidative phosphorylation in renal and cerebellar tissues, systemic oxygen extraction, and global tissue perfusion (pCO2-gap). Sepsis induced significant organ dysfunction, impaired hemodynamics, reduced oxygen utilization, and decreased mitochondrial respiration. CH4 inhalation improved survival when administered early, restored cerebellar mitochondrial respiration during the intermediate phase, and in the late phase reduced ROFA scores and MPO levels, while attenuating mitochondrial dysfunction in renal and cerebellar tissues. All CH4-treated groups demonstrated improved renal function and enhanced tissue oxygenation. Targeted CH4 inhalation during sepsis confers protective effects by preserving mitochondrial function, reducing inflammation, and improving oxygen dynamics, suggesting promising therapeutic potential.
1. Introduction
Sepsis is a life-threatening syndrome caused by a dysregulated host response to infection, causing organ failures [1]. As sepsis progresses, an oxygen delivery (DO2)–consumption (VO2) mismatch transpires, with cellular deficits in oxygen extraction (ExO2) limiting mitochondrial oxygen availability [2]. Mitochondrial dysfunction is crucial to pathology, impairing ATP production, shifting metabolism to anaerobic pathways, and generating reactive oxygen species (ROS), all contributing to tissue damage and organ failure [3,4]. Cardiovascular dysfunction is an early clinical complication, resulting in central nervous system abnormalities in up to 70% of patients, as measured using the Sequential Organ Failure Assessment (SOFA) score [5,6]. Another severe complication that is associated with significantly higher mortality rates is sepsis-induced acute kidney injury (AKI), driven by renal hypoperfusion and inflammation [7,8]. Despite progress in critical care, novel treatment strategies targeting sepsis are still needed [9,10]. As conventional respiratory and circulatory support frequently fails to address sepsis-induced organ alterations, drug delivery efficacy remains a key determinant of therapeutic success [11,12]. Considering that subcellular, cellular, and intercellular membrane components are central to sepsis pathophysiology, agents that can traverse various biological barriers are particularly well-suited for this application. Although the anti-inflammatory, antipyroptotic, and antiapoptotic effects of methane (CH4) have been well-characterized [13,14], as well as several components of the signaling mechanisms, including its ability to suppress cytokines and inhibit NF-κB activation [15,16,17], its role in sepsis-induced mitochondrial dysfunction and organ failure remains unexplored. This is the first study to investigate the therapeutic role of inhaled CH4 in experimental sepsis, focusing on mitochondrial preservation and organ function. Notably, inhaled CH4 can be directly delivered to the lungs, and CH4 (2.2%) administered in a normoxic air mixture has been shown to preserve renal function in preclinical models of extracorporeal perfusion and veno–venous membrane oxygenation [18]. Based on previous findings, we hypothesized that inhaled CH4, a biologically active gas that can cross cellular and subcellular membranes [13], could enhance mitochondrial function and concurrently alleviate organ dysfunction in experimental sepsis. We aimed to evaluate the temporal dynamics of mitochondrial respiratory impairment and organ dysfunction over a 24-h period in a polymicrobial rat model of sepsis, both with and without CH4 treatment [19]. Furthermore, we sought to determine the optimal therapeutic window for CH4 administration. Septic animals were randomized into untreated and CH4-treated groups, with identical treatment durations applied at distinct stages of sepsis progression (3–6, 16–19, and 19–22 h following sepsis induction).
2. Materials and Methods
Experiments were conducted on 11–12 weeks old male Sprague Dawley rats (n = 44; 380 ± 30 g). Animals were housed under controlled conditions, appropriate temperature (21–23 °C), and humidity (45–55%) in plastic cages with a 12/12 h dark/light cycle and access to standard rodent food and water ad libitum. These conditions were maintained throughout their lifetime to ensure consistent living conditions. The study was conducted in a randomized, double-blind manner. Neither the individual performing the experimental procedures nor the investigator analyzing the samples was aware of the groups of animal allocations. This approach allowed both the execution of the experiments and the analysis of the data to be performed without bias. All procedures followed the National Institutes of Health guidelines and EU Directive on the care and use of laboratory animals, with the study protocol approved by the local Institutional Review Board and the National Scientific Ethical Committee for Animal Experimentation in Hungary (license V./2884/2022, approved: 14 November 2022). The study design and data presentation followed the Minimum Quality Threshold in Preclinical Sepsis Studies (MQTiPSS) recommendations [20] and ARRIVE guidelines (https://arriveguidelines.org/).
2.1. Experimental Protocol
Sample size estimation was performed, assuming approximately 20% mortality after 24 h. If the presumed true hazard ratio of septic subjects relative to controls is 0.2 with a power of 1-β = 0.8 and the Type I error probability is α = 0.05, the inclusion of 9 animal/group was recommended.
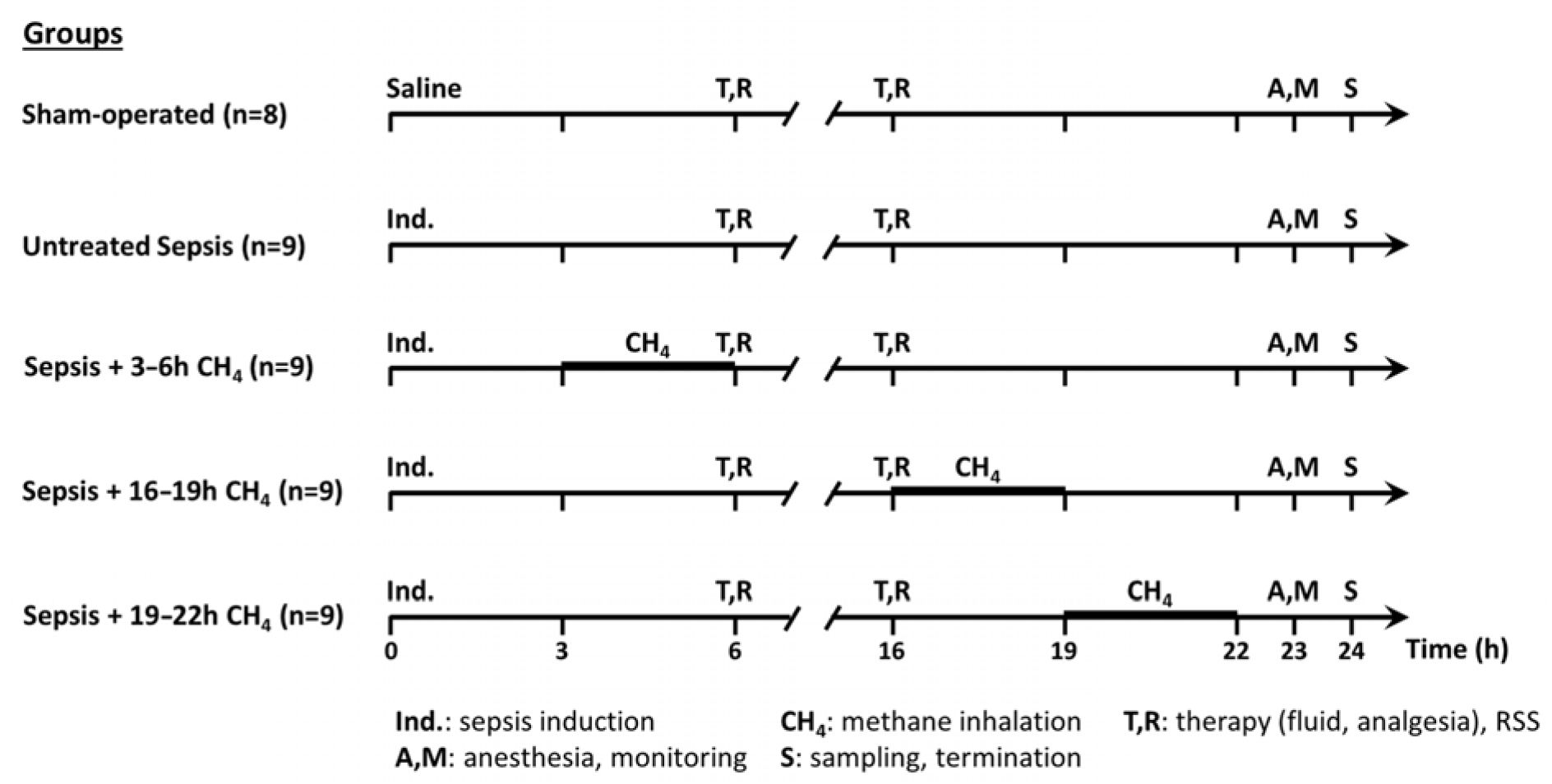
The experimental animals were randomly assigned to a sham-operated group (n = 8) or septic groups (n∑ = 36) [19]. The septic groups were intraperitoneally (i.p.) injected fecal inoculum (t = 0 h) to induce peritonitis, ensuring the rapid onset of polymicrobial sepsis. Previously, fresh feces were randomly collected from healthy rats (n = 5). Four grams of fecal material were mixed with 36 mL of saline and incubated for 6 h at 37 °C. The resulting suspension was filtered, and a 5 mL/kg inoculum was i.p. administered using a 21-G needle. The septic groups were further categorized into an untreated group (n = 9) and three CH4-treated septic groups (see below). The sham-operated group received an equivalent volume of sterile physiological saline (Figure 1).
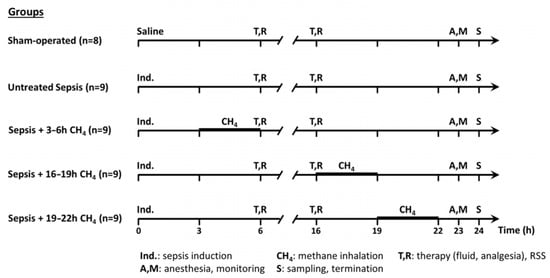
Figure 1.
Scheme of the experimental protocol (groups, interventions, and assessments).
Sepsis progression was monitored using a modified rat-specific well-being-related sickness (RSS) score system [19] at baseline and at 6 and 16 h post-induction. At the time of the RSS assessments, the animals received 10 mL/kg crystalloid solution subcutaneously (sc) (Ringerfundin, B. Braun, Hungary) to avoid dehydration and 15 µg/kg buprenorphine sc (Bupaq, Merck, USA) to maintain analgesia according to the MQTiPSS recommendations [20]. At 23 h post-induction, the animals were anesthetized with a mixture of ketamine (45.7 mg/kg; Medicus Partner Ltd., Biatorbágy Hungary) and xylazine (9.12 mg/kg; Produlab Pharma, Raamsdonksveer, The Netherlands) i.p. Subsequently, the animals were positioned supine on a heated operating table to maintain normothermia (37 °C). To secure patent airway, tracheostomy was performed. A PE50 cannula was inserted into the right external jugular vein for continuous anesthesia (ketamine 12 mg/kg/h, xylazine 2.4 mg/kg/h and diazepam 0.576 mg/kg/h; Richter Pharma, Hungary), fluid replacement (10 mL/kg/h; Ringerfundin, B. Braun, Budapest, Hungary), and venous blood sampling. Moreover, the left carotid artery was cannulated for continuous heart rate and mean arterial pressure (MAP) monitoring (SPEL Advanced Cardiosys 1.4, Experimetria Ltd., Budapest, Hungary), as well as for arterial blood gas and lactate analysis. At 24 h post-induction, median laparotomy was performed. Venous blood was collected from the inferior vena cava in tubes containing EDTA (for myeloperoxidase [MPO] level analysis). The left kidney was removed, and the animals were subsequently euthanized by rapid decapitation under deep anesthesia. The skull was opened along the sagittal suture, the cerebellum was separated from the adjacent brain tissue, and cerebellar and renal tissue samples were obtained for biochemical and mitochondrial analyses.
2.2. Methane Treatment
For CH4 treatment, conscious animals were placed in a sealed chamber with a pressure-compensating valve at 3-h intervals at their respective group timepoints (t = 3–6, 16–19, or 19–22 h post-induction). The chamber was presaturated with normoxic artificial air containing 2.2% CH4, 21% O2, and 76.8% N2. A continuous flow rate of 250 mL/min was maintained and monitored using a rotameter. Bedding, food, and water were provided throughout the inhalation period.
2.3. Organ Failure Assessment
The SOFA scoring system, originally developed for humans, was previously adapted by our group to incorporate rodent-specific parameters [21]. Respiratory function was assessed using the ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2, set at 0.21 for room air). MAP was used for evaluating cardiovascular status. Blood lactate levels indicated metabolic imbalance due to anaerobic metabolism. Kidney injury severity was assessed on the basis of plasma urea concentrations; plasma alanine aminotransferase (ALT) levels, measured using a Roche/Hitachi 917 analyzer (F. Hoffmann–La Roche AG, Basel, Switzerland), were used for liver dysfunction evaluation. Animals with ROFA scores of >2 were classified as septic. The resulting Rat-Specific Organ Failure Assessment (ROFA) scores were used for evaluating multiple organ dysfunction in animals, with each component assigned a score of 0–4 on the basis of predefined thresholds (Supplementary Material, Supplementary Table S1).
2.4. Measurements of the Oxygen Dynamics
Using a Cobas b 123 blood gas analyzer (Roche Ltd., Basel, Switzerland), blood gas variables were measured from heparinized arterial and venous samples. Simplified ExO2 was calculated using the formula (SaO2 − SvO2)/SaO2, based on arterial (SaO2) and venous oxygen saturation (SvO2). Tissue perfusion quality was assessed by determining the pCO2 gap, defined as the difference between venous and arterial CO2 partial pressures (pCO2 gap = PvCO2 − PaCO2).
2.5. Mitochondrial Respiration Assessment
Mitochondrial oxygen consumption linked to mitochondrial Complexes I and II (C-I and C-II) was evaluated in cerebellar and kidney homogenate samples using high-resolution respirometry (Oxygraph-2k; Oroboros Instruments, Innsbruck, Austria) [21]. Briefly, LEAK respirations were evaluated after oxidation of the following complex specific substrates: 10 mM glutamate, 2 mM malate (C-I–linked respiration; LEAKGM), or 10 mM succinate (C-II–linked respiration; LEAKs). Before adding succinate, C-I–linked respiration was inhibited with rotenone (0.5 μM). Maximal capacities of oxidative phosphorylation (OXPHOS I and II) were achieved by saturating the ADP concentration (2.5 mM). Exogenous cytochrome c (CytC; 10 μM) was used after adding ADP to assess the outer membrane integrity. ATP synthase was inhibited by oligomycin (2.5 μM) to evaluate LEAK respiration in a nonphosphorylating state (LEAKOmy) of mitochondrial respiration. Electron transport-independent respiration (or residual mitochondrial oxygen consumption, ROX) was determined following C-III inhibition with antimycin A (2.5 μM). All measurements were performed under continuous stirring (750 rpm) at 37 °C in a 2 mL Mir05 respiration buffer. Mitochondrial oxygen consumption was expressed in pmol/s/mL. All respiratory substrates and inhibitors were purchased from Sigma–Aldrich (St. Louis, MO, USA). The DatLab 7 software (version 7.4.0.4.; Oroboros Instruments, Innsbruck, Austria) was used for online respirometry data display and analysis.
2.6. Plasma MPO Measurement
Circulating MPO activity, used as an indicator of systemic neutrophil activation, was measured as described by Kuebler et al. [22].
2.7. Statistical Analysis
Data were evaluated using the SigmaStat 13.0 software package (Systat Software, San Jose, CA, USA). The Shapiro–Wilk test was performed to analyze the normality of the data distribution. The Kruskal–Wallis analysis of variance (ANOVA) of the ranks, with Dunn’s post-hoc test, or two-way repeated measures ANOVA completed with Holm–Sidak’s post-hoc test, was employed for calculating the differences between the groups. The median values with 25th and 75th percentiles are provided in the figures; p < 0.05 was considered statistically significant.
3. Results
3.1. Changes in Well-Being
Animal well-being markedly declined at 6 h post-sepsis induction across all the septic groups (Supplementary Material, Supplementary Figure S1), as indicated by increasing RSS score, which continued to increase during the later stages of sepsis. Animals that reached predefined humane endpoints were euthanized at 16 h, and their RSS data were excluded from the statistical analysis. Specifically, two animals per septic group were euthanized, except in the 3–6 h CH4-treated group, wherein no animals met the euthanasia criteria. Considering these circumstances, untreated septic animals demonstrated the highest RSS at 16 h, whereas animals that received CH4 treatment at 3–6 h exhibited significantly lower scores than untreated septic controls.
3.2. Organ Function Alterations
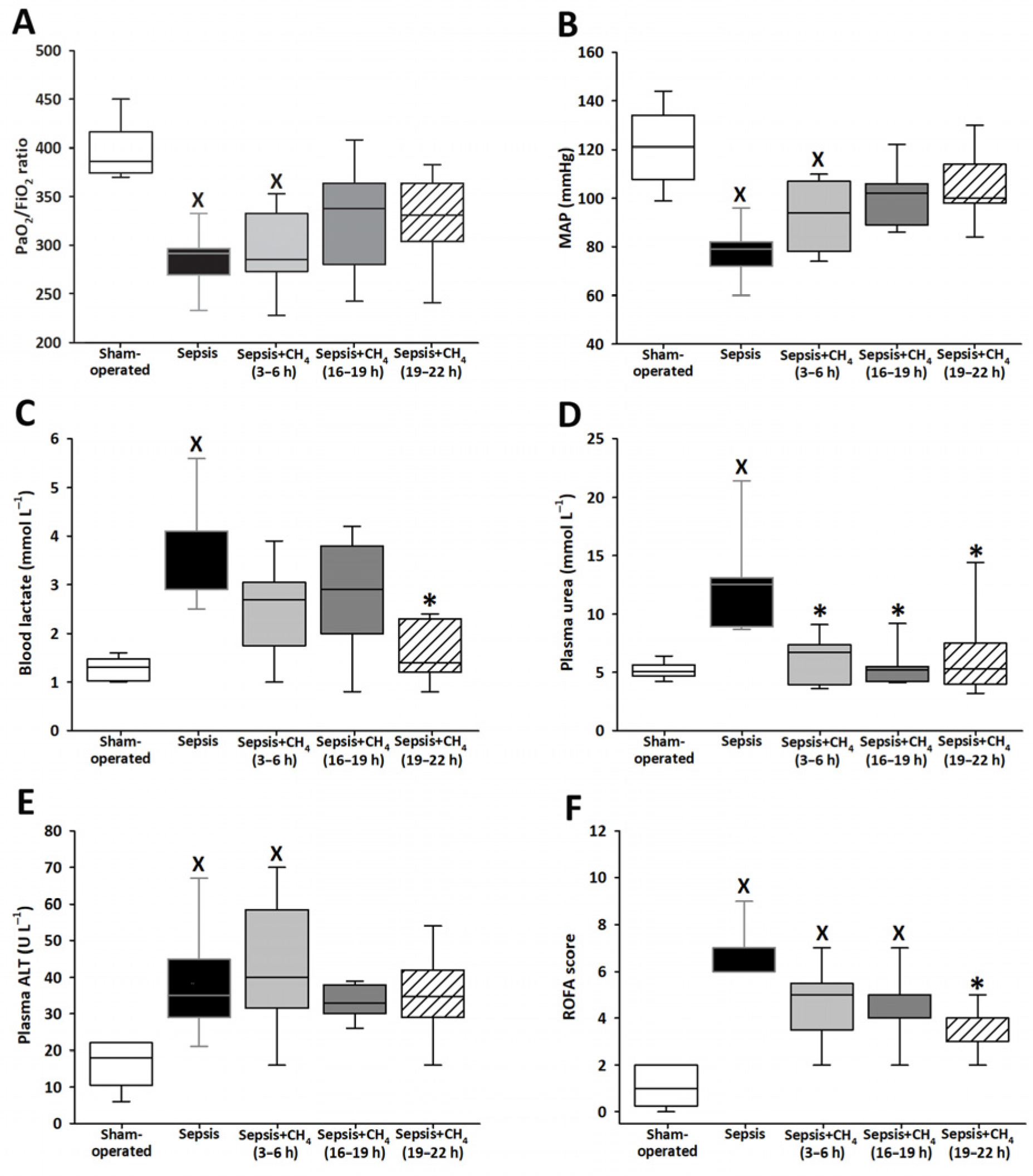
Various ROFA score components were determined at t = 24 h. The untreated septic group exhibited significantly lower PaO2/FiO2 ratios and MAP values than the sham-operated controls, indicating that sepsis-induced marked respiratory dysfunction and systemic hypotension (Figure 2A,B). Septic animals that received CH4 treatment at t = 3–6 h demonstrated comparable reductions in these parameters. In contrast, PaO2/FiO2 ratios and MAP values in the groups receiving CH4 treatment at t = 16–19 and 19–22 h did not significantly differ from those of the sham-operated group.
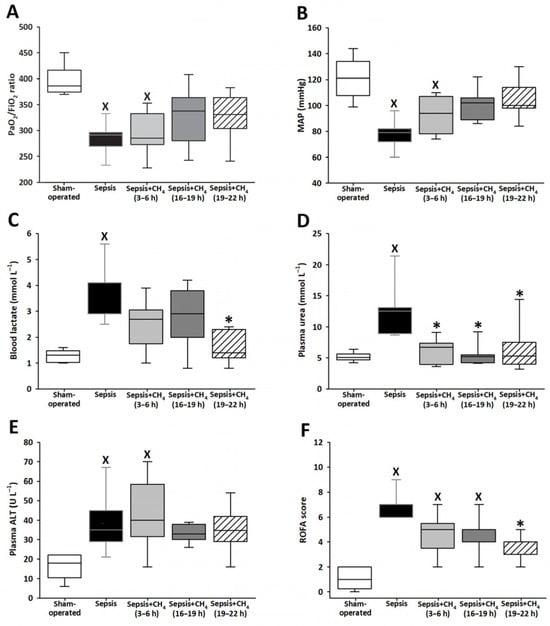
Figure 2.
PaO2/FiO2 ratio (A), mean arterial pressure (MAP) (B), blood lactate (C), plasma urea (D), plasma alanine aminotransferase (ALT) (E), and Rat-specific Organ Failure Assessment (ROFA) scores (F) in the sham-operated group and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group.
Compared with sham-operated animals, only the untreated septic group showed elevated blood lactate and plasma urea levels. Compared with untreated sepsis, CH4 treatment administered in the latest stage significantly decreased the lactate levels (Figure 2C), whereas CH4 treatment administered during any of the three stages led to significantly decreased plasma urea levels (Figure 2D). The untreated septic group and the group receiving early CH4 treatment demonstrated significantly elevated plasma ALT levels, whereas ALT levels following CH4 administration at 16–19 and 19–22 h were comparable to those observed in the sham-operated group (Figure 2E). All the septic groups exhibited significantly elevated ROFA scores, which reflect the aggregate of systemic organ dysfunction parameters, compared with the sham-operated controls. Notably, CH4 treatment administered at the latest timepoint (t = 19–22 h) significantly reduced the ROFA scores compared with the untreated septic group (Figure 2F).
3.3. Oxygen Dynamics Alterations
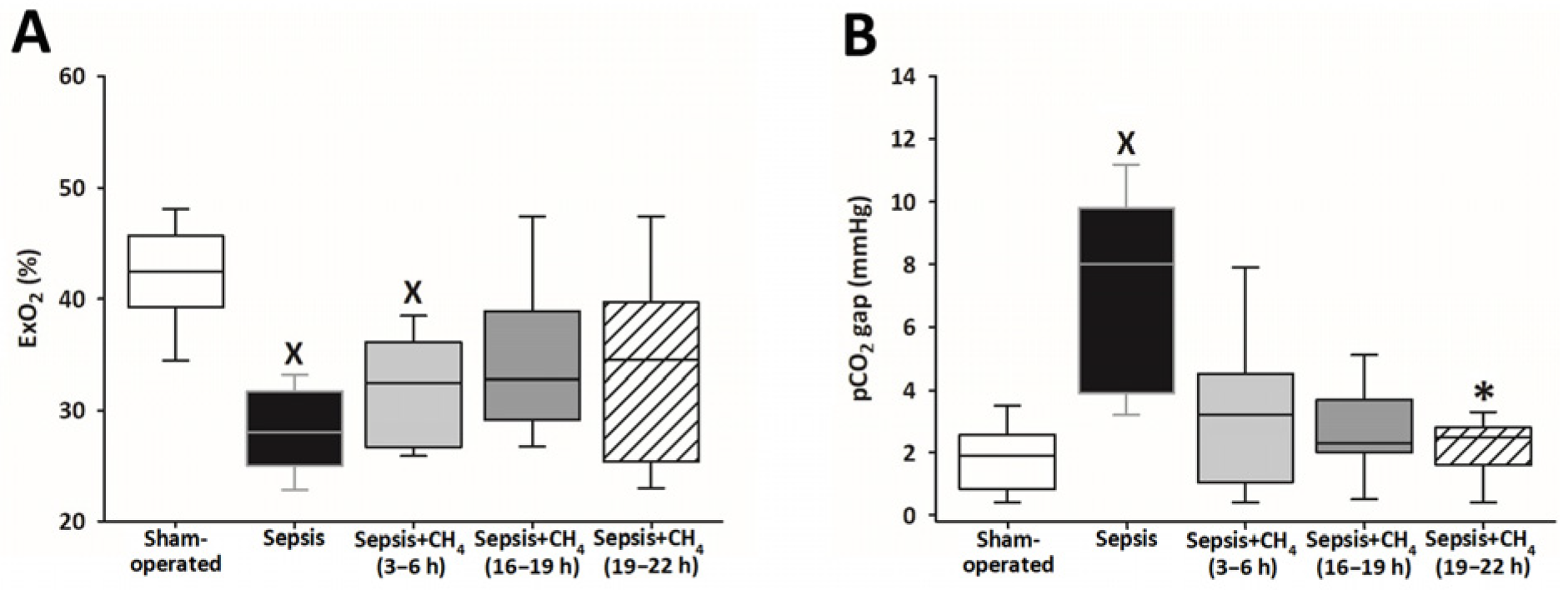
Systemic ExO2 and the pCO2 gap were measured to evaluate sepsis-induced alterations in oxygen dynamics and tissue hypoperfusion (Supplementary Material). The untreated septic group and the early CH4-treated group (t = 3–6 h) showed significantly lower ExO2 values than the sham-operated group, whereas the groups receiving CH4 treatment at later timepoints (t = 16–19 and 19–22 h) exhibited no significant differences (Figure 3A). Sepsis resulted in a significantly increased pCO2 gap solely in the untreated septic animals compared with that in the sham-operated controls, whereas the CH4-treated septic groups exhibited no such elevations. Furthermore, CH4 administration at the latest timepoint significantly decreased the pCO2 gap compared with the untreated septic group (Figure 3B).
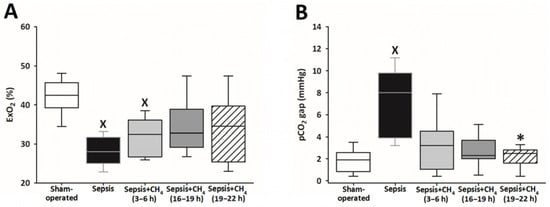
Figure 3.
Oxygen extraction (ExO2) (A) and partial carbon dioxide gap (pCO2 gap) (B) in the sham-operated group and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group.
3.4. Cerebellar and Renal Mitochondrial Function Alterations
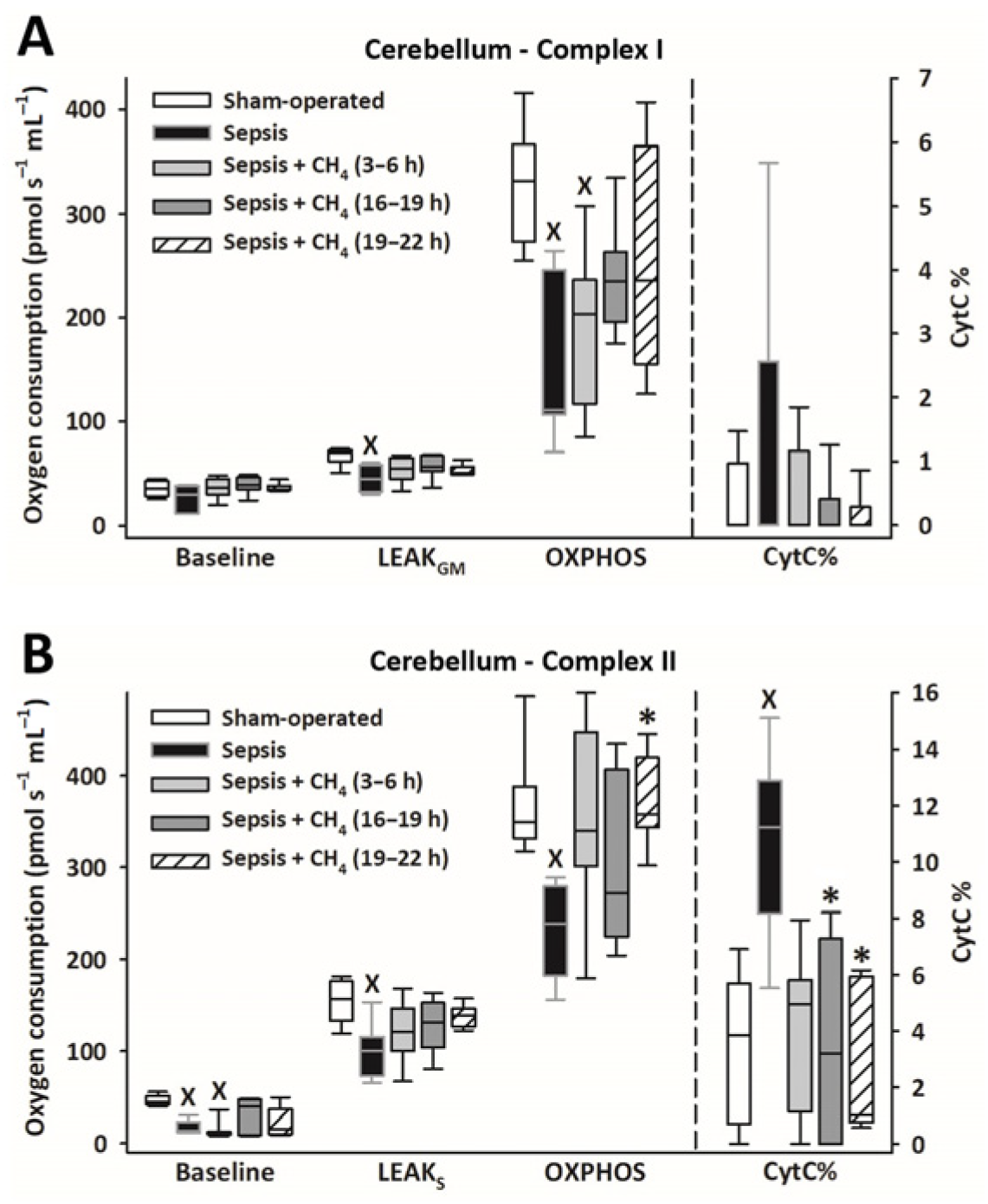
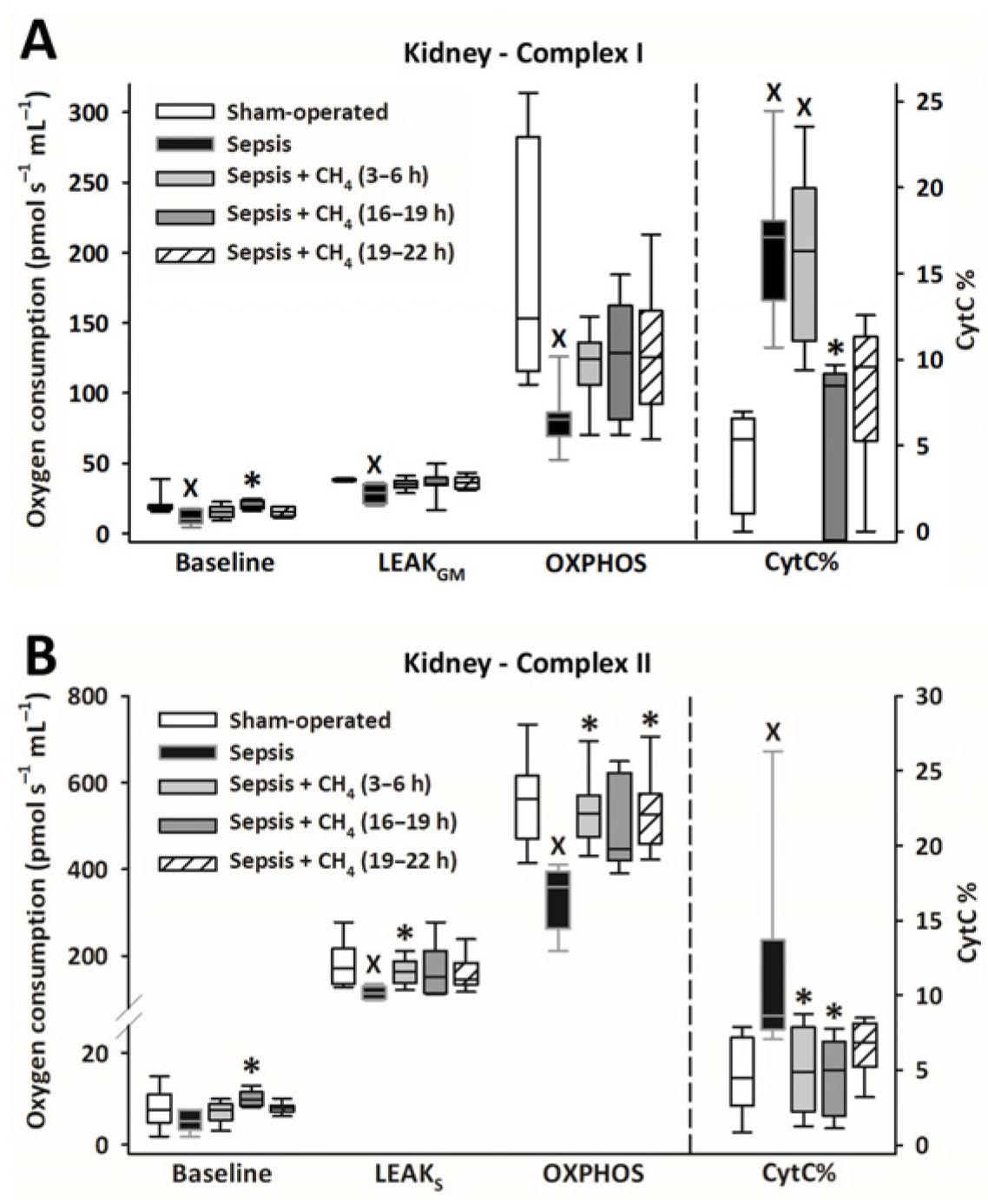
Owing to sepsis, mitochondrial respiration was markedly reduced in the cerebellum (Figure 4) and kidney (Figure 5), as evidenced by significantly lower LEAKGM, LEAKS, and OXPHOS values than those in the sham-operated group.
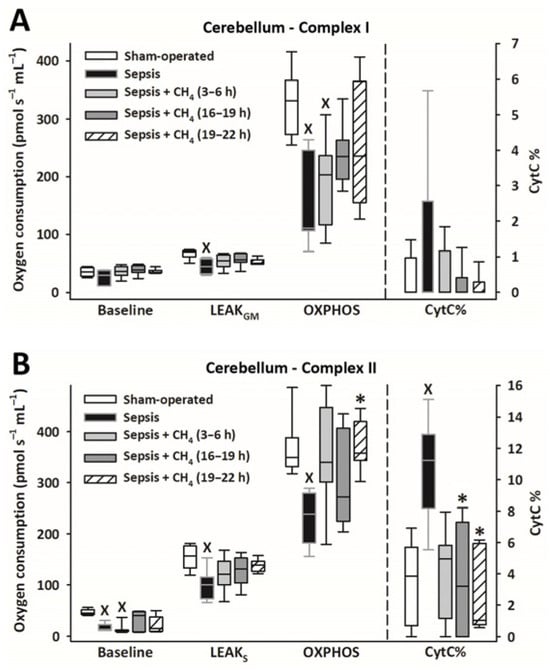
Figure 4.
Mitochondrial respiration linked to Complex I (A) and II (B) in the cerebellum in the sham-operated group and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group. Respiratory states: Baseline: respiration without external substrates and inhibitors; LEAKGM: glutamate-malate-supported respiration; LEAKS: succinate-supported respiration capacity; OXPHOS: ADP-stimulated respiration capacity; CytC%: increase in oxygen consumption after addition of CytC, expressed as a percentage of OXPHOS respiration.
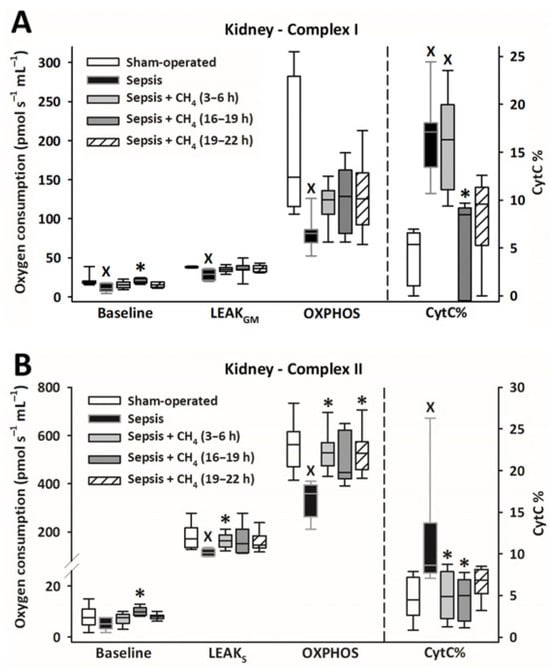
Figure 5.
Mitochondrial respiration linked to Complex I (A) and II (B) in the kidney in the sham-operated group and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group. Respiratory states: Baseline: respiration without external substrates and inhibitors; LEAKGM: glutamate-malate-supported respiration; LEAKS: succinate-supported respiration capacity; OXPHOS: ADP-stimulated respiration capacity; CytC%: increase in oxygen consumption after addition of CytC, expressed as a percentage of OXPHOS respiration.
Compared with the untreated septic group, CH4 treatment did not significantly improve C-I–linked respiration in the cerebellum and kidney. However, the C-II–linked OXPHOS values significantly improved in the cerebellum following the latest (t = 19–22 h) CH4 treatment (Figure 4B). In the kidney, significant improvements in C-II–linked OXPHOS were observed following early (t = 3–6 h) and late (t = 19–22 h) CH4 treatments (Figure 5B). Mitochondrial oxygen consumption increased following the addition of cytochrome c (CytC; expressed as a proportion of OXPHOS: CytC%), indicating significant deterioration of the outer mitochondrial membrane permeability in both organs in the untreated septic group. This increase was apparent for C-II in the cerebellum (Figure 4B) and both C-I and -II in the kidney (Figure 5B). In the cerebellum, CytC% values for C-II were comparable to those of the sham-operated group following CH4 treatment applied at all stages of sepsis, and CH4 administered at t = 16–19 and t = 19–22 h showed significantly lower C-II–related CytC% values than untreated sepsis. Similarly, a time-dependent protective effect of CH4 on the outer mitochondrial membrane integrity was observed in the kidney for C-I (Figure 5A), whereas CH4 treatment at all stages conferred a beneficial effect on C-II (Figure 5B).
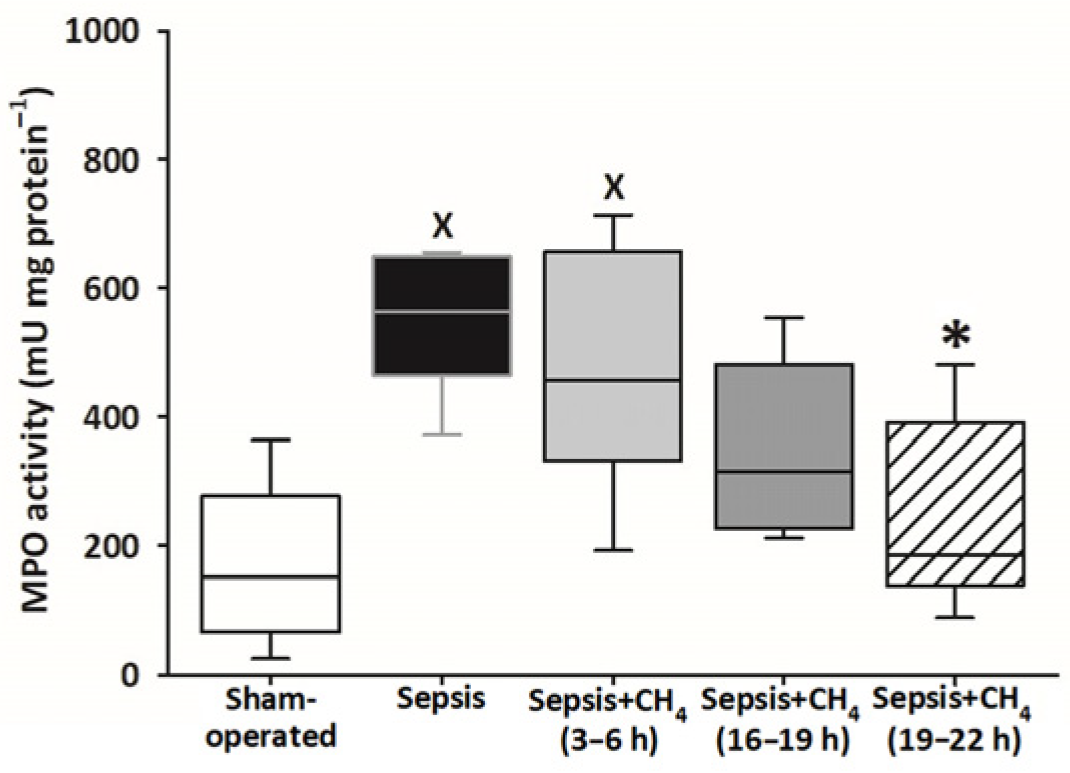
3.5. Neutrophil Granulocyte Activation
Compared with sham-operated animals, MPO activity in the plasma was significantly elevated only in the untreated septic group and the group receiving CH4 treatment at t = 3–6 h. The latest CH4 treatment (t = 19–22 h) resulted in a significantly lower MPO activity compared with the untreated septic group (Figure 6).
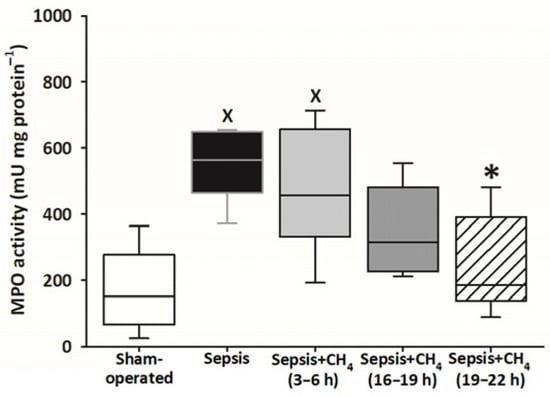
Figure 6.
Plasma myeloperoxidase (MPO) activity in the sham-operated group and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group.
4. Discussion
This study demonstrated the efficacy of CH4 inhalation therapy in mitigating organ dysfunction, neutrophil activation, and mitochondrial respiratory impairment at various stages of a standardized intra-abdominal sepsis model [19]. To evaluate the influence of intervention timing on therapeutic outcomes, CH4 was administered at distinct timepoints following sepsis induction. Early (3–6 h) administration prevented sepsis-related mortality, whereas treatments initiated at later stages improved MAP; enhanced respiratory, hepatic, and renal functions, as evidenced by enhanced PaO2/FiO2 ratios and reduced urea and ALT levels; and attenuated metabolic acidosis, as indicated by decreased lactate levels.
Notably, late-stage CH4 treatment significantly reduced multiple organ dysfunction, as evidenced by lower ROFA scores, and yielded the greatest improvements in ExO2 and reductions in the pCO2 gap, with some benefits observed across all treated groups. These findings suggest that the attenuation of tissue hypoxia and enhancement of ExO2 can originate from improved DO2 and microcirculatory function, which is consistent with previous studies [23].
The potential transient benefits of early CH4 treatment remain uncertain because organ function parameters were only assessed at the endpoint. Nevertheless, our results suggest that early administration cannot significantly impact organ function during the initial and relatively preserved stage of sepsis and that its benefits can be short-lived. In contrast, CH4 inhalation was most effective when applied following organ dysfunction onset, supporting the concept of a time-dependent therapeutic window.
In this study, the exact mechanisms underlying the benefits of CH4 inhalation could not be fully understood. However, improved mitochondrial functions may play a key role (see later sections). Moreover, the anti-inflammatory properties of CH4 are probable contributors; however, comprehensive cytokine profiling was not performed, and only MPO activity was employed for assessing neutrophil activation. However, previous studies have demonstrated that CH4 suppresses proinflammatory cytokine production and inhibits NF-κB activation [17,24,25].
It should be noted that conventional sepsis therapies remain of limited effectiveness. In sepsis, alternative treatments have emerged, such as methylene blue, which improved mean arterial pressure, increased oxygenation and reduced mortality in adult patients with septic shock [26] or high-dose ascorbic acid, which has been shown to have antioxidant and anti-inflammatory effects in sepsis [27,28]. CH4 shows significant antioxidant activity, as indicated by the reduced activation of xanthine oxidoreductase during ischemia reperfusion [23,29]. Accordingly, CH4, a gaseous molecule, may broaden the scope of alternative interventions in the treatment of sepsis.
Furthermore, CH4 administration decreases the tissue levels of nitric oxide and nitrotyrosine [23], both of which can impair mitochondrial respiration by competing with oxygen or modifying the respiratory chain components via nitrosylation and oxidation [30]. Additionally, CH4 inhalation has been reported to improve organ function in an Nrf2-dependent manner during early reperfusion [31].
Despite its chemical inertness, CH4 exerts substantial biological activity, probably because of its apolar and hydrophobic nature, which facilitates accumulation at membrane interfaces and may modulate transmembrane proteins, ion channels, and enzyme activity [29]. Additionally, hydrocarbon gases, including CH4, have been demonstrated to influence membrane dynamics, affect cell–cell junction integrity, and modulate erythrocyte deformability and aggregation, particularly under oxidative stress conditions [32,33].
Sepsis-associated encephalopathy (SAE), a life-threatening complication of systemic infection, is characterized by a complex interplay of oxidative and nitrosative stress, neuroinflammation, and blood–brain barrier disruption. These pathophysiological changes ultimately result in neuronal cell death, impaired neurotransmission, and mitochondrial dysfunction within the neurons [6,34]. Mitochondrial injury, primarily induced by oxidative stress, promotes CytC release and mitochondrial permeability transition pore opening, thereby triggering apoptosis and neuronal loss [35]. In our study, CH4 inhalation preserved mitochondrial membrane integrity and enhanced oxidative phosphorylation efficiency, resulting in improved cellular energetics and organ function in the treated animals. The 19–22 h treated group exhibited the most pronounced benefits, including improved cerebellar mitochondrial respiration, outer mitochondrial membrane permeability stabilization, and enhanced ATP-generating capacity. These findings are consistent with those of earlier studies demonstrating that normoxic CH4 exposure attenuates CytC release and preserves mitochondrial respiration [14]. Complementary evidence from ischemia–reperfusion models shows that the administration of CH4-enriched saline not only reduces neuronal apoptosis by suppressing CytC release [36] but also mitigates hippocampal microglial activation, oxidative stress, and behavioral deficits in murine models of SAE [25].
Sepsis-induced microcirculatory dysfunction also contributes to impaired renal DO2 and nutrient supply, predisposing renal tubular epithelial cells, which are among the most metabolically active cells in the body, to mitochondrial damage and cellular injury [8,37,38]. The degree of mitochondrial dysfunction, characterized by impaired oxidative phosphorylation and increased uncoupling, is strongly associated with sepsis-induced AKI severity [39]. Methane therapy has previously been demonstrated to exert renoprotective effects by attenuating oxidative stress, enhancing mitochondrial respiration, and modulating endoplasmic reticulum stress, thereby restoring energy homeostasis in renal cells [16]. In the present study, CH4 inhalation across all treatment stages significantly decreased serum urea levels, reduced CytC release, and normalized C-II–linked oxidative phosphorylation in kidney tissues. These findings support the hypothesis that CH4 preserves mitochondrial membrane integrity, possibly by stabilizing the outer membrane, thereby preventing apoptosis and maintaining renal function during sepsis.
Methane therapy administered during the early, intermediate, and late stages of sepsis exerted organ-specific protective effects on mitochondrial function in the brain and kidneys. In the kidneys, all treatment windows exhibited beneficial effects, whereas in the cerebellum, efficacy was restricted to late-stage interventions. Notably, each treatment window conferred the following distinct advantages: early CH4 administration (3–6 h) was associated with complete survival, whereas the intermediate (16–19 h) and late (19–22 h) groups demonstrated extensive mitochondrial function enhancements.
Collectively, these findings suggest that prolonged or repeated inhaled CH4 administration following sepsis diagnosis can represent the most rational and effective therapeutic strategy. Our recent study further supports this concept, demonstrating that 24-h continuous CH4 inhalation during veno–venous extracorporeal membrane oxygenation not only significantly increased renal blood flow and urine output but also reduced proinflammatory cytokine levels in swine [18]. These results suggest that prolonged inhalation of CH4, initiated at an earlier stage of sepsis, could provide even more pronounced organ protection.
Some limitations of our sepsis model should be acknowledged. First, although the polymicrobial rat model used in this study provided valuable mechanistic insights, species-specific differences in the immune response, metabolism, and disease progression may have limited the direct clinical translation of these findings. Notably, broad-spectrum antibiotics, including standard first-line agents in sepsis management, were intentionally excluded from our model, as a previous study suggests that they can adversely influence mitochondrial function [40]. Second, the 24-h timeframe of this model did not enable pathogen identification and targeted antimicrobial therapy, which are integral to clinical sepsis management. The potential synergistic effects of CH4 treatment when combined with conventional treatments, including antibiotics and immunomodulatory agents, should be explored in future studies.
5. Conclusions
This study demonstrates that inhaled CH4 treatment, administered at different stages of early sepsis, effectively mitigates sepsis-induced organ dysfunction, mitochondrial damage, and inflammatory responses. Notably, CH4 treatment during the advanced stage of multiple organ dysfunction (19–22 h post-induction) was the most effective, as evidenced by improved organ function and enhanced mitochondrial performance in the cerebellum and kidneys, mainly by preserving mitochondrial oxygen consumption, particularly via C-II–linked respiration. Furthermore, CH4 treatment improved oxygen dynamics, with the intervention timing strongly influencing treatment efficacy.
Overall, these findings underscore the therapeutic potential of CH4 in sepsis management. However, to enhance translational relevance, future studies should integrate standard clinical interventions, including antibiotics and immunomodulatory agents, to assess potential synergistic effects and further optimize treatment approaches.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14070814/s1, Table S1. Threshold values for components of the Rat-specific Organ Failure Assessment scoring system; Figure S1. Time course of the Rat-Specific Sickness (RSS) score in sham-operated and in various groups of septic animals (untreated or treated with CH4 at t = 3–6 h, 16–19 h, or 19–22 h). Median with 25th and 75th percentiles; X p < 0.05 versus sham-operated group; * p < 0.05 versus untreated septic group.
Author Contributions
Conceptualization: L.F.G. and A.R.; Investigation: L.F.G., A.R., L.J., B.L.C. and S.P.T.; Validation: S.P.T., J.K. and M.Z.P., Visualization: L.J., A.S. and M.Z.P.; Writing—original draft: J.K., A.S., L.F.G., A.R., L.J. and S.P.T.; Writing—review and editing: J.K., S.P.T., A.S. and M.B. A.R. and L.F.G. contributed equally to this work as first authors, and M.Z.P. and S.P.T. contributed equally to this work as last authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hungarian National Research, Development and Innovation Office (NKFI) under grant number ADVANCED 149858 and by Szent-Györgyi Fellowships provided by the Albert Szent-Györgyi Medical School, University of Szeged (grant numbers SZTE/SZGYA-5S864, 5S774, and 5S775). Additional support was provided by the University of Szeged Open Access Fund (grant number 7820). This research work was conducted with the support of the National Academy of Scientist Education Program of the National Biomedical Foundation under the sponsorship of the Hungarian Ministry of Culture and Innovation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the National Institutes of Health guidelines and EU Directive, and approved by the local Institutional Review Board and the National Scientific Ethical Committee for Animal Experimentation in Hungary (license V./2884/2022 approved: 14 November 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to generate the figures in the manuscript are fully included in the Supplementary Excel file. These cover all quantitative results presented. Additional raw data—such as outputs from specialized instruments (Oxygraph-2k (O2k, OROBOROS INSTRUMENTS, Austria, etc.)—are stored in proprietary formats that require specific software and expert interpretation. Therefore, these raw datasets are available upon reasonable request.
Acknowledgments
We are grateful to Csilla Mester and Natália Dudás for their skillful assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKI | Acute Kidney Injury |
| ALT | Alanine Aminotransferase |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| ATP | Adenosine Triphosphate |
| C-I, C-II | Mitochondrial Complex I and II |
| CH4 | Methane |
| CytC% | Cytochrome c–induced oxygen consumption as a percentage of OXPHOS |
| DO2 | Oxygen Delivery |
| ECMO | Extracorporeal Membrane Oxygenation |
| ExO2 | Oxygen Extraction |
| FiO2 | Fraction of Inspired Oxygen |
| LEAKGM | Glutamate-Malate–supported Leak Respiration |
| LEAKS | Succinate-supported Leak Respiration |
| MAP | Mean Arterial Pressure |
| MQTiPSS | Minimum Quality Threshold in Preclinical Sepsis Studies |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| OXPHOS | ADP-Stimulated Oxidative Phosphorylation |
| PaO2 | Partial Pressure of Arterial Oxygen |
| PaO2/FiO2 | Arterial oxygen partial pressure/fraction of inspired oxygen |
| pCO2-gap | Central Venous-to-Arterial Carbon Dioxide Gap |
| ROFA | Rat-Specific Organ Failure Assessment |
| ROS | Reactive Oxygen Species |
| RSS | Rat-Specific Sickness Score |
| SOFA | Sequential Organ Failure Assessment |
| VO2 | Oxygen Consumption |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9 (Suppl. S4), S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nedel, W.; Deutschendorf, C.; Portela, L.V.C. Sepsis-induced mitochondrial dysfunction: A narrative review. World J. Crit. Care Med. 2023, 12, 139–152. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Lyu, J.; Zheng, G.; Chen, Z.; Wang, B.; Tao, S.; Xiang, D.; Xie, M.; Huang, J.; Liu, C.; Zeng, Q. Sepsis-induced brain mitochondrial dysfunction is associated with altered mitochondrial Src and PTP1B levels. Brain Res. 2015, 1620, 130–138. [Google Scholar] [CrossRef]
- Manrique-Caballero, C.L.; Rio-Pertuz, G.D.; Gomez, H. Sepsis-associated acute kidney injury. Crit. Care Clin. 2021, 37, 279–301. [Google Scholar] [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int. J. Mol. Sci. 2021, 22, 11253. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019: Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Prest, J.; Sathananthan, M.; Jeganathan, N. Current trends in sepsis-related mortality in the United States. Crit. Care Med. 2021, 49, 1276–1284. [Google Scholar] [CrossRef]
- Moore, J.P.R.; Dyson, A.; Singer, M.; Fraser, J. Microcirculatory dysfunction and resuscitation: Why, when, and how. Br. J. Anaesth. 2015, 115, 366–375. [Google Scholar] [CrossRef]
- Napolitano, L.M. Sepsis 2018: Definitions and guideline changes. Surg. Infect. 2018, 19, 117–125. [Google Scholar] [CrossRef]
- Boros, M.; Keppler, F. Methane production and bioactivity − A link to oxido-reductive stress. Front. Physiol. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Juhász, L.; Tallósy, S.P.; Nászai, A.; Varga, G.; Érces, D.; Boros, M. Bioactivity of inhaled CH4 and interactions with other biological gases. Front. Cell Dev. Biol. 2022, 9, 824749. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Liu, C.; Zhang, J. Methane medicine: A rising star gas with powerful anti-inflammation, antioxidant, and antiapoptosis properties. Oxid. Med. Cell. Longev. 2018, 2018, 1912746. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, Z.; Feng, Y.; Cui, R.; Dong, Y.; Zhang, X.; Xiang, X.; Qu, K.; Liu, C.; Zhang, J. Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2018, 2018, 4756846. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, Y.; Feng, Y.; Cui, R.; Wang, Z.; Qu, K.; Liu, C.; Zhang, J. Methane-rich saline protects against sepsis-induced liver damage by regulating the PPAR-γ/NF-κB signaling pathway. Shock 2019, 52, e163–e172. [Google Scholar] [CrossRef] [PubMed]
- Vida, N.; Varga, Z.; Szabó-Biczók, A.; Bari, G.; Vigyikán, G.; Hodoniczki, Á.; Gajda, Á.; Rutai, A.; Juhász, L.; Tallósy, S.P.; et al. Methane administration during oxygenation mitigates acute kidney injury in a pig model of 24-h veno-venous extracorporeal membrane oxygenation. Shock 2025, 63, 935–943. [Google Scholar] [CrossRef]
- Tallósy, S.P.; Poles, M.Z.; Rutai, A.; Fejes, R.; Juhász, L.; Burián, K.; Sóki, J.; Szabó, A.; Boros, M.; Kaszaki, J. The microbial composition of the initial insult can predict the prognosis of experimental sepsis. Sci. Rep. 2021, 11, 22772. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Alfred, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.S.; Drechsler, S.; et al. Minimum quality threshold in pre-clinical sepsis studies (MQTIPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Shock 2018, 50, 377–380. [Google Scholar] [CrossRef]
- Juhász, L.; Rutai, A.; Fejes, R.; Tallósy, S.P.; Poles, M.Z.; Szabó, A.; Szatmári, I.; Fülöp, F.; Vécsei, L.; Boros, M.; et al. Divergent effects of the N-methyl-D-aspartate receptor antagonist kynurenic acid and the synthetic analog SZR-72 on microcirculatory and mitochondrial dysfunction in experimental sepsis. Front. Med. 2020, 7, 566582. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, W.M.; Abels, C.; Schuerer, L. Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int. J. Microcirc. Clin. Exp. 1996, 16, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Bódi, N.; Bagyánszki, M.; Fekete, É.; Mészáros, A.T.; Varga, G.; Szűcs, S.; Nászai, A.; Kiss, L.; Kozlov, A.V.; et al. Reduction of nitrosative stress by CH4: Neuroprotection through xanthine oxidoreductase inhibition in a rat model of mesenteric ischemia-reperfusion. Free Radic. Biol. Med. 2018, 120, 160–169. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Shao, H.; Meng, Y.; Wang, L.; Wu, Q.; Yao, Y.; Li, J.; Bian, J.; Zhang, Y.; et al. Methane limits LPS-induced NF-κB/MAPKs signal in macrophages and suppresses immune response in mice by enhancing PI3K/AKT/GSK-3β-mediated IL-10 expression. Sci. Rep. 2016, 6, 29359. [Google Scholar]
- Wang, Y.; Wang, C.; Zhang, D.; Wang, L.; Wang, H.; Hu, B.; Bo, L. Methane-rich saline protects against sepsis-associated cognitive deficits in mice. Brain Res. 2022, 1791, 148000. [Google Scholar] [CrossRef]
- Ng, K.T.; Kwok, P.E.; Lim, W.E.; Teoh, W.Y.; Hasan, M.S.; Zainal Abidin, M.F. The use of methylene blue in adult patients with septic shock: A systematic review and meta-analysis. Braz. J. Anesthesiol. 2025, 75, 844580. [Google Scholar] [CrossRef]
- Alissa, A.; Alrashed, M.A.; Alshaya, A.I.; Al Sulaiman, K.; Alharbi, S. Reevaluating vitamin C in sepsis and septic shock: A potential benefit in severe cases? Front. Med. 2024, 11, 1476242. [Google Scholar] [CrossRef]
- Juneja, D.; Nasa, P.; Jain, R. Current role of high dose vitamin C in sepsis management: A concise review. World J. Crit. Care Med. 2022, 11, 349–363. [Google Scholar] [CrossRef]
- Boros, M.; Ghyczy, M.; Érces, D.; Varga, G.; Tőkés, T.; Kupai, K.; Torday, C.; Kaszaki, J. The anti-inflammatory effects of CH4. Crit. Care Med. 2012, 40, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta 2017, 1858, 573–581. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Li, G.; Zhao, H.; Wang, X.; Yin, Y.; Yu, J.; Meng, C. Methane inhalation protects against lung ischemia-reperfusion injury in rats by regulating pulmonary surfactant via the Nrf2 pathway. Front. Physiol. 2021, 12, 615974. [Google Scholar] [CrossRef] [PubMed]
- Batliwala, H.; Somasundaram, T.; Uzgiris, E.E. Methane-induced haemolysis of human erythrocytes. Biochem. J. 1995, 307, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Mayne, C.G.; Arcario, M.J.; Mahinthichaichan, P.; Baylon, J.L.; Vermaas, J.V.; Navidpour, L.; Wen, P.C.; Thangapandian, S.; Tajkhorshid, E. The cellular membrane as a mediator for small molecule interaction with membrane proteins. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Mei, X.L.; Zhao, Y.N. Sepsis and cerebral dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox. Res. 2021, 39, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Giridharan, V.V.; Catalão, C.H.R.; Ritter, C.; Dal-Pizzol, F. Neurochemical effects of sepsis on the brain. Clin. Sci. 2023, 137, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, Y.; He, R.; Meng, Y.; Li, N.; Zhang, D.; Xu, J.; Chen, O.; Cui, J.; Bian, J.; et al. Methane ameliorates spinal cord ischemia-reperfusion injury in rats: Antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic. Biol. Med. 2017, 103, 69–86. [Google Scholar] [CrossRef]
- Exline, M.C.; Crouser, E.D. Mitochondrial mechanisms of sepsis-induced organ failure. Front. Biosci. 2008, 13, 5030–5041. [Google Scholar] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Chvojka, J.; Ledvinova, L.; Benes, J.; Tuma, Z.; Grundmanova, M.; Jedlicka, J.; Kuncova, J.; Matejovic, M. Renal mitochondria response to sepsis: A sequential biopsy evaluation of experimental porcine model. Intensive Care Med. Exp. 2025, 13, 25. [Google Scholar] [CrossRef]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).