Argan Callus Extract Restores Skin Cells via AMPK-Dependent Regulation of Energy Metabolism, Autophagy, and Inflammatory Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Compound Treatment
2.2. Skin-Derived Precursor Cells and Stem Cells
2.3. Senescence-Associated β-Galactosidase Assay
2.4. Determination of Cell Number, Cumulative Population Doubling, and Cytotoxicity
2.5. BrDU Staining
2.6. Western Blot
2.7. Cryopreservation and Cryosectioning
2.8. Immunocytochemistry
2.9. Measurement of ROS
2.10. Autophagy Activity
2.11. Measurement of Cellular ATP
2.12. Antioxidant ABTS Assay
2.13. Elastase Inhibition Assay
2.14. Gene Expression Analysis
2.15. Statistical Evaluation and Graphics
3. Results
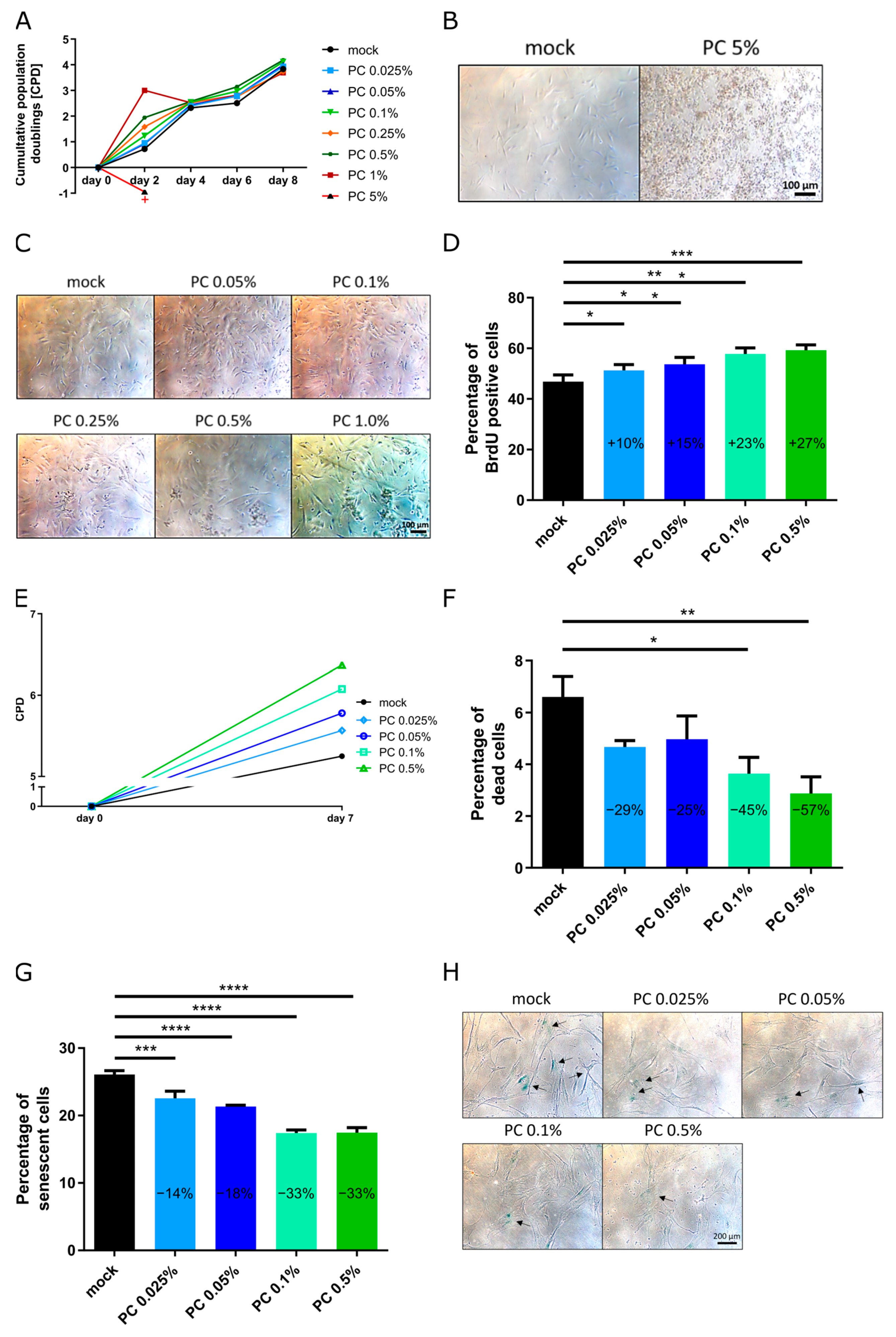
3.1. Optimization of Effectiveness of Working Concentrations
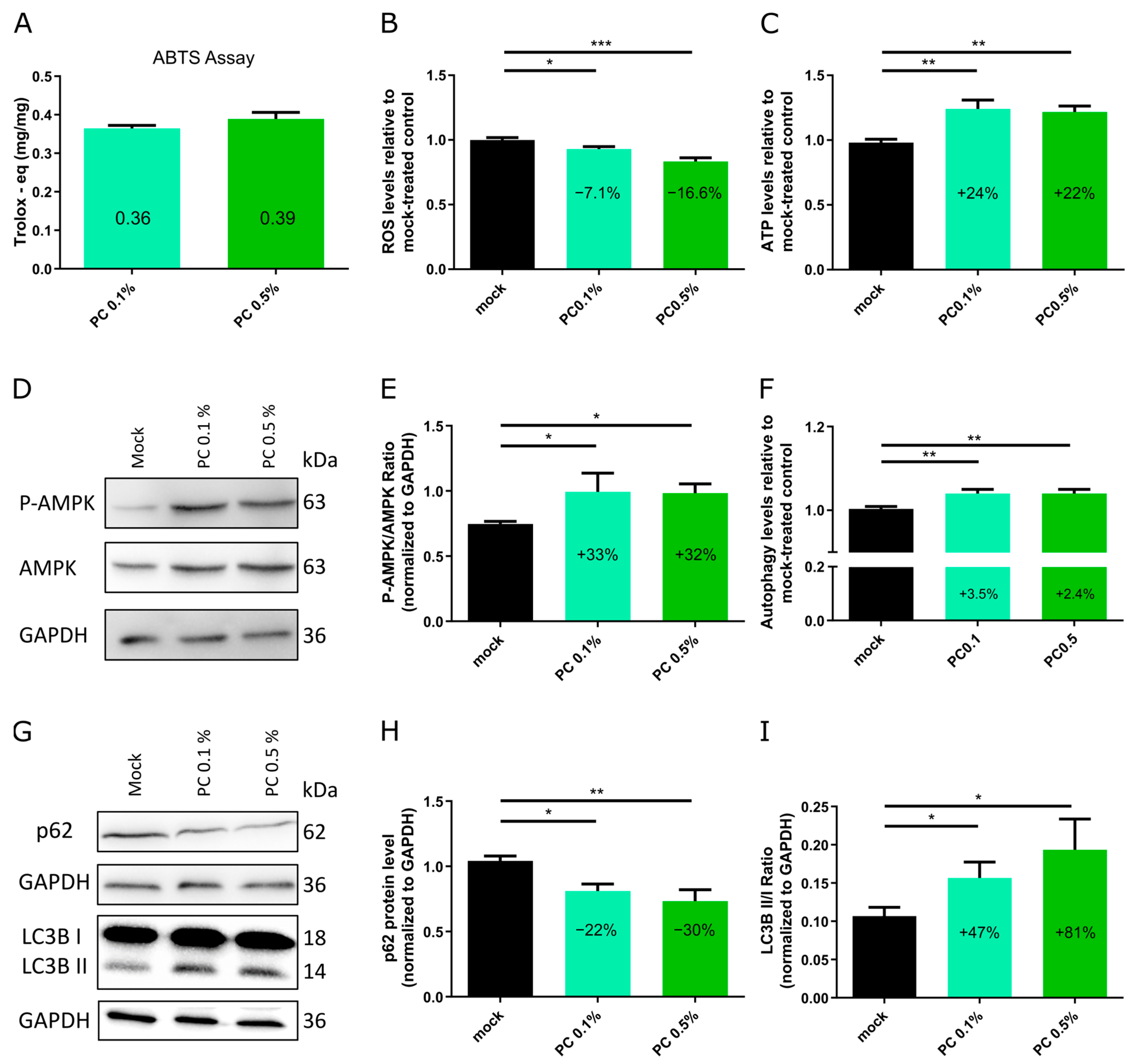
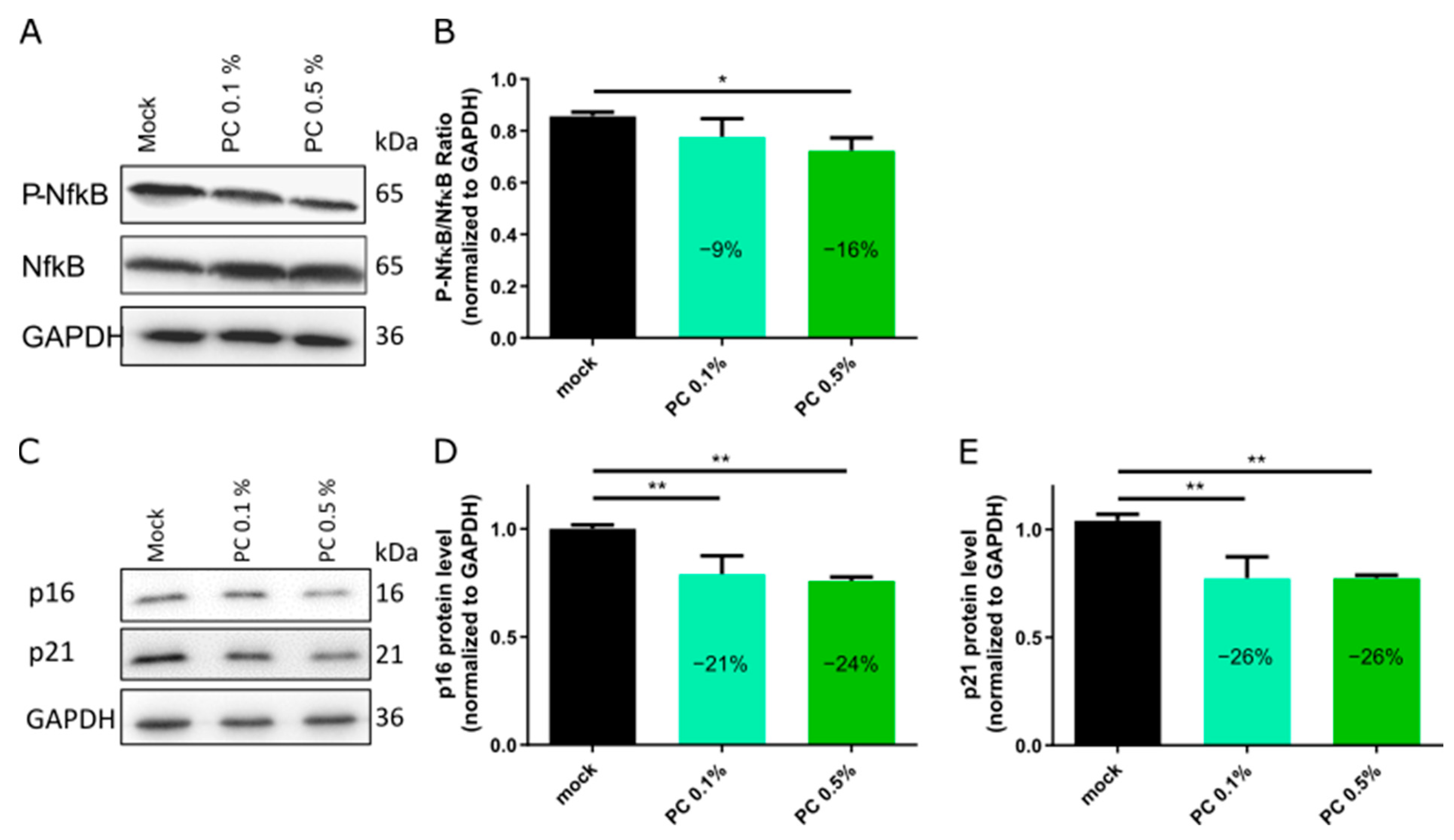
3.2. Functional Characterization and Biological Effects
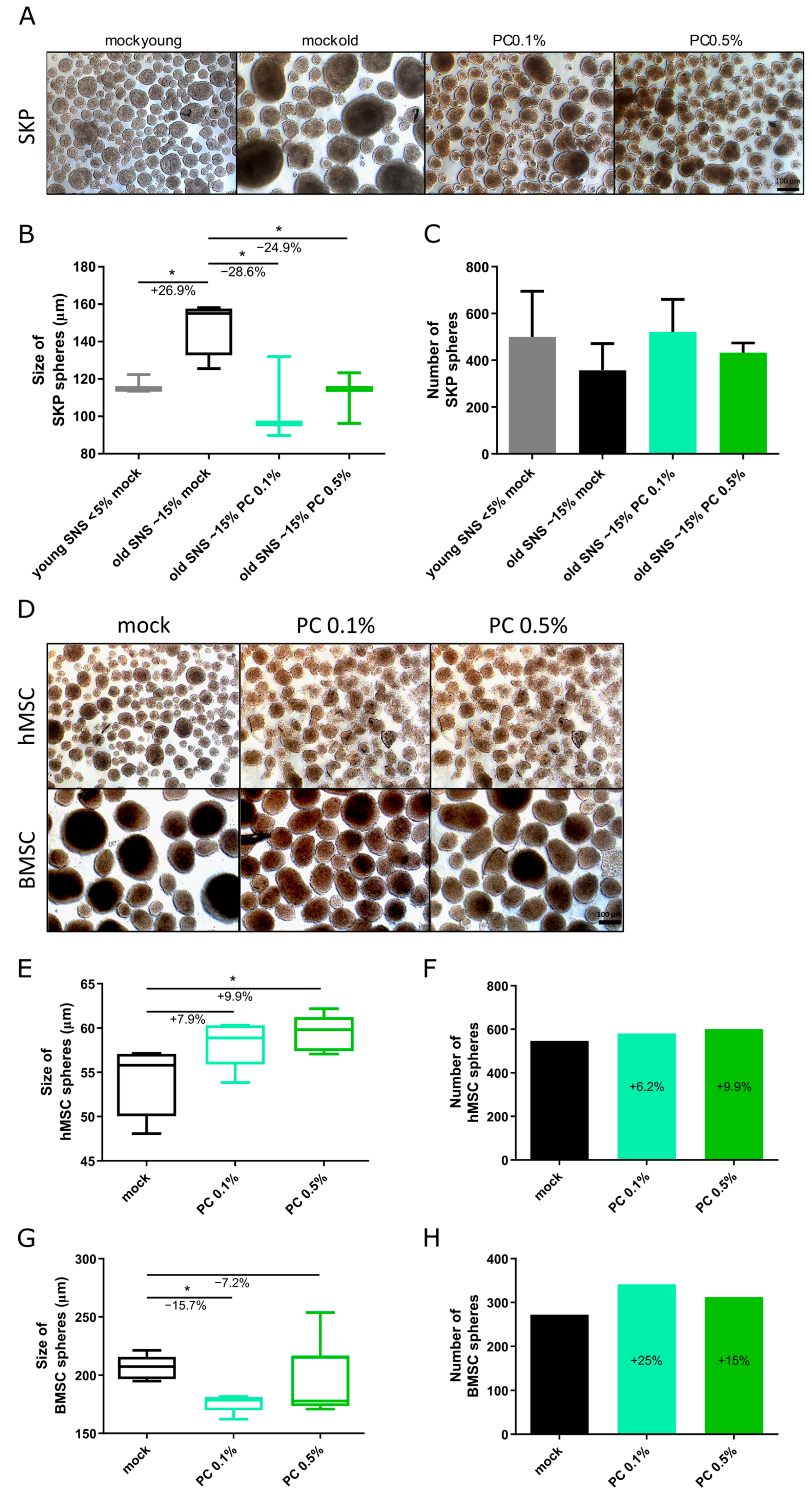
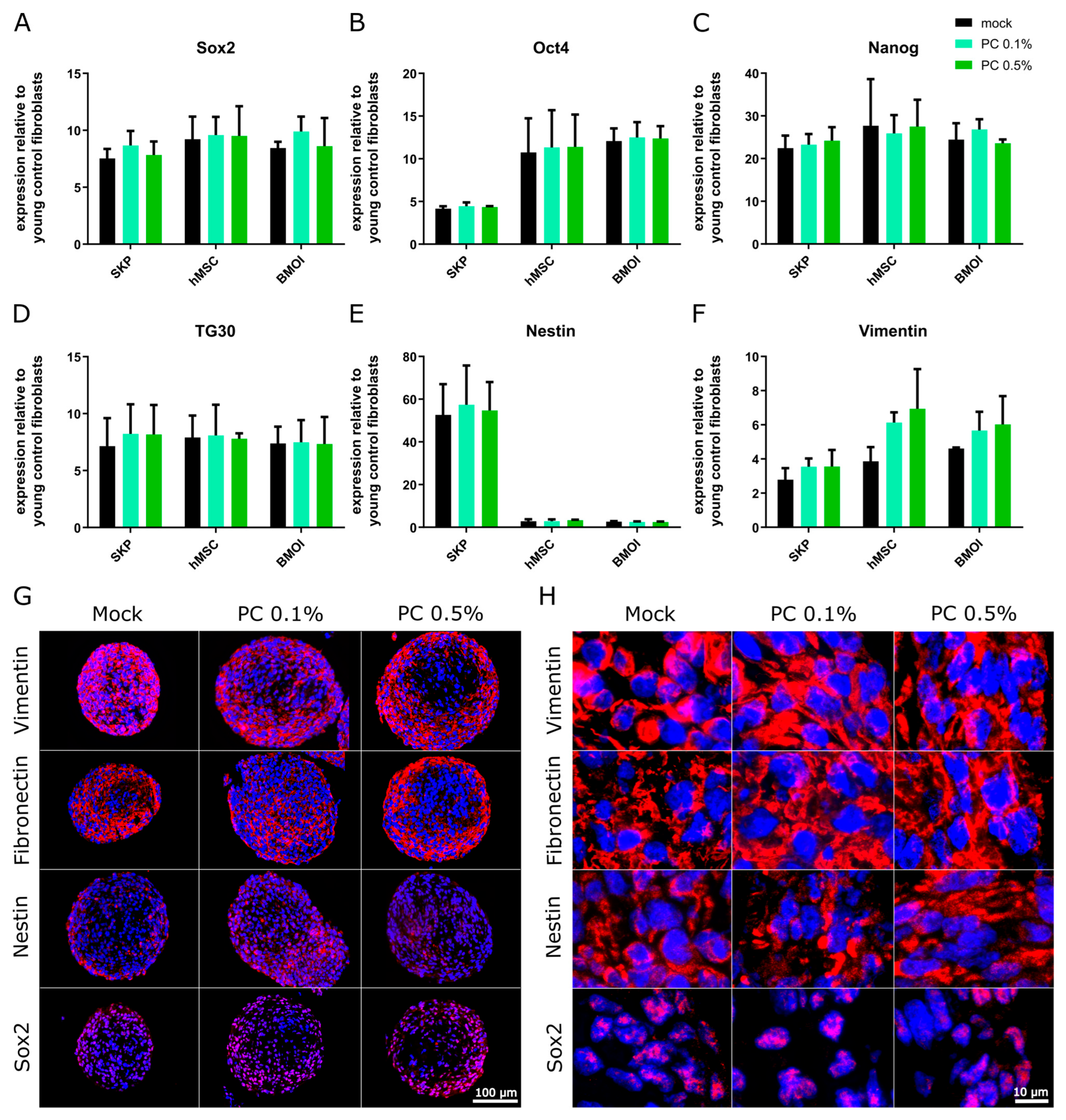
3.3. Effect of PhytoCell on Human Adult Stem Cells
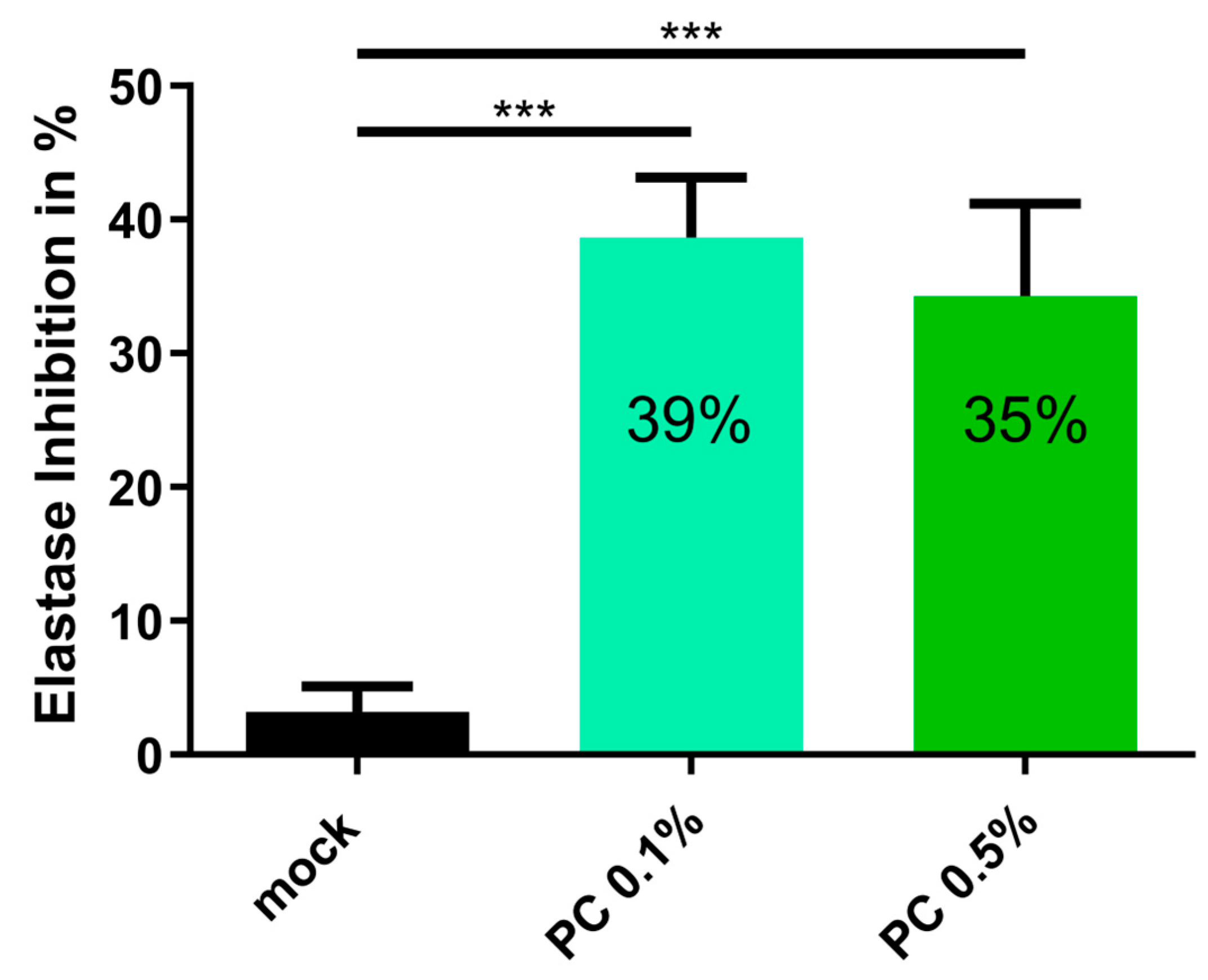
3.4. Anti-Aging Effect of PC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| BrdU | 5-Bromo-2′-deoxyuridine |
| BMSC | Bone marrow stem cell |
| CPD | Cumulative population doubling |
| DCF | Dichlorofluorescein |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| hMSC | Human mesenchymal stem cell |
| MDC | Monodansylcadaverine |
| OCT | Optimal cutting medium |
| PC | PhytoCellTec™ Argan |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SNS | Senescence |
| SKP | Skin-derived precursor cell |
| TEAC | TROLOX Equivalent Antioxidant Capacity |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef]
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, 4. [Google Scholar] [CrossRef]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Gravitz, L. Skin. Nature 2018, 563, S83. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35, quiz 36-27. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Zou, Z.; Long, X.; Zhao, Q.; Zheng, Y.; Song, M.; Ma, S.; Jing, Y.; Wang, S.; He, Y.; Esteban, C.R.; et al. A Single-Cell Transcriptomic Atlas of Human Skin Aging. Dev. Cell 2021, 56, 383–397.e388. [Google Scholar] [CrossRef]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Gomes, R.N.; Manuel, F.; Nascimento, D.S. The bright side of fibroblasts: Molecular signature and regenerative cues in major organs. NPJ Regen. Med. 2021, 6, 43. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Chen, J.; Liu, W.; Qiao, P.; Quan, H.; Li, Z.; Dang, E.; Wang, G.; Shao, S. Epidermal stem cells: Skin surveillance and clinical perspective. J. Transl. Med. 2024, 22, 779. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Adjaye, J.; Akamatsu, H.; Moe-Behrens, G.; Niemann, C. Human skin stem cells and the ageing process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.S.; Lee, Y.; Song, J.H.; Park, T.; Lee, J.K.; Kim, M.; Park, E.; Kim, G.N. Anti-aging Potential of Extracts Prepared from Fruits and Medicinal Herbs Cultivated in the Gyeongnam Area of Korea. Prev. Nutr. Food Sci. 2014, 19, 178–186. [Google Scholar] [CrossRef] [PubMed]
- El Kebbaj, R.; Bouchab, H.; Tahri-Joutey, M.; Rabbaa, S.; Limami, Y.; Nasser, B.; Egbujor, M.C.; Tucci, P.; Andreoletti, P.; Saso, L.; et al. The Potential Role of Major Argan Oil Compounds as Nrf2 Regulators and Their Antioxidant Effects. Antioxidants 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Serrafi, A.; Chegdani, F.; Bennis, F.; Kepinska, M. The Importance of Argan Oil in Medicine and Cosmetology. Nutrients 2024, 16, 3573. [Google Scholar] [CrossRef]
- Lamaoui, M.; Chakhchar, A.; Benlaouane, R.; El Kharrassi, Y.; Farissi, M.; Wahbi, S.; El Modafar, C. Uprising the antioxidant power of Argania spinosa L. callus through abiotic elicitation. C R. Biol. 2019, 342, 7–17. [Google Scholar] [CrossRef]
- El Oumari, F.E.; Bousta, D.; Imtara, H.; Lahrichi, A.; Elhabbani, R.; El Mouhri, G.; Al Kamaly, O.; Saleh, A.; Parvez, M.K.; Grafov, A.; et al. Chemical Composition and Anti-Urolithiatic Activity of Extracts from Argania spinosa (L.) Skeels Press-Cake and Acacia senegal (L.) Willd. Molecules 2022, 27, 3973. [Google Scholar] [CrossRef]
- Khallouki, F.; Eddouks, M.; Mourad, A.; Breuer, A.; Owen, R.W. Ethnobotanic, Ethnopharmacologic Aspects and New Phytochemical Insights into Moroccan Argan Fruits. Int. J. Mol. Sci. 2017, 18, 2277. [Google Scholar] [CrossRef]
- Chafchaouni-Moussaoui, I.; Charrouf, Z.; Guillaume, D. Triterpenoids from Argania spinosa: 20 years of research. Nat. Prod. Commun. 2013, 8, 43–46. [Google Scholar] [CrossRef]
- Simões, T.; Ferreira, J.; Lemos, M.F.L.; Augusto, A.; Félix, R.; Silva, S.F.J.; Ferreira-Dias, S.; Tecelão, C. Argan Oil as a Rich Source of Linoleic Fatty Acid for Dietetic Structured Lipids Production. Life 2021, 11, 1114. [Google Scholar] [CrossRef]
- Poljšak, N.; Kočevar Glavač, N. Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Front. Pharmacol. 2022, 13, 868461. [Google Scholar] [CrossRef]
- El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; El Kharrassi, Y.; Vamecq, J.; Mandard, S.; Saih, F.E.; Latruffe, N.; El Kebbaj, M.S.; Lizard, G.; et al. Argan oil prevents down-regulation induced by endotoxin on liver fatty acid oxidation and gluconeogenesis and on peroxisome proliferator-activated receptor gamma coactivator-1α, (PGC-1α), peroxisome proliferator-activated receptor α (PPARα) and estrogen related receptor α (ERRα). Biochim. Open 2015, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Gardiki, V.; Pavlou, P.; Siamidi, A.; Papageorgiou, S.; Papadopoulos, A.; Iakovou, K.; Varvaresou, A. Plant Stem Cells in Cosmetic Industry. Plants 2025, 14, 433. [Google Scholar] [CrossRef]
- Zabari, E. Cosmetic Topical Skin Treatment Formulation. WO2014167557A1, 16 October 2014. [Google Scholar]
- Budel, L.; Djabali, K. Rapid isolation and expansion of skin-derived precursor cells from human primary fibroblast cultures. Biol. Open 2017, 6, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Gabriel, D.; Roedl, D.; Gordon, L.B.; Djabali, K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell 2015, 14, 78–91. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; Wang, Y. BrdU Incorporation Assay to Analyze the Entry into S Phase. Methods Mol. Biol. 2022, 2579, 209–226. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Quan, Y.; Xin, Y.; Tian, G.; Zhou, J.; Liu, X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxid. Med. Cell Longev. 2020, 2020, 9423593. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Umemoto, E.; Miyasaka, M. The role of extracellular ATP in homeostatic immune cell migration. Curr. Opin. Pharmacol. 2023, 68, 102331. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. 2021, 26, 50–96. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the hallmarks of aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Invest. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- Murase, D.; Kusaka-Kikushima, A.; Hachiya, A.; Fullenkamp, R.; Stepp, A.; Imai, A.; Ueno, M.; Kawabata, K.; Takahashi, Y.; Hase, T.; et al. Autophagy Declines with Premature Skin Aging resulting in Dynamic Alterations in Skin Pigmentation and Epidermal Differentiation. Int. J. Mol. Sci. 2020, 21, 5708. [Google Scholar] [CrossRef]
- Marafon, B.B.; Pinto, A.P.; da Rocha, A.L.; Rovina, R.L.; Pauli, J.R.; De Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; Da Silva, A.S.R. Hepatic LC3 II/I ratio is not modulated in exercised mice. Physiol. Res. 2020, 69, 1103–1111. [Google Scholar] [CrossRef]
- Hwang, H.J.; Ha, H.; Lee, B.S.; Kim, B.H.; Song, H.K.; Kim, Y.K. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022, 13, 1436. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Najdi, F.; Krüger, P.; Djabali, K. Impact of Progerin Expression on Adipogenesis in Hutchinson-Gilford Progeria Skin-Derived Precursor Cells. Cells 2021, 10, 1598. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Kandarakov, O.; Belyavsky, A.; Semenova, E. Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells. Int. J. Mol. Sci. 2022, 23, 4462. [Google Scholar] [CrossRef]
- Mori, M.; Ikeda, N.; Kato, Y.; Minamino, M.; Watabe, K. Inhibition of elastase activity by essential oils in vitro. J. Cosmet. Dermatol. 2002, 1, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement. Alternat Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H. Identification of Plant Extracts that Inhibit Cellular Senescence in Human Fibroblasts, Endothelial Cells, and Vascular Smooth Muscle Cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 584–592. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cha, H.J.; Nam, K.H.; Yoon, Y.; Lee, H.; An, S. Centella asiatica extracts modulate hydrogen peroxide-induced senescence in human dermal fibroblasts. Exp. Dermatol. 2011, 20, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Taarji, N.; Saito, M.; Nakajima, M.; Isoda, H. Argan (Argania Spinosa) press cake extract enhances cell proliferation and prevents oxidative stress and inflammation of human dermal papilla cells. J. Dermatol. Sci. 2021, 103, 33–40. [Google Scholar] [CrossRef]
- Samane, S.; Noël, J.; Charrouf, Z.; Amarouch, H.; Haddad, P.S. Insulin-sensitizing and anti-proliferative effects of Argania spinosa seed extracts. Evid. Based Complement. Alternat Med. 2006, 3, 317–327. [Google Scholar] [CrossRef]
- Drissi, A.; Bennani, H.; Giton, F.; Charrouf, Z.; Fiet, J.; Adlouni, A. Tocopherols and saponins derived from Argania spinosa exert, an antiproliferative effect on human prostate cancer. Cancer Invest. 2006, 24, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef]
- Brahma, D.; Sarkar, T.; Kaushik, R.; Sarangi, A.N.; Gupta, A.N. Structural rearrangement of elastin under oxidative stress. Colloids Surf. B Biointerfaces 2024, 233, 113663. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, A.; Sahri, N.; Mahdi, I.; Fahsi, N.; El Herradi, E.H.; Sobeh, M. Argan: Phytochemical profiling and evaluation of the antioxidant, hypoglycemic, and antibacterial properties of its fruit pulp extracts. Heliyon 2024, 10, e23612. [Google Scholar] [CrossRef]
- Çelik, S.E.; Asfoor, A.; Şenol, O.; Apak, R. Screening Method for Argan Oil Adulteration with Vegetable Oils: An Online HPLC Assay with Postcolumn Detection Utilizing Chemometric Multidata Analysis. J. Agric. Food Chem. 2019, 67, 8279–8289. [Google Scholar] [CrossRef]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Invest. Dermatol. 2001, 117, 671–677. [Google Scholar] [CrossRef]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Lee, K.K.; Kim, J.H.; Cho, J.J.; Choi, J.D. Inhibitory Effects of 150 Plant Extracts on Elastase Activity, and Their Anti-inflammatory Effects. Int. J. Cosmet. Sci. 1999, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lim, C.J.; Chung, H.J.; Kim, J.H.; Huh, Y.H.; Park, K.; Jeong, S. Autophagy Activation by Crepidiastrum Denticulatum Extract Attenuates Environmental Pollutant-Induced Damage in Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 517. [Google Scholar] [CrossRef]
- Machihara, K.; Kageyama, S.; Oki, S.; Makino, H.; Sasaki, M.; Iwahashi, H.; Namba, T. Lotus germ extract rejuvenates aging fibroblasts via restoration of disrupted proteostasis by the induction of autophagy. Aging 2022, 14, 7662–7691. [Google Scholar] [CrossRef] [PubMed]
- Sutkowska-Skolimowska, J.; Brańska-Januszewska, J.; Strawa, J.W.; Ostrowska, H.; Botor, M.; Gawron, K.; Galicka, A. Rosemary Extract-Induced Autophagy and Decrease in Accumulation of Collagen Type I in Osteogenesis Imperfecta Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 10341. [Google Scholar] [CrossRef]
- Vidali, S.; Feichtinger, R.G.; Emberger, M.; Brunner, S.M.; Gaisbauer, S.; Blatt, T.; Smiles, W.J.; Kreutzer, C.; Weise, J.M.; Kofler, B. Ageing is associated with a reduction in markers of mitochondrial energy metabolism in the human epidermis. Exp. Dermatol. 2023, 32, 900–905. [Google Scholar] [CrossRef]
- Zhuang, Y.; Jiang, S.; Deng, X.; Lao, A.; Hua, X.; Xie, Y.; Jiang, L.; Wang, X.; Lin, K. Energy metabolism as therapeutic target for aged wound repair by engineered extracellular vesicle. Sci. Adv. 2024, 10, eadl0372. [Google Scholar] [CrossRef]
- Haga, M.; Okada, M. Systems approaches to investigate the role of NF-κB signaling in aging. Biochem. J. 2022, 479, 161–183. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yang, Z.; Wang, Y.; Zhao, T.; Luo, W.; Liang, T.; Yang, Z. Traditional herbs: Mechanisms to combat cellular senescence. Aging 2023, 15, 14473–14505. [Google Scholar] [CrossRef] [PubMed]
- Zumerle, S.; Sarill, M.; Saponaro, M.; Colucci, M.; Contu, L.; Lazzarini, E.; Sartori, R.; Pezzini, C.; Rinaldi, A.; Scanu, A.; et al. Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice. Nat. Aging 2024, 4, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Menni, H.B.; Belarbi, M.; Menni, D.B.; Bendiab, H.; Kherraf, Y.; Ksouri, R.; Djebli, N.; Visioli, F. Anti-inflammatory activity of argan oil and its minor components. Int. J. Food Sci. Nutr. 2020, 71, 307–314. [Google Scholar] [CrossRef]
- Metallo, C.M.; Mohr, J.C.; Detzel, C.J.; de Pablo, J.J.; Van Wie, B.J.; Palecek, S.P. Engineering the stem cell microenvironment. Biotechnol. Prog. 2007, 23, 18–23. [Google Scholar] [CrossRef]
- Birbrair, A. Stem Cell Microenvironments and Beyond. Adv. Exp. Med. Biol. 2017, 1041, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ra’em, T.; Cohen, S. Microenvironment design for stem cell fate determination. Adv. Biochem. Eng. Biotechnol. 2012, 126, 227–262. [Google Scholar] [CrossRef]
- Sanap, A.; Chandravanshi, B.; Shah, T.; Tillu, G.; Dhanushkodi, A.; Bhonde, R.; Joshi, K. Herbal pre-conditioning induces proliferation and delays senescence in Wharton’s Jelly Mesenchymal Stem Cells. Biomed. Pharmacother. 2017, 93, 772–778. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, H.; Wu, Y.; Deng, S.; Ge, F.; Ma, W.; Zhou, X.; Songyang, Z. Rosa roxburghii Fruit Extracts Upregulate Telomerase Activity and Ameliorate Cell Replicative Senescence. Foods 2024, 13, 1673. [Google Scholar] [CrossRef]
- Li, C.; Cui, Z.; Deng, S.; Chen, P.; Li, X.; Yang, H. The potential of plant extracts in cell therapy. Stem Cell Res. Ther. 2022, 13, 472. [Google Scholar] [CrossRef]
- Leung, Y.; Kandyba, E.; Chen, Y.B.; Ruffins, S.; Chuong, C.M.; Kobielak, K. Bifunctional ectodermal stem cells around the nail display dual fate homeostasis and adaptive wounding response toward nail regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 15114–15119. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Alfarafisa, N.M.; Ikezawa, M.; Khairani, A.F. Progress in the Development of Stem Cell-Derived Cell-Free Therapies for Skin Aging. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3383–3406. [Google Scholar] [CrossRef]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Erlank, H.; Elmann, A.; Kohen, R.; Kanner, J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011, 51, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
| Cell Strain | Passage Number | Senescence |
|---|---|---|
| GM05757C | Passage 9–24 | 4.1–25.0% |
| GM05565A | Passage 10–23 | 4.4–24.6% |
| HGFDFN369 | Passage 7–21 | 3.1–23.7% |
| GM01651C | Passage 11–21 | 5.0–19.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartinger, R.; Fenzl, F.Q.; Nalewaja, V.M.; Djabali, K. Argan Callus Extract Restores Skin Cells via AMPK-Dependent Regulation of Energy Metabolism, Autophagy, and Inflammatory Pathways. Antioxidants 2025, 14, 804. https://doi.org/10.3390/antiox14070804
Hartinger R, Fenzl FQ, Nalewaja VM, Djabali K. Argan Callus Extract Restores Skin Cells via AMPK-Dependent Regulation of Energy Metabolism, Autophagy, and Inflammatory Pathways. Antioxidants. 2025; 14(7):804. https://doi.org/10.3390/antiox14070804
Chicago/Turabian StyleHartinger, Ramona, Felix Quirin Fenzl, Vanessa Martina Nalewaja, and Karima Djabali. 2025. "Argan Callus Extract Restores Skin Cells via AMPK-Dependent Regulation of Energy Metabolism, Autophagy, and Inflammatory Pathways" Antioxidants 14, no. 7: 804. https://doi.org/10.3390/antiox14070804
APA StyleHartinger, R., Fenzl, F. Q., Nalewaja, V. M., & Djabali, K. (2025). Argan Callus Extract Restores Skin Cells via AMPK-Dependent Regulation of Energy Metabolism, Autophagy, and Inflammatory Pathways. Antioxidants, 14(7), 804. https://doi.org/10.3390/antiox14070804








