Influence of Kombucha Fermentation on Antioxidant and Antimicrobial Activity of Monofloral Rapeseed Bee-Collected Pollen
Abstract
1. Introduction
2. Materials and Methods
2.1. List of Reagents
2.2. Bee-Collected Pollen Sample Collection
2.3. Pollen-Enriched Kombucha Sample Preparation
2.4. Proximate Phytochemical Characterization and UHPLC Q-ToF-MS Analysis
2.5. Determination of Antioxidant Properties
2.6. Determination of Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Phytochemical Composition of Prepared Samples
3.2. The Obtained UHPLC Q-ToF-MS Profile of Selected Samples
3.3. Antioxidant Properties of Obtained Samples
3.4. Antimicrobial Activity of Obtained Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuso, P.J.; Ismail, M.; Ha, B.P.; Bartolotto, C. Nutritional Update for Physicians: Plant-Based Diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hever, J.; Cronise, R.J. Plant-Based Nutrition for Healthcare Professionals: Implementing Diet as a Primary Modality in the Prevention and Treatment of Chronic Disease. J. Geriatr. Cardiol. 2017, 14, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Nájera Espinosa, S.; Hadida, G.; Jelmar Sietsma, A.; Alae-Carew, C.; Turner, G.; Green, R.; Pastorino, S.; Picetti, R.; Scheelbeek, P. Mapping the Evidence of Novel Plant-Based Foods: A Systematic Review of Nutritional, Health, and Environmental Impacts in High-Income Countries. Nutr. Rev. 2025, 83, e1626–e1646. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Ecem Bayram, N.; Kostic, A.Ž.; Can Gercek, Y. (Eds.) Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-47562-7. [Google Scholar]
- Kacemi, R.; Campos, M.G. Translational Research on Bee Pollen as a Source of Nutrients: A Scoping Review from Bench to Real World. Nutrients 2023, 15, 2413. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-Rich Bee Pollen: A Treasure Trove of Active Natural Metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Kacemi, R.; Campos, M.G. Bee Pollen as a Source of Pharmaceuticals: Where Are We Now? In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 319–336. [Google Scholar]
- Kaur, J.; Rasane, P.; Kumar, V.; Nanda, V.; Bhadariya, V.; Kaur, S.; Singh, J. Exploring the Health Benefits of Bee Pollen and Its Viability as a Functional Food Ingredient. Rev. Agric. Sci. 2024, 12, 65–78. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Deng, Z.; Li, G.; Zhang, J.; Li, H. Effect of Wall-Disruption on Nutrient Composition and in Vitro Digestion of Camellia and Lotus Bee Pollens. Food Sci. Hum. Well. 2024, 13, 1567–1577. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented Foods, Their Microbiome and Its Potential in Boosting Human Health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Tachie, C.Y.E.; Onuh, J.O.; Aryee, A.N.A. Nutritional and Potential Health Benefits of Fermented Food Proteins. J. Sci. Food Agric. 2024, 104, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee Bread and Bee Pollen of Different Plant Sources: Determination of Phenolic Content, Antioxidant Activity, Fatty Acid and Element Profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.Ž.; Dramićanin, A.M.; Özkök, A. Phenolic and Free Amino Acid Profiles of Bee Bread and Bee Pollen with the Same Botanical Origin—Similarities and Differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Geana, E.-I.; Chircov, C.; Ficai, A.; Ditu, L.-M.; Oprea, E. Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties-First Report on Some Antimicrobial Features. Antioxidants 2024, 13, 353. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Mora-Adames, W.I.; Fuenmayor, C.A.; Benavides-Martín, M.A.; Algecira-Enciso, N.A.; Quicazán, M.C. Bee Pollen as a Novel Substrate in Pilot-Scale Probiotic-Mediated Lactic Fermentation Processes. LWT Food Sci. Technol. 2021, 141, 110868. [Google Scholar] [CrossRef]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.; Ștefan, L.; Mănoiu, S.; Vrăjmașu, V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef]
- Tritean, N.; Dima, Ș.O.; Trică, B.; Stoica, R.; Ghiurea, M.; Moraru, I.; Cimpean, A.; Oancea, F.; Constantinescu-Aruxandei, D. Selenium-Fortified Kombucha–Pollen Beverage by In Situ Biosynthesized Selenium Nanoparticles with High Biocompatibility and Antioxidant Activity. Antioxidants 2023, 12, 1711. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen Composition and Standardisation of Analytical Methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Nedić, N.; Gašić, U.M.; Špirović Trifunović, B.; Vojt, D.; Tešić, Ž.L.; Pešić, M.B. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants 2021, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef]
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and Flavonoid Glycoside Profiles of 20 Types of Monofloral Bee Pollen. Food Chem. 2023, 405, 134800. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Li, W.; Zhao, L.; Meng, F.; Wang, Y.; Tan, H.; Yang, H.; Wei, C.; Wan, X.; et al. Tissue-Specific, Development-Dependent Phenolic Compounds Accumulation Profile and Gene Expression Pattern in Tea Plant (Camellia sinensis). PLoS ONE 2013, 8, e62315. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Akermi, M.; Ünlü, F.Y.; Mihaleva, V.; Roldan, V.G.; Bino, R.J.; de Vos, R.C.H.; Vervoort, J. Structural Annotation and Elucidation of Conjugated Phenolic Compounds in Black, Green, and White Tea Extracts. J. Agric. Food Chem. 2012, 60, 8841–8850. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, H.; Li, C.; Wang, Y.; Zhang, J.; Tang, X.; Zhang, T.; Liu, Y. A Combined Drying Process Involving Hot Air and Roasting for Improving Summer Congou Black Tea Quality. Food Res. Int. 2025, 201, 115584. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects Against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, Y.; Haubruge, E.; Wang, K.; El-Seedi, H.R.; Dong, J.; Xu, X.; Zhang, H. New Insights into Bee Pollen: Nutrients, Phytochemicals, Functions and Wall-Disruption. Food Res. Int. 2024, 178, 113934. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Zhu, X.; Liu, R.; Lu, Q. Metabolomics Reveals That Phenolamides Are the Main Chemical Components Contributing to the Anti-Tyrosinase Activity of Bee Pollen. Food Chem. 2022, 389, 133071. [Google Scholar] [CrossRef]
- Sknepnek, A.; Pantić, M.; Matijašević, D.; Miletić, D.; Lević, S.; Nedović, V.; Niksic, M. Novel Kombucha Beverage from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum, with Antibacterial and Antioxidant Effects. Int. J. Med. Mushrooms 2018, 20, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and Microbiological Impacts on the Chemical and Flavor Profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.D.S.D.; Barth, O.M.; Almeida-Muradian, L.B.D. Phenolic Profile by HPLC-MS, Biological Potential, and Nutritional Value of a Promising Food: Monofloral Bee Pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; de Carvalho, C.; Campos, M.d.G.R. Phytochemical Analysis and Botanical Origin of Apis mellifera Bee Pollen from the Municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2018, 21, e2016176. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic Profile and Antioxidant Properties of Bee-Collected Pollen from Sunflower (Helianthus annuus L.) Plant. LWT Food Sci. Technol. 2019, 112, 108244. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Çobanoğlu, D.N.; Milinčić, D.D.; Felek, I.; Düzdaban, P.; Pešić, M.B. Could Phenylamides Emerge as a Powerful Chemotaxonomic Tool? A Case Study of Rapeseed Bee-Collected Pollen Originating From Serbia and Türkiye. Chem. Biodivers. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and Anti-Tyrosinase Activities of Phenolic Extracts from Rape Bee Pollen and Inhibitory Melanogenesis by CAMP/MITF/TYR Pathway in B16 Mouse Melanoma Cells. Front. Pharmacol. 2017, 8, 104. [Google Scholar] [CrossRef]
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef]
- Marques, V.; Farah, A. Chlorogenic Acids and Related Compounds in Medicinal Plants and Infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F.A. Liquid Chromatography–Tandem Mass Spectrometry Reveals the Widespread Occurrence of Flavonoid Glycosides in Honey, and Their Potential as Floral Origin Markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Song, W.O.; Chun, O.K. Tea Is the Major Source of Flavan-3-Ol and Flavonol in the U.S. Diet. J. Nutr. 2008, 138, 1543S–1547S. [Google Scholar] [CrossRef] [PubMed]
- De La Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Segura-Carretero, A. Pine Bark and Green Tea Concentrated Extracts: Antioxidant Activity and Comprehensive Characterization of Bioactive Compounds by HPLC-ESI-QTOF-MS. Int. J. Mol. Sci. 2014, 15, 20382–20402. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Barčauskaitė, K.; Kaškonas, P.; Maruška, A. The Impact of Fermentation on Bee Pollen Polyphenolic Compounds Composition. Antioxidants 2022, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef]
- Aylanc, V.; Larbi, S.; Calhelha, R.; Barros, L.; Rezouga, F.; Rodríguez-Flores, M.S.; Seijo, M.C.; El Ghouizi, A.; Lyoussi, B.; Falcão, S.I.; et al. Evaluation of Antioxidant and Anticancer Activity of Mono- and Polyfloral Moroccan Bee Pollen by Characterizing Phenolic and Volatile Compounds. Molecules 2023, 28, 835. [Google Scholar] [CrossRef]
- Kim, S.B.; Liu, Q.; Ahn, J.H.; Jo, Y.H.; Turk, A.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Polyamine Derivatives from the Bee Pollen of Quercus mongolica with Tyrosinase Inhibitory Activity. Bioorg. Chem. 2018, 81, 127–133. [Google Scholar] [CrossRef]
- Glavnik, V.; Bensa, M.; Vovk, I.; Guzelmeric, E. High-Performance Thin-Layer Chromatography-Multi-Stage Mass Spectrometry Methods for Analyses of Bee Pollen Botanically Originating from Sweet Chestnut (Castanea sativa Mill.). JPC J. Planar Chrom. Mod. TLC 2023, 36, 471–482. [Google Scholar] [CrossRef]
- dos Santos, G.S.; de Almeida Veiga, A.; Carlotto, J.; Mello, R.G.; Serrato, R.V.; de Souza, L.M. Identification and Fingerprint Analysis of Novel Multi-Isomeric Lycibarbarspermidines and Lycibarbarspermines from Lycium barbarum L. by Liquid Chromatography with High-Resolution Mass Spectrometry (UHPLC-Orbitrap). J. Food Compos. Anal. 2022, 105, 104194. [Google Scholar] [CrossRef]
- Gardana, C.; Del Bo’, C.; Quicazán, M.C.; Corrrea, A.R.; Simonetti, P. Nutrients, Phytochemicals and Botanical Origin of Commercial Bee Pollen from Different Geographical Areas. J. Food Compos. Anal. 2018, 73, 29–38. [Google Scholar] [CrossRef]
- Qian, D.; Xi, J.; Nie, Y.; Zou, M.; Zhang, J.; Alseekh, S.; Fernie, A.R.; Chen, J.; Huang, L. Chemical Characterization of Dicaffeoyl Polyamine Derivatives to Understand Specific Secondary Metabolites in Goji Berry. J. Food Compos. Anal. 2023, 122, 105434. [Google Scholar] [CrossRef]
- Chesnov, S.; Bigler, L.; Hesse, M. Detection and Characterization of Natural Polyamines by High-Performance Liquid Chromatography-Atmospheric Pressure Chemical Ionization (Electrospray Ionization) Mass Spectrometry. Eur. J. Mass Spectrom. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Ahad, H.; Jin, H.; Liu, Y.; Wang, J.; Sun, G.; Liang, X.; Akber Aisa, H. Chemical Profiling of Spermidines in Goji Berry by Strong Cation Exchange Solid-Phase Extraction (SCX-SPE) Combined with Ultrahigh-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MS/MS). J. Chrom. B 2020, 1137, 121923. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C.; Rojo, S.; Vilas-Boas, M.; Falcão, S.I. Phenolic Profile of Castanea Bee Pollen from the Northwest of the Iberian Peninsula. Separations 2023, 10, 270. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Cheng, L.; Zhang, X. Interactions of Tea Catechins with Intestinal Microbiota and Their Implication for Human Health. Food Sci. Biotechnol. 2019, 28, 1617–1625. [Google Scholar] [CrossRef]
- Hsu, P.-S.; Wu, T.-H.; Huang, M.-Y.; Wang, D.-Y.; Wu, M.-C. Nutritive Value of 11 Bee Pollen Samples from Major Floral Sources in Taiwan. Foods 2021, 10, 2229. [Google Scholar] [CrossRef]
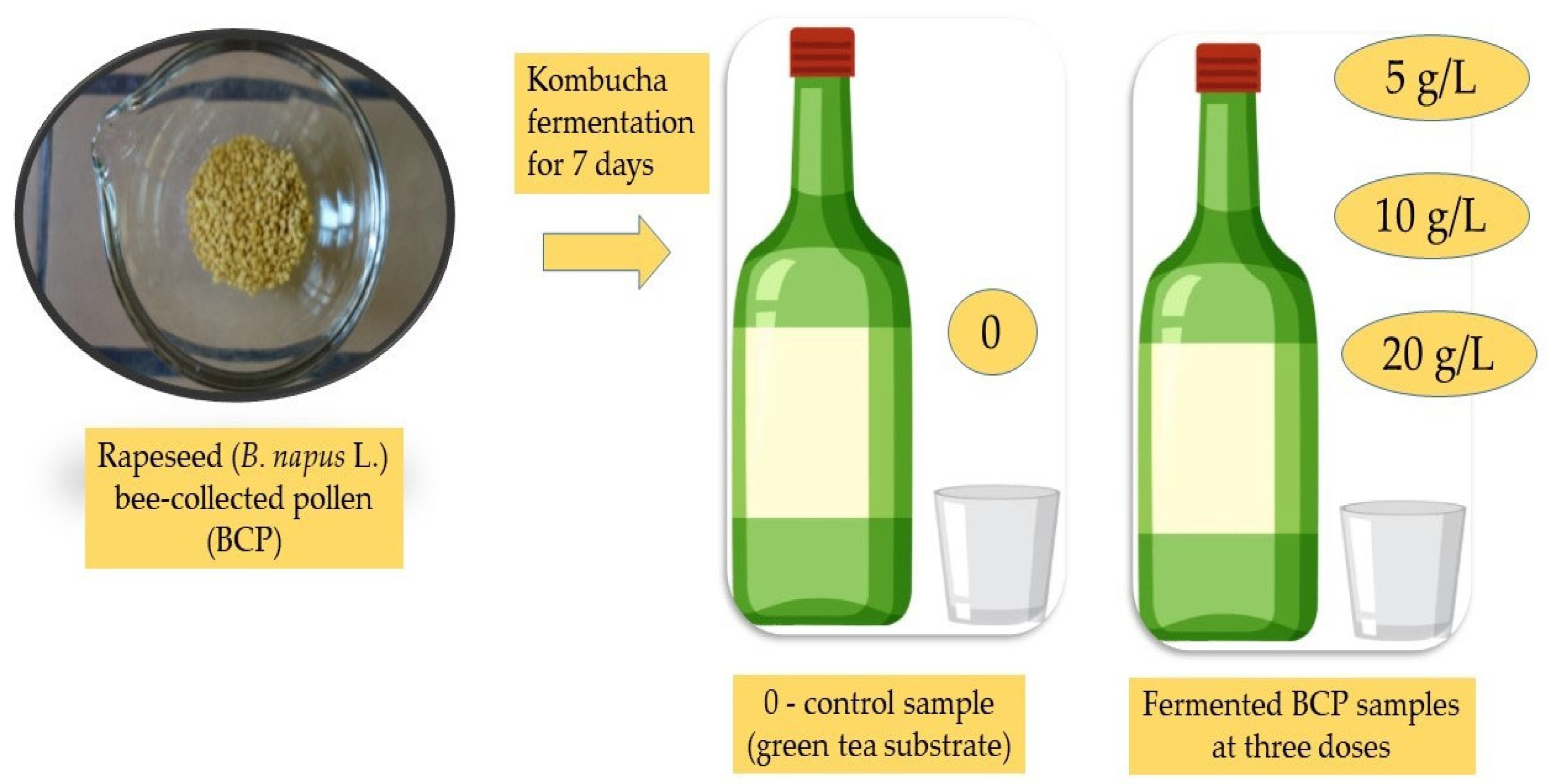
| Sample | TPC * (GAE **) | TFC (QE) | TAC (AAE) | FRP (AAE) | CUPRAC (AAE) | DPPH• (TE) | ABTS•+ (TE) |
|---|---|---|---|---|---|---|---|
| 0 | 16.98 ± 0.11 e | 31.97 ± 0.14 a | 156.46 ± 0.59 c | 46.70 ± 0.13 a | 424.20 ± 16.85 a | 31.34 ± 0.89 a | 34.87 ± 0.51 a |
| 1 | 21.94 ± 0.17 d | 12.84 ± 0.93 c | 212.66 ± 0.37 a | 13.96 ± 0.16 d | 213.76 ± 4.06 c | 10.14 ± 0.10 b,c | 18.08 ± 0.71 d |
| K1 | 23.45 ± 0.33 c | 11.99 ± 0.21 c | 188.42 ± 1.58 b | 15.85 ± 0.36 b | 272.35 ± 14.47 b | 10.69 ± 0.07 b | 22.16 ± 0.54 b |
| 2 | 25.31 ± 0.35 b | 12.70 ± 0.36 c | 180.70 ± 4.42 b | 11.10 ± 0.19 f | 196.52 ± 27.28 c | 9.81 ± 0.06 b,c | 16.66 ± 0.57 e |
| K2 | 24.66 ± 0.21 b | 11.77 ± 1.29 c | 159.81 ± 3.78 c | 12.67 ± 0.36 e | 204.05 ± 12.82 c | 10.33 ± 0.01 b,c | 18.68 ± 0.37 c,d |
| 3 | 27.03 ± 0.59 a | 12.56 ± 0.07 c | 155.93 ± 1.34 c | 13.66 ± 0.37 d | 212.44 ± 5.03 c | 9.53 ± 0.01 c | 18.21 ± 0.03 d |
| K3 | 26.46 ± 0.32 a | 14.77 ± 0.43 b | 114.43 ± 3.88 d | 14.88 ± 0.14 c | 212.44 ± 5.03 c | 9.99 ± 0.03 b,c | 19.56 ± 0.03 c |
| RT | Compound Name | Formula | Calculated Mass | m/z Exact Mass | mDa | Fragments (MS2) | Refs. * | Samples (µg/100 mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | K3 | ||||||||
| Phenolic acids and derivatives | ||||||||||
| 5.73 | Hydroxybenzoic acid isomer I b | C7H5O3− | 137.02390 | 137.02480 | −0.90 | / | [25,38] | <LOQ | <LOQ | 25.73 |
| 8.83 | Hydroxybenzoic acid isomer II b | C7H5O3− | 137.02390 | 137.02509 | −1.19 | / | [25,38] | <LOQ | <LOQ | <LOQ |
| 7.75 | Cinnamic acid b | C9H7O2− | 147.04460 | 147.04543 | −0.83 | 101.0412(68), 103.05682(7), 117.03663(100), 118.03995(10), 119.05082(7) | [35,39] | - | 46.58 | 74.58 |
| 3.44 | Dihydroxybenzoic acid b | C7H5O4− | 153.01880 | 153.01970 | −0.90 | 108.02333(100), 109.03079(77) | [25,37] | - | 45.29 | <LOQ |
| 1.68 | Gallic acid a | C7H5O5− | 169.01370 | 169.01470 | −1.00 | 107.01514(10), 108.02348(9), 123.00827(12), 124.01806(84), 125.02585(100) | Standard [27,35] | <LOQ | 51.81 | 26.48 |
| 5.69 | Methyl gallate b | C8H7O5− | 183.02930 | 183.02985 | −0.55 | 123.01056(35), 124.01831(100), 125.02362(6), 168.00384(2) | [29] | <LOQ | <LOQ | <LOQ |
| 6.33 | Sinapic acid b | C11H11O5− | 223.06060 | 223.06260 | −2.00 | 109.03047(46), 121.032(21), 123.04656(11), 125.02461(9), 135.04836(13), 137.02591(100), 138.03402(99), 151.04375(10), 161.02543(10), 179.03436(8) | [40] | <LOQ | <LOQ | <LOQ |
| 2.39 | Gallic acid hexoside b | C13H15O10− | 331.06650 | 331.06970 | −3.20 | 124.0185(18), 125.02615(96), 168.00922(100), 169.01397(17), 331.07253(7) | [27] | <LOQ | 27.37 | 34.50 |
| 6.47 | Coumaroylquinic acid isomer I b | C16H17O8− | 337.09230 | 337.09549 | −3.19 | 119.05185(100), 120.05465(10), 163.04224(45), 164.0453(5), 173.04689(3), 191.05832(15), 192.06292(2) | [27,28] | <LOQ | - | <LOQ |
| 7.35 | Coumaroylquinic acid isomer II b | C16H17O8− | 337.09230 | 337.09515 | −2.85 | 111.0467(23), 119.05188(52), 137.0275(11), 163.04257(28), 173.04812(100), 191.05846(7) | [27,28] | <LOQ | - | <LOQ |
| 2.09 | Galloylquinic acid isomer I b | C14H15O10− | 343.06650 | 343.06970 | −3.20 | 125.02662(1), 127.04233(3), 137.02634(6), 173.04847(2), 191.05879(100) | [27,28] | <LOQ | 27.74 | 26.54 |
| 3.30 | Galloylquinic acid isomer II b | C14H15O10− | 343.06650 | 343.06970 | −3.20 | 107.01476(10), 111.04708(8), 124.01873(3), 125.02625(89), 137.02579(5), 169.01627(100), 173.0471(27), 191.05852(45) | [27,28] | <LOQ | <LOQ | <LOQ |
| 5.93 | Caffeoylquinic acid b | C16H17O9− | 353.08730 | 353.09044 | −3.14 | 134.04159(10), 135.047(97), 136.05015(12), 161.02425(6), 173.04671(5), 179.03619(43), 191.05764(100), 192.06487(10) | [27,41] | <LOQ | <LOQ | - |
| 7.55 | Feruloylquinic acid b | C17H19O9− | 367.10290 | 367.10760 | −4.70 | 111.04851(12), 129.05678(7), 130.08874(19), 134.04015(41), 173.04998(24), 174.04921(9), 175.04191(7), 191.05723(100), 192.05995(10), 193.05302(23) | [41] | - | <LOQ | <LOQ |
| 6.88 | Galloyl-HHDP-hexose b | C27H21O18− | 633.07280 | 633.07770 | −4.90 | 169.01712(3), 249.04386(3), 275.02233(9), 301.00272(100), 302.00625(18), 331.07316(2), 463.05704(8), 633.0797(33) | [28,29] | 26.09 | <LOQ | <LOQ |
| ∑ | 26.09 | 198.78 | 187.83 | |||||||
| Flavan-3-ols and derivatives | ||||||||||
| 6.74 | Catechin a | C15H13O6− | 289.07120 | 289.07420 | −3.00 | 109.03155(95), 121.03162(28), 122.03861(16), 123.04722(100), 125.02644(43), 137.02677(28), 151.04223(32), 161.06179(13), 203.07402(20), 221.08493(13) | Standard [27] | 7628.17 | 711.36 | 1283.74 |
| 7.35 | Epicatechin a | C15H13O6− | 289.07120 | 289.07420 | −3.00 | 109.03111(93), 121.03105(28), 123.04687(100), 125.02618(45), 137.02672(25), 149.02669(15), 151.04176(29), 161.06232(13), 203.07438(19), 221.08592(11) | Standard [27] | 1504.70 | 447.58 | 510.25 |
| 3.91 | Gallocatechin c | C15H13O7− | 305.06610 | 305.06920 | −3.10 | 109.03095(15), 111.04691(22), 121.03207(5), 123.01092(12), 124.018(9), 125.02624(100), 137.02557(33), 139.04209(29), 165.02075(14), 167.03728(21), 219.06784(7) | [27] | 709.41 | 783.37 | 581.65 |
| 4.92 | Epigallocatechin c | C15H13O7− | 305.06610 | 305.06920 | −3.10 | 109.03141(15), 111.04717(20), 123.01039(11), 124.01854(9), 125.02647(100), 137.02679(36), 139.04187(33), 165.02131(19), 167.03747(21), 219.06795(7) | [27] | 1109.42 | 856.09 | 1233.48 |
| 8.83 | Epiafzelechin 3-O-gallate c | C22H17O9− | 425.08730 | 425.09098 | −3.68 | 124.01793(45), 125.02621(57), 137.02573(16), 149.02736(10), 168.00816(10), 169.01729(74), 187.08013(18), 189.05883(21), 205.08878(23), 211.07995(15), 229.08791(19), 255.07135(37), 273.07992(100), 274.08329(13) | [27] | 107.99 | - | - |
| 8.29 | Epicatechin gallate c | C22H17O10− | 441.08220 | 441.08710 | −4.90 | 109.03134(5), 124.01855(11), 125.02618(45), 169.0168(100), 170.01997(8), 179.03774(4), 203.07401(6), 205.05352(5), 245.085(13), 289.07557(20), 290.07856(4) | [27] | 2203.25 | 234.44 | 282.18 |
| 7.48 | Epigallocatechin gallate c | C22H17O11− | 457.07710 | 457.08234 | −5.24 | 124.01823(2), 125.02634(48), 169.01665(100), 170.01985(9), 305.07011(3) | [27] | 829.36 | 138.32 | 158.26 |
| 4.18 | Gallocatechin hexoside c | C21H23O12− | 467.11900 | 467.12140 | −2.40 | 125.02606(91), 137.02621(47), 165.0216(37), 167.03661(54), 179.03724(28), 219.06938(27), 261.08123(35), 305.07077(100), 306.07254(21), 467.12336(10) | [27] | 71.56 | 40.61 | 25.07 |
| 6.40 | Epigallocatechin hexoside c | C21H23O12− | 467.11900 | 467.12390 | −4.90 | 125.02594(76), 137.02617(51), 161.02611(20), 165.02483(39), 167.0384(86), 169.02013(19), 179.0352(51), 261.07905(32), 305.07145(100) | [27] | 73.80 | - | - |
| 7.01 | Procyanidin dimer B type c | C30H25O12− | 577.13460 | 577.14030 | −5.70 | 125.02592(91), 137.0266(16), 151.04409(11), 161.02718(26), 205.05107(12), 245.08291(24), 273.04275(10), 289.07448(100), 407.0819(73), 408.08588(28) | [27] | 404.62 | 26.77 | 54.98 |
| 7.35 | Chalcan-flavan 3-ol dimer c | C30H27O12− | 579.15080 | 579.15600 | −5.20 | 125.02547(15), 137.02661(10), 167.03743(10), 205.05567(14), 245.08484(38), 289.07504(100), 290.07943(22), 369.06652(9), 459.10279(6), 579.14044(6) | [28] | - | 60.44 | - |
| 6.26 | Epigallocatechin-epigallocatechin 3-O-gallate c | C37H29O18− | 761.13540 | 761.14290 | −7.50 | 125.02502(12), 169.01804(11), 297.04335(15), 299.05776(11), 327.056(34), 339.05854(16), 435.07646(11), 453.08687(47), 465.08847(25), 471.09785(13), 573.10382(11), 591.11999(100), 592.12505(36), 609.13553(70), 610.13481(32) | [27] | 199.77 | - | - |
| ∑ | 14,842.1 | 3298.98 | 4129.60 | |||||||
| Flavonol aglycones and glycosides | ||||||||||
| 10.74 | Kaempferol d | C15H9O6− | 285.03990 | 285.04190 | −2.00 | 143.05241(8), 151.00639(6), 159.04741(9), 171.04757(7), 185.06338(13), 187.04283(11), 211.04312(8), 229.05402(11), 239.03849(9), 285.04486(100), 286.04783(19) | [38] | <LOQ | 1635.77 | 1617.70 |
| 9.91 | Quercetin a | C15H9O7− | 301.03480 | 301.03600 | −1.20 | 107.01558(46), 121.03135(43), 149.02665(9), 151.0061(100), 152.00988(10), 179.00107(13), 187.04291(3), 245.04782(4), 301.04008(4) | Standard [39] | 88.55 | 461.76 | 449.41 |
| 10.85 | Isorhamnetin a | C16H11O7− | 315.05050 | 315.05420 | −3.70 | 107.01561(19), 151.00586(49), 152.01066(7), 227.03772(6), 243.03308(6), 255.03296(7), 271.02837(11), 283.02806(8), 300.03166(100), 301.03448(20) | Standard [42] | <LOQ | 99.61 | 105.72 |
| 9.06 | Kaempferol-3-O-rhamnoside d | C21H19O10− | 431.09780 | 431.10553 | −7.73 | 227.04101(24), 255.03527(41), 256.03883(21), 257.04198(7), 271.08023(5), 284.03663(100), 285.04091(75), 431.10621(6) | [38] | <LOQ | - | - |
| 8.56 | Kaempferol-3-O-hexoside d | C21H19O11− | 447.09270 | 447.09790 | −5.20 | 227.03763(21), 228.04433(3), 255.03369(32), 257.04587(3), 284.03646(100), 285.04159(41), 447.10039(13) | [37] | 29.65 | 120.87 | 163.17 |
| 8.22 | Quercetin 3-O-hexoside d | C21H19O12− | 463.08770 | 463.09320 | −5.50 | 151.00521(4), 179.00053(3), 243.03319(2), 255.03379(6), 256.03636(1), 271.02862(11), 300.03139(100), 301.03764(48) | [27,38] | 63.82 | <LOQ | <LOQ |
| 7.75 | Myricetin 3-O-hexoside d | C21H19O13− | 479.08260 | 479.08780 | −5.20 | 151.0069(1), 179.00116(3), 271.02806(8), 287.02337(4), 316.02649(100), 317.03185(29), 318.0332(5), 479.08636(4) | [27] | 30.94 | - | - |
| 8.89 | Kaempferol 3-O-(6″-acetyl)hexoside d | C23H21O12− | 489.10330 | 489.10871 | −5.41 | 227.04146(5), 229.05163(5), 255.03556(11), 256.03807(5), 257.04548(7), 284.03729(100), 285.04252(96), 489.28126(3) | [29] | <LOQ | <LOQ | <LOQ |
| 8.89 | Kaempferol 3-O-(6″-O-malonyl)hexoside d | C24H21O14− | 533.09310 | 533.10034 | −7.24 | 227.03607(2), 229.05413(2), 255.03368(7), 257.04871(2), 284.03563(94), 285.04255(100), 286.04646(18), 287.04729(2), 489.10903(5), 490.11036(2) | [26] | <LOQ | - | <LOQ |
| 8.14 | Kaempferol 3-O-(2″-O-pentosyl)hexoside d | C26H27O15− | 579.13500 | 579.13861 | −3.61 | 227.03675(3), 255.03401(10), 257.04532(4), 284.03524(100), 285.04112(35), 429.08863(4), 579.14137(23), 580.14395(9) | [26] | - | <LOQ | <LOQ |
| 8.36 | Kaempferol 3-O-(6″-O-rhamnosyl)hexoside d | C27H29O15− | 593.15060 | 593.15660 | −6.00 | 227.03779(2), 255.03327(5), 256.03779(2), 257.04991(2), 284.03638(50), 285.04383(100), 286.04755(17), 593.1586(44), 594.16158(16) | [28,38] | 30.94 | 32.55 | 60.58 |
| 7.75 | Isorhamnetin 3-O-(6″-O-hexosyl)hexoside d | C28H31O17− | 639.15610 | 639.16235 | −6.25 | 271.02798(6), 299.02295(44), 300.02956(19), 301.03072(3), 314.04657(100), 315.0526(42), 459.09849(5), 639.16315(58), 640.16622(23) | [26] | <LOQ | - | <LOQ |
| 8.35 | Kaempferol 3-O-(2″-pentosyl)-malonyl-pentoside d | C29H31O17− | 651.15610 | 651.15920 | −3.10 | 135.04763(40), 145.03209(11), 255.0313(9), 284.03597(100), 285.04397(45), 399.20994(52), 509.28402(13), 519.26712(52), 535.2655(8), 651.16299(40) | / | - | <LOQ | <LOQ |
| 8.02 | Kaempferol 3-O-(2″-rhamnosyl-6″-hexosyl)hexoside d | C33H39O20− | 755.20350 | 755.20606 | −2.56 | 229.05162(2), 255.03363(2), 284.03595(12), 285.04393(100), 286.04858(17), 449.05875(4), 467.06532(5), 605.10631(2), 755.21071(84), 756.21563(38) | [26,43] | - | 142.53 | 142.68 |
| 7.82 | Quercetin 3-O-(6″-rhamnosyl-hexosyl)hexoside d | C33H39O21− | 771.19840 | 771.20540 | −7.00 | 151.00583(1), 179.00097(1), 271.02833(1), 300.03146(16), 301.03879(24), 771.20752(100), 772.21109(44), 773.21283(13) | [27,43] | 212.29 | 30.26 | 81.36 |
| 6.57 | Kaempferol 3-O-(6″-hexosyl)hexoside-7-O-hexoside d | C33H39O21− | 771.19840 | 771.20839 | −9.99 | 255.03458(2), 283.0282(6), 284.03554(13), 285.04319(10), 446.09042(9), 447.09619(4), 609.15384(100), 610.15678(36), 651.16692(1), 771.20778(1) | [38] | - | <LOQ | 47.14 |
| 6.40 | Quercetin 3-O-(2″-hexosyl)hexoside-7-O-hexoside d | C33H39O22− | 787.19330 | 787.20056 | −7.26 | 178.99502(3), 299.02313(26), 300.02955(9), 301.04217(11), 445.08287(3), 462.08742(58), 463.09198(49), 625.14705(88), 626.15142(31), 787.20256(100), 788.20381(38) | [38] | - | <LOQ | <LOQ |
| 8.02 | Quercetin 3-O-(6″-rhamnosyl)hexoside d | C27H29O16− | 609.14569 | 609.15110 | −5.41 | 151.00589(3), 179.00092(3), 255.03315(3), 271.02923(6), 300.031640(100), 301.03816(73) | [27,37] | 164.79 | 155.44 | 193.02 |
| 7.88 | Kaempferol 3-O-(2″-hexosyl)hexoside d | C27H29O16− | 609.14569 | 609.15299 | −7.30 | 227.03677(3), 255.03261(8), 284.03562(100), 285.04182(49), 429.08801(5) | [38] | - | 167.83 | 181.06 |
| 7.54 | Quercetin 3-O-(2″-hexosyl)hexoside d | C27H29O17− | 625.14058 | 625.14793 | −7.35 | 179.00092(4), 271.02771(5), 300.03166(100), 301.03668(30), 445.08254(2), 463.09269(2) | [38] | - | 225.47 | 293.15 |
| 9.30 | Quercetin 3-O-(2″-rhamnosyl-3″-pentosyl-6″-coumaroyl)hexoside d | C41H43O22− | 887.22465 | 887.23370 | −9.05 | 151.00495(3), 179.00133(3), 299.02251(12), 300.03151(29), 301.03918(83), 723.18393(3), 741.1968(20), 887.23568(100) | [28] | 39.06 | - | - |
| 9.23 | Quercetin 3-O-(2″-rhamnosyl-3″-hexosyl-6″-coumaroyl)hexoside d | C42H45O23− | 917.23527 | 917.24240 | −7.13 | 169.01477(3), 178.99951(3), 299.01998(9), 300.03042(21), 301.03909(49), 609.14184(3), 753.19589(4), 771.20409(8), 917.24439(100) | [28] | <LOQ | <LOQ | <LOQ |
| ∑ | 660.03 | 3072.07 | 3334.97 | |||||||
| Flavone aglycone | ||||||||||
| 10.58 | Apigenin a | C15H9O5− | 269.04500 | 269.04763 | −2.63 | 107.01539(26), 119.05147(20), 133.03093(53), 135.04631(16), 151.0058(12), 159.04666(22), 181.06752(15), 183.04727(15), 201.05826(11), 224.05064(12), 225.05784(9), 269.04913(100), 270.05167(21) | Standard | <LOQ | - | <LOQ |
| 9.87 | Luteolin a | C15H9O6− | 285.03990 | 285.04310 | −3.20 | 107.01603(18), 121.03196(5), 133.03111(100), 134.03446(11), 151.00573(31), 175.04358(15), 199.04331(12), 217.05309(7), 285.04402(45), 286.04861(8) | Standard | 364.29 | - | - |
| ∑ | 364.29 | 0 | 0 | |||||||
| Flavanone aglycone | ||||||||||
| 10.58 | Naringenin a | C15H11O5− | 271.06060 | 271.06123 | −0.63 | 107.01562(30), 119.05205(100), 120.05542(11), 151.00569(31), 152.00874(3), 161.06313(3), 165.02226(2), 177.02197(3), 187.04301(5) | Standard | <LOQ | 477.61 | 395.35 |
| 9.06 | Eridictyol e | C15H11O6− | 287.05560 | 287.05723 | −1.63 | 107.01722(11), 125.02643(100), 126.02753(11), 131.05415(11), 151.00542(17), 152.01339(16), 173.06236(8), 177.06071(27), 201.05888(6), 241.04914(6), 259.06193(10) | [37] | - | <LOQ | <LOQ |
| ∑ | 0 | 477.61 | 395.35 | |||||||
| ∑∑ | 15,892.5 | 7047.44 | 8047.76 | |||||||
| No | RT | Compounds (Proposed Phenylamide Name) | Formula | Calculated Mass | m/z Exact Mass | mDa | Fragments (MS2) | Refs. * | Samples (%) | Ratio 3/K3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | K3 | ||||||||||
| 1 | 7.41 | Benzoiyl putrescin (N1-benzoyl putrescin) | C11H17N2O+ | 193.13410 | 193.13470 | −0.60 | 105.07078(9), 108.08172(12), 120.08237(11), 134.09717(8), 136.11229(27), 148.11206(13), 150.0926(43), 164.10833(100), 165.11111(19) | / | - | 0.46 | 2.72 | 0.17 |
| 2 | 7.92 | Coumaroyl spermidine (N1-coumaroyl spermidine) | C16H26N3O2+ | 292.20250 | 292.20449 | −1.99 | 119.05032(15), 146.16608(1), 147.04553(100), 148.0485(14), 204.10387(7) | [38] | - | 2.55 | 2.62 | 0.96 |
| 3 | 8.01 | Dicoumaroyl spermidine (N1,N5-dicoumaroyl spermidine) | C25H32N3O4+ | 438.23930 | 438.24160 | −2.30 | 119.05045(8), 129.14018(4), 147.04577(95), 204.10406(100), 205.10683(22), 218.11927(16), 275.17809(15), 292.20439(39), 293.20749(10) | [26,38] | - | 56.04 | 55.41 | 1.00 |
| 4 | 8.02 | Feruloyl coumaroyl spermidine (N1-feruloyl-N5-coumaroyl spermidine) | C26H34N3O5+ | 468.24980 | 468.25352 | −3.72 | 145.02926(19), 147.04528(51), 177.0557(100), 178.05894(13), 204.10298(71), 205.10605(12), 234.11379(63), 235.11715(12), 275.17737(8), 292.20353(32), 293.20609(7), 322.21375(22), 468.25352(15) | [38] | - | 1.65 | 1.59 | 1.02 |
| 5 | 9.90 | Tricoumaroyl spermidine (N1,N5,N10-tri-coumaroyl spermidine) | C34H38N3O6+ | 584.27610 | 584.28269 | −6.59 | 119.05086(3), 147.0457(41), 204.10425(50), 275.1783(15), 292.20513(32), 293.20743(8), 420.23277(15), 421.22839(9), 438.24348(100), 439.2466(40) | [26,38] | - | 0.02 | 0.01 | 1.63 |
| 6 | 8.96 | Tricoumaroyl spermine (N1,N5,N10-tri-coumaroyl spermine) | C37H45N4O6+ | 641.33390 | 641.33760 | −3.70 | 129.13967(7), 147.04515(36), 203.1194(14), 204.10365(84), 205.10674(13), 275.17735(91), 477.28941(17), 495.29994(54), 496.30279(23), 641.33788(100), 642.34076(59) | [38] | - | 2.04 | 1.96 | 1.03 |
| 7 | 8.62 | Dicoumaroyl caffeoyl spermine (N1,N5-di-coumaroyl-N10-caffeoyl spermine) | C37H45N4O7+ | 657.32880 | 657.33260 | −3.80 | 147.04542(22), 204.10404(45), 205.1068(7), 220.09925(17), 275.1777(45), 276.18055(11), 291.17267(27), 292.17816(6), 349.2620(2), 365.2564(1), 511.29547(35), 512.29859(15), 640.3124(2), 657.334(100), 658.33662(57) | [26,38] | - | 4.82 | 4.64 | 1.03 |
| 8 | 8.96 | Dicoumaroyl feruloyl spermine (N1,N5-di-coumaroyl-N10-feruloyl spermine) | C38H47N4O7+ | 671.34450 | 671.34820 | −3.70 | 147.04525(19), 177.056(11), 204.10379(41), 234.11506(16), 275.17771(43), 305.18806(23), 451.2254(3), 495.2997(10), 525.31078(32), 526.31495(15), 671.34824(100), 672.35241(61) | [26,38] | - | 3.14 | 3.08 | 1.01 |
| 9 | 8.62 | Dicoumaroyl hydroxyferuloyl spermine (N1,N10-di-coumaroyl-N5-hydroxyferuloyl spermine) | C38H47N4O8+ | 687.33940 | 687.34340 | −4.00 | 147.04555(16), 193.05083(5), 204.10339(39), 205.10675(6), 250.11019(14), 275.17709(39), 321.18326(23), 495.30019(11), 541.30598(32), 687.34338(100), 688.34674(63) | [26,38] | - | 16.96 | 16.39 | 1.02 |
| 10 | 8.96 | Diferuloyl coumaroyl spermine (N1,N10-di-feruloyl-N5-coumaroyl spermine) | C39H49N4O8+ | 701.35500 | 701.35750 | −2.50 | 147.0452(11), 177.05649(12), 204.10372(30), 234.1151(9), 275.17743(34), 276.18067(7), 305.18736(14), 335.20036(9), 525.30953(13), 555.3214(18), 701.35917(100), 702.36096(65), 703.35572(30) | [38] | - | 1.37 | 1.21 | 1.12 |
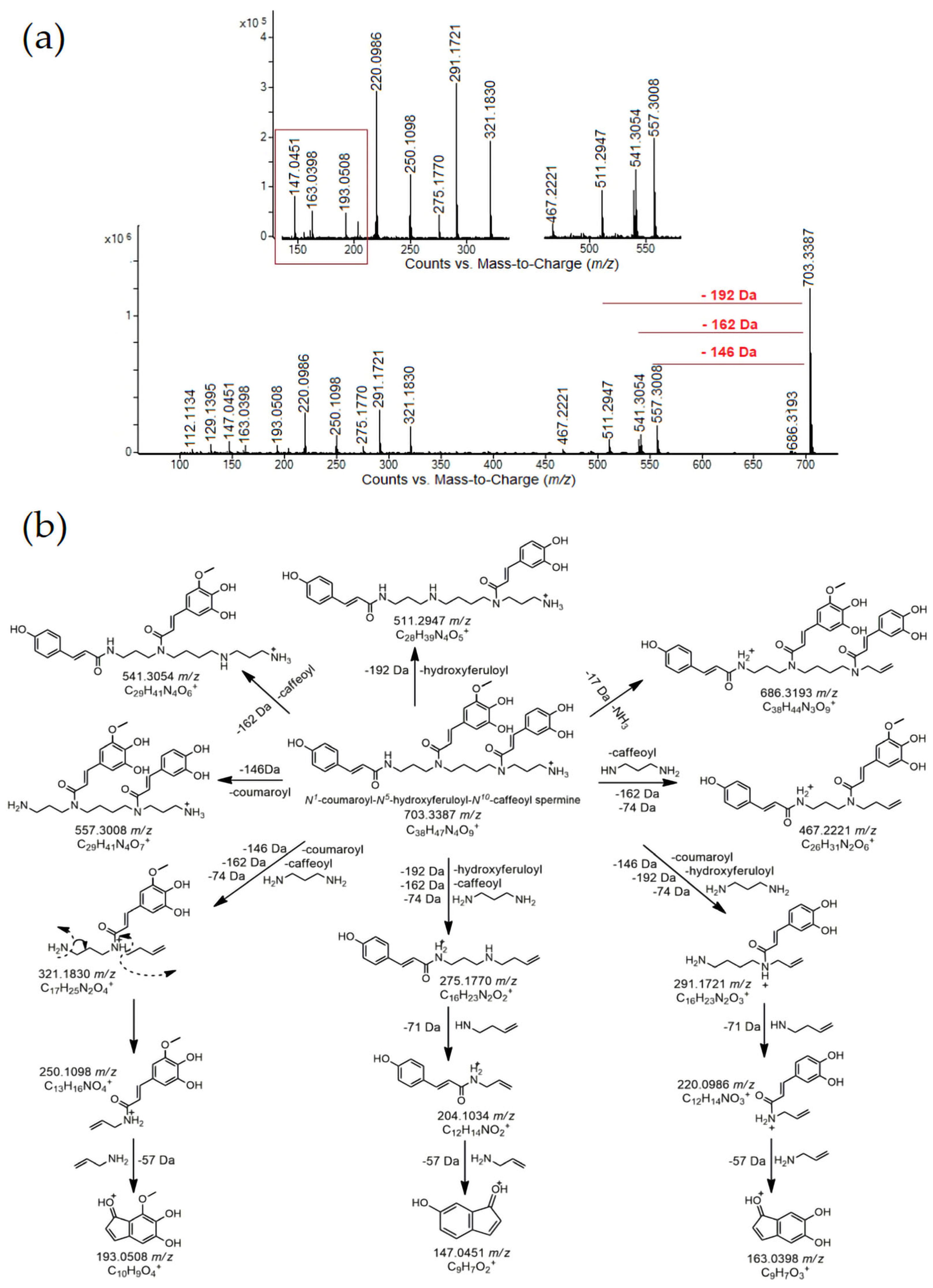
| 11 | 8.36 | Coumaroyl hydroxyferuloyl caffeoyl spermine (N1-coumaroyl-N5-hydroxyferuloyl-N10-caffeoyl spermine) | C38H47N4O9+ | 703.33430 | 703.33870 | −4.40 | 147.0451(7), 204.1034(3), 220.0986(24), 250.1098(11), 275.1770(4), 291.1721(26), 321.1830(17), 467.2221(2), 511.2947(8), 541.3054(11), 557.3008(17), 686.3193(1), 703.3387 (100), 704.3422(64) | [26,38] | - | 4.38 | 4.32 | 1.00 |
| 12 | 8.69 | Coumaroyl hydroxyferuloyl feruloyl spermine (N1-coumaroyl-N5-hydroxyferuloyl-N10-feruloyl spermine) | C39H49N4O9+ | 717.35000 | 717.35390 | −3.90 | 147.04485(5), 177.05619(10), 204.10388(10), 234.11428(15), 250.11079(9), 275.17762(13), 305.18827(15), 321.1835(15), 525.31035(7), 541.30497(11), 553.30564(5), 571.31718(12), 717.35426(100), 718.35733(62) | [26,38] | - | 6.10 | 5.64 | 1.07 |
| 13 | 9.57 | Tricaffeoyl hydroxyferuloyl spermine | C47H53N4O13+ | 881.36090 | 881.36498 | −4.08 | 220.09787(4), 250.10682(3), 291.17122(6), 321.18306(4), 557.29922(7), 689.32273(4), 701.32262(5), 719.33302(100), 720.33609(64), 881.36498(9) | [26,38] | - | 0.47 | 0.41 | 1.14 |
| ∑Total area 3/∑Total area K3 | 0.99 | |||||||||||
| Sample | P. aeruginosa | S. Enteritidis | K. aerogenes | A. baumannii | E. coli | S. aureus ATCC | S. aureus Clinical Isolate | L. monocytogenes | B. spizizenii | C. albicans | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic/Antimycotic * (mg/mL) | MIC | 2.5 ± 0.0 | <0.05 | 1.7 ± 0.7 | 1.6 ± 0.0 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.3 ± 0.0 |
| MBC | 13.3 ± 5.7 | 0.05 ± 0.0 | 6.7 ± 2.9 | 2.5 ± 0.0 | 0.05 ± 0.0 | <0.05 | <0.05 | <0.05 | <0.05 | 0.3 ± 0.0 | |
| 0 | MIC | 50.0 ± 0.0% a | - | 6.3 ± 0.0% d | - | - | - | 50.0 ± 0.0% a | - | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| 0N | MIC | - | - | - | - | - | - | 50.0 ± 0.0% a | - | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| 1 | MIC | 6.3 ± 0.0% d | 1.6 ± 0.0% e | 6.3 ± 0.0% d | 6.3 ± 0.0% d | 3.1 ± 0.0% c | 3.1 ± 0.0% e | 3.1 ± 0.0% e | 12.5 ± 0.0% c | 25.0 ± 0.0% b | 50.0 ± 0.0% a |
| MBC/ MFC | 12.5 ± 0.0% c | 25.0 ± 0.0% a | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 12.5 ± 0.0% a | 25.0 ± 0.0% b | 25.0 ± 0.0% b | 50.0 ± 0.0% a | 25.0 ± 0.0% b | 50.0 ± 0.0% a | |
| 1N | MIC | 50.0 ± 0.0% a | 3.1 ± 0.0% d | 12.5 ± 0.0% c | 50.0 ± 0.0% a | 6.3 ± 0.0% b | - | - | 25.0 ± 0.0% b | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| K1 | MIC | 3.1 ± 0.0% e | 1.6 ± 0.0% e | 6.3 ± 0.0% d | 6.3 ± 0.0% d | 3.1 ± 0.0% c | 12.5 ± 0.0% c | 12.5 ± 0.0% c | 6.3 ± 0.0% d | 12.5 ± 0.0% c | 50.0 ± 0.0% a |
| MBC/ MFC | 6.3 ± 0.0% d | 6.3 ± 0.0% c | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 12.5 ± 0.0% a | 50.0 ± 0.0% a | 12.5 ± 0.0% c | 25.0 ± 0.0% b | 12.5 ± 0.0% c | - | |
| K1N | MIC | 50.0 ± 0.0% a | 3.1 ± 0.0% d | - | 50.0 ± 0.0% a | 12.5 ± 0.0% a | - | - | 25.0 ± 0.0% b | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| 2 | MIC | 3.1 ± 0.0% e | 1.6 ± 0.0% e | 6.3 ± 0.0% d | 6.3 ± 0.0% d | 3.1 ± 0.0% c | 12.5 ± 0.0% c | 1.6 ± 0.0% f | 6.3 ± 0.0% d | 12.5 ± 0.0% c | 25.0 ± 0.0% b |
| MBC/ MFC | 6.3 ± 0.0% d | 6.3 ± 0.0% c | 12.5 ± 0.0% c | 6.3 ± 0.0% d | 6.3 ± 0.0% b | 50.0 ± 0.0% a | 25.0 ± 0.0% b | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 50.0 ± 0.0% a | |
| 2N | MIC | 50.0 ± 0.0% a | 3.1 ± 0.0% d | 12.5 ± 0.0% c | 50.0 ± 0.0% a | 6.3 ± 0.0% b | - | - | 25.0 ± 0.0% b | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| K2 | MIC | 3.1 ± 0.0% e | 1.6 ± 0.0% e | 6.3 ± 0.0% d | 3.1 ± 0.0% e | 3.1 ± 0.0% c | 12.5 ± 0.0% c | 6.3 ± 0.0% d | 6.3 ± 0.0% d | 12.5 ± 0.0% c | 25.0 ± 0.0% b |
| MBC/ MFC | 6.3 ± 0.0% d | 12.5 ± 0.0% b | 12.5 ± 0.0% c | 6.3 ± 0.0% d | 6.3 ± 0.0% b | 50.0 ± 0.0% a | 6.3 ± 0.0% d | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 25.0 ± 0.0% b | |
| K2N | MIC | 50.0 ± 0.0% a | 3.1 ± 0.0% d | 12.5 ± 0.0% c | 25.0% b | 12.5 ± 0.0% a | 50.0 ± 0.0% a | 50.0 ± 0.0% a | 25.0 ± 0.0% b | - | - |
| MBC/ MFC | - | - | - | - | - | - | - | - | - | - | |
| 3 | MIC | 3.1 ± 0.0% d | 1.6 ± 0.0% e | 3.1 ± 0.0% e | 3.1% e | 3.1 ± 0.0% c | 6.3 ± 0.0% d | 1.6 ± 0.0% f | 6.3 ± 0.0% d | 12.5 ± 0.0% c | 25.0 ± 0.0% b |
| MBC/ MFC | 3.1 ± 0.0% e | 12.5 ± 0.0% b | 12.5 ± 0.0% c | 6.3% d | 6.3 ± 0.0% b | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 25.0 ± 0.0% b | 12.5 ± 0.0% c | 25.0 ± 0.0% b | |
| 3N | MIC | 12.5 ± 0.0% c | 3.1 ± 0.0% d | 12.5 ± 0.0% c | 25.0% b | 6.3 ± 0.0% b | 25.0 ± 0.0% b | 50.0 ± 0.0% a | 25.0 ± 0.0% b | - | - |
| MBC/ MFC | - | -. | - | - | - | - | - | - | - | - | |
| K3 | MIC | 3.1 ± 0.0% e | 1.6 ± 0.0% e | 3.1 ± 0.0% e | 3.1% e | 3.1 ± 0.0% c | 6.3 ± 0.0% d | 1.6 ± 0.0% f | 6.3 ± 0.0% d | 6.3 ± 0.0% d | 25.0 ± 0.0% b |
| MBC/ MFC | 6.3 ± 0.0% d | 12.5 ± 0.0% b | 12.5 ± 0.0% c | 6.3% d | 6.3 ± 0.0% b | 50.0 ± 0.0% a | 6.3 ± 0.0% d | 12.5 ± 0.0% c | 12.5 ± 0.0% c | 25.0 ± 0.0% b | |
| K3N | MIC | 12.5 ± 0.0% c | 3.1 ± 0.0% d | 12.5 ± 0.0% c | 25% b | 12.5 ± 0.0% a | 50.0 ± 0.0% a | 50.0 ± 0.0% a | 25.0 ± 0.0% b | 50.0 ± 0.0% a | - |
| MBC/ MFC | 25.0 ± 0.0% b | - | 50.0 ± 0.0% a | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, A.Ž.; Sknepnek, A.; Milinčić, D.D.; Gašić, U.; Kilibarda, S.; Pešić, M.B. Influence of Kombucha Fermentation on Antioxidant and Antimicrobial Activity of Monofloral Rapeseed Bee-Collected Pollen. Antioxidants 2025, 14, 752. https://doi.org/10.3390/antiox14060752
Kostić AŽ, Sknepnek A, Milinčić DD, Gašić U, Kilibarda S, Pešić MB. Influence of Kombucha Fermentation on Antioxidant and Antimicrobial Activity of Monofloral Rapeseed Bee-Collected Pollen. Antioxidants. 2025; 14(6):752. https://doi.org/10.3390/antiox14060752
Chicago/Turabian StyleKostić, Aleksandar Ž., Aleksandra Sknepnek, Danijel D. Milinčić, Uroš Gašić, Sofija Kilibarda, and Mirjana B. Pešić. 2025. "Influence of Kombucha Fermentation on Antioxidant and Antimicrobial Activity of Monofloral Rapeseed Bee-Collected Pollen" Antioxidants 14, no. 6: 752. https://doi.org/10.3390/antiox14060752
APA StyleKostić, A. Ž., Sknepnek, A., Milinčić, D. D., Gašić, U., Kilibarda, S., & Pešić, M. B. (2025). Influence of Kombucha Fermentation on Antioxidant and Antimicrobial Activity of Monofloral Rapeseed Bee-Collected Pollen. Antioxidants, 14(6), 752. https://doi.org/10.3390/antiox14060752








