Food Grade Synthesis of Hetero-Coupled Biflavones and 3D-Quantitative Structure–Activity Relationship (QSAR) Modeling of Antioxidant Activity
Abstract
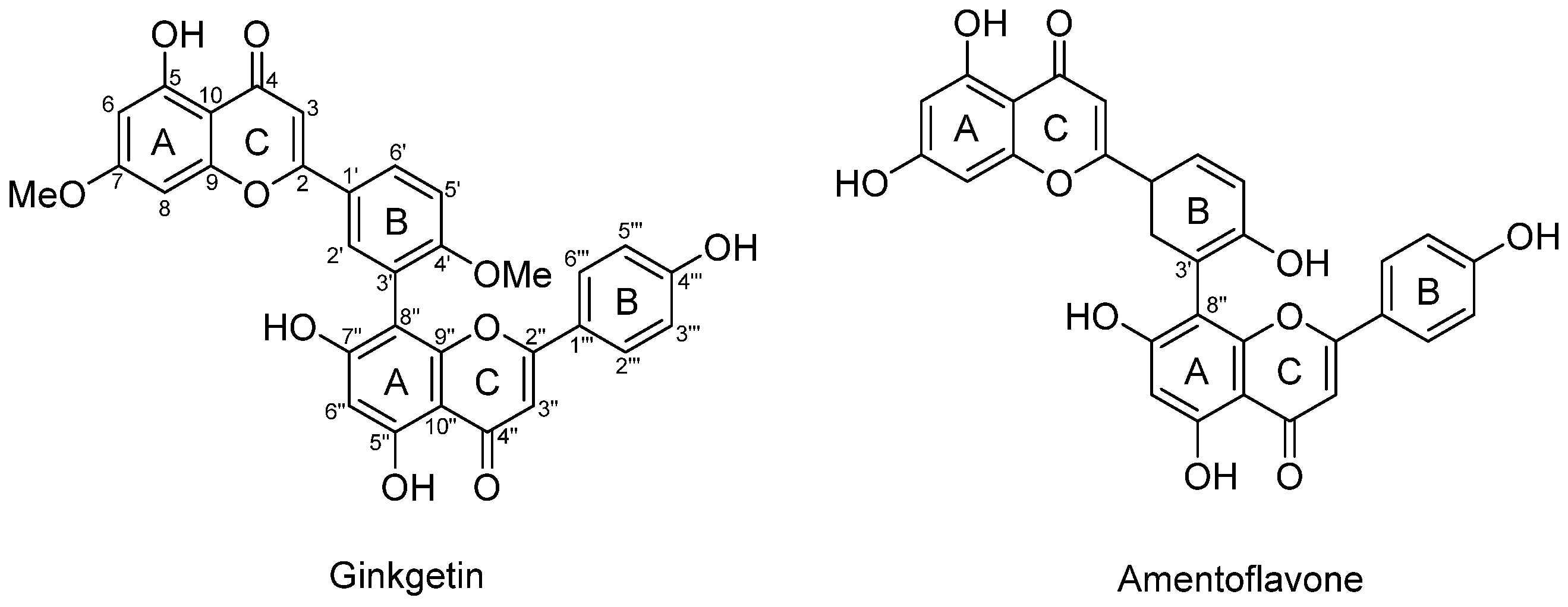
1. Introduction
2. Materials and Methods
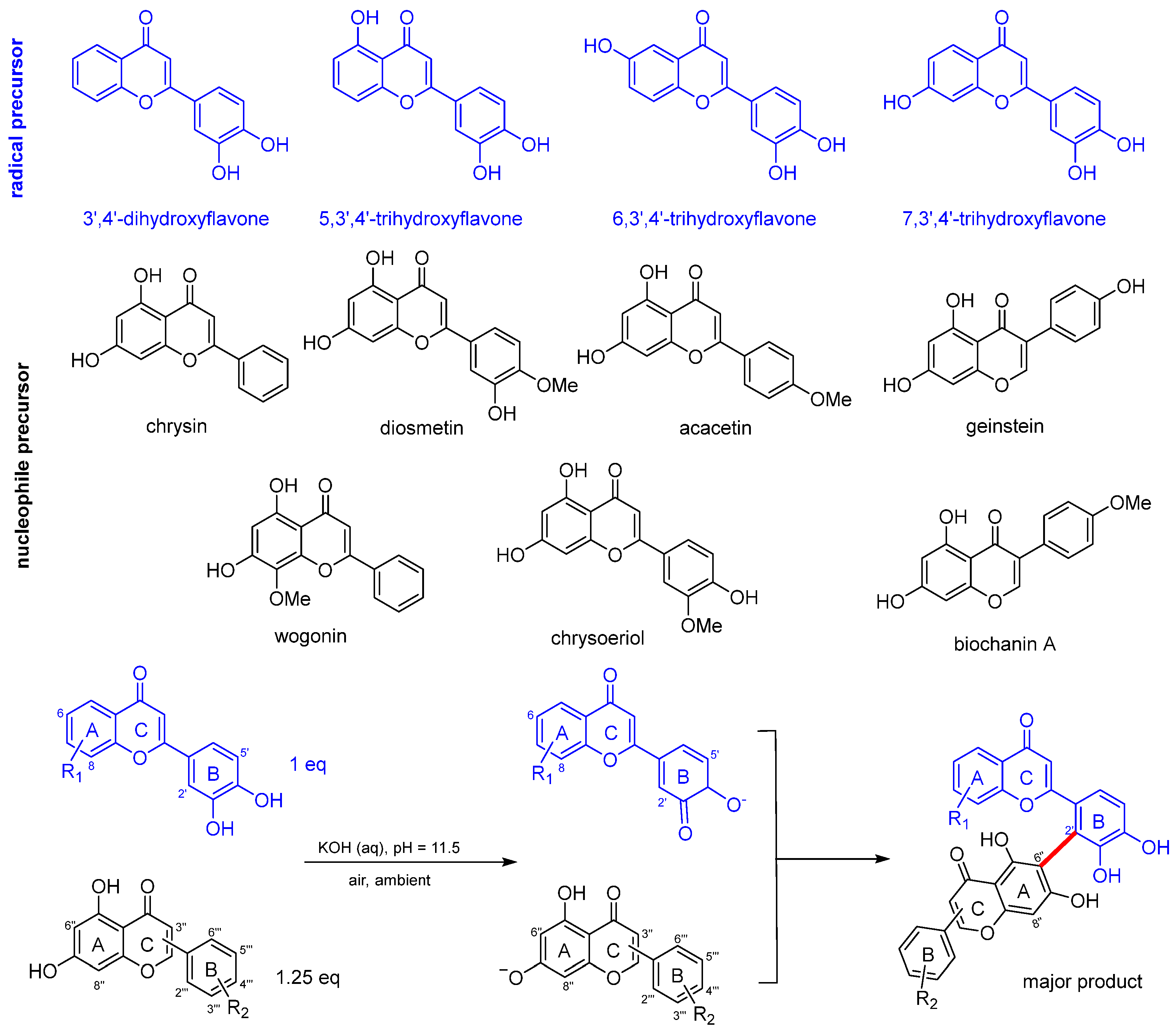
2.1. Synthesis of Biflavonoids
2.2. HPLC Separation of Reaction Mixtures
2.3. Characterization of Biflavonoids with 1H NMR, 13C NMR, and MS
2.4. DPPH Assay
2.5. Data Set Preparation, Alignment, and 3D-QSAR Modeling by Partial Least Squares (PLS) Analysis
2.6. Density Functional Theory (DFT) Calculation
3. Results and Discussion
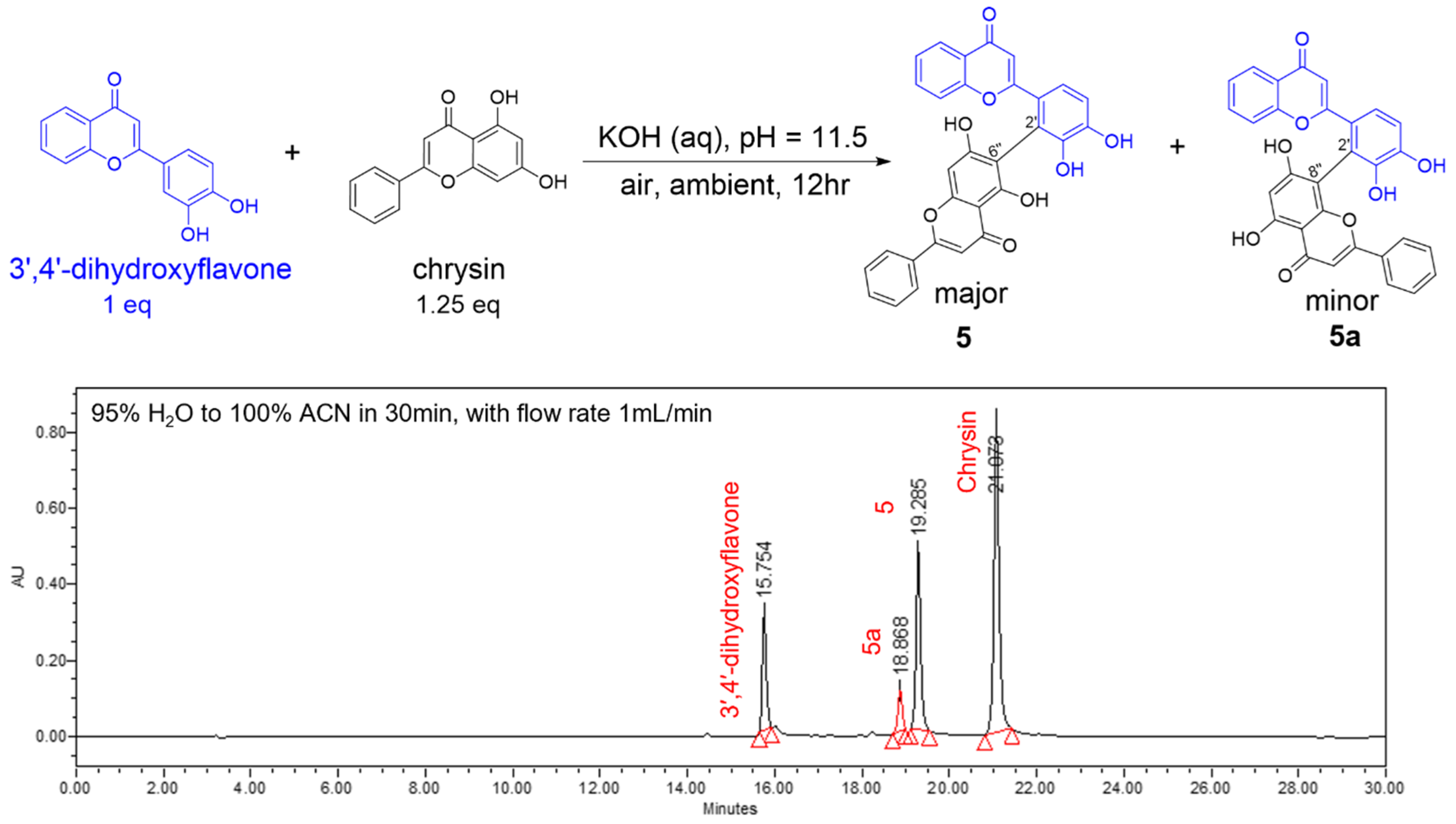
3.1. Synthesis of Biflavonoids Under Alkaline Conditions
3.2. HPLC Analysis of the Reaction Profiles
3.3. Separation and Characterization of Biflavones
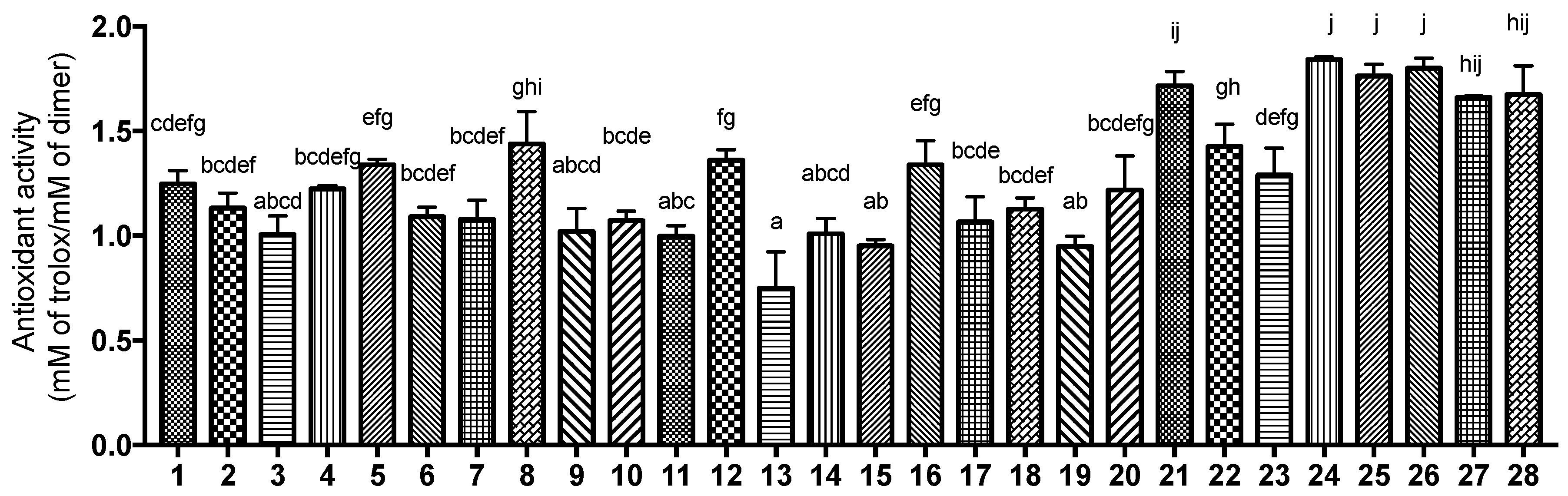
3.4. Antioxidant Activity Measured by DPPH Assay
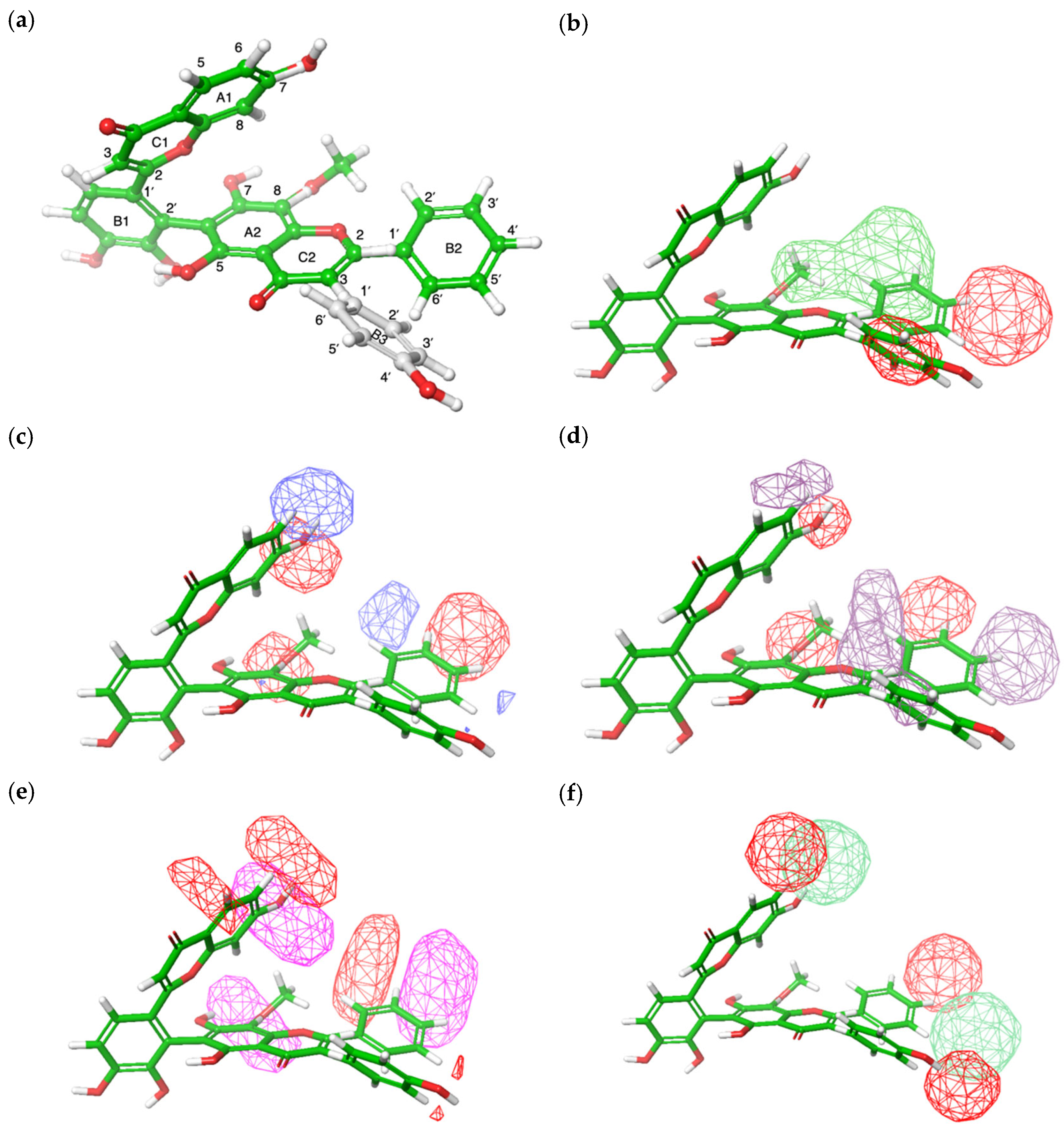
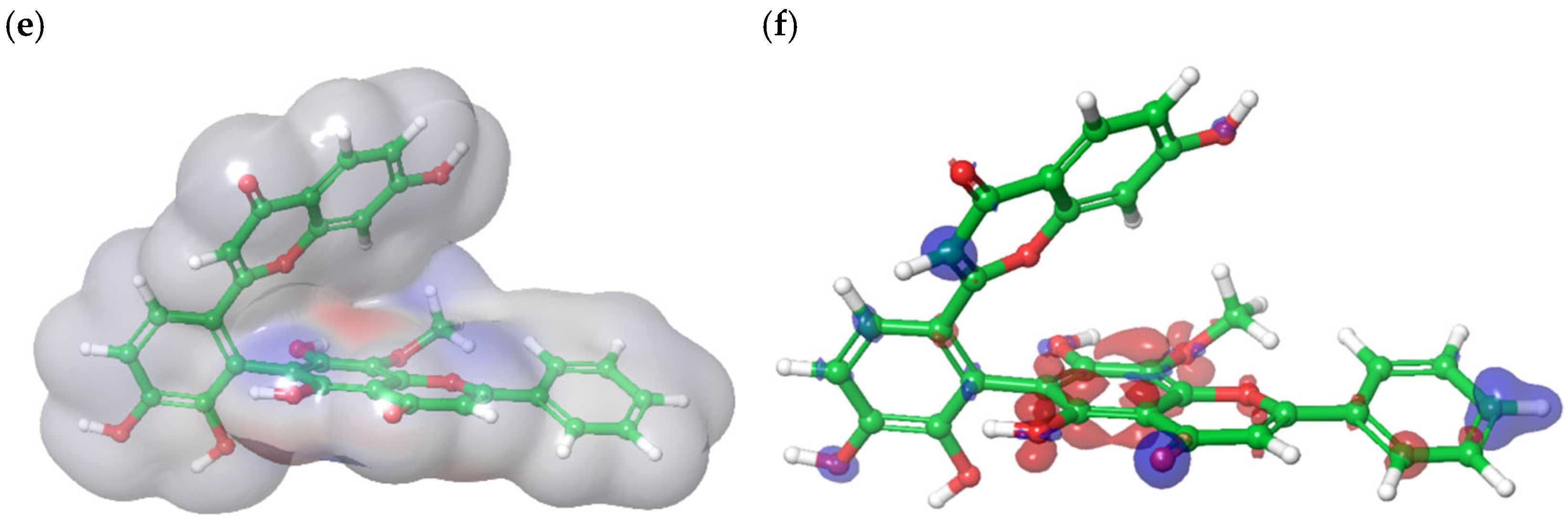
3.5. Field-Based 3D-QSAR Analysis
3.6. Density Functional Theory Calculation of Molecular Orbital Energies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclature
References
- Frota, L.S.; Alves, D.R.; Marinho, M.M.; da Silva, L.P.; Almeida Neto, F.W.Q.; Marinho, E.S.; de Morais, S.M. Antioxidant and anticholinesterase activities of amentoflavone isolated from Ouratea fieldingiana (Gardner) Engl. through in vitro and chemical-quantum studies. J. Biomol. Struct. Dyn. 2023, 41, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Hwang, L.; Lee, M.; Lee, K.Y.; Ahn, M.J.; Sung, S.H. Neuroprotective biflavonoids of Chamaecyparis obtusa leaves against glutamate-induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 2014, 64, 397–402. [Google Scholar] [CrossRef]
- Li, Q.; Ye, T.; Long, T.; Peng, X. Ginkgetin exerts anti-inflammatory effects on cerebral ischemia/reperfusion-induced injury in a rat model via the TLR4/NF-kappaB signaling pathway. Biosci. Biotechnol. Biochem. 2019, 83, 675–683. [Google Scholar] [CrossRef]
- Alharbi, M.O.; Dutta, B.; Goswami, R.; Sharma, S.; Lei, K.Y.; Rahaman, S.O. Identification and functional analysis of a biflavone as a novel inhibitor of transient receptor potential vanilloid 4-dependent atherogenic processes. Sci. Rep. 2021, 11, 8173. [Google Scholar] [CrossRef]
- Coulerie, P.; Eydoux, C.; Hnawia, E.; Stuhl, L.; Maciuk, A.; Lebouvier, N.; Canard, B.; Figadere, B.; Guillemot, J.C.; Nour, M. Biflavonoids of Dacrydium balansae with potent inhibitory activity on dengue 2 NS5 polymerase. Planta Med. 2012, 78, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Flavin, M.T.; Schure, R.; Chen, F.-C.; Sidwell, R.; Barnard, D.I.; Huffmann, J.H.; Kern, E.R. Antiviral activities of biflavonoids. Planta Med. 1999, 65, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Park, I.; Lee, J.; Shukla, S.; Nile, S.H.; Chun, H.S.; Khan, I.; Oh, S.Y.; Lee, H.; Huh, Y.S.; et al. Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 2019, 271, 239–247. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.; Liu, S.; Wang, L.; Wang, J. Anticytotoxin effects of amentoflavone to pneumolysin. Biol. Pharm. Bull. 2017, 40, 61–67. [Google Scholar] [CrossRef]
- Jung, H.J.; Sung, W.S.; Yeo, S.-H.; Kim, H.S.; Lee, I.-S.; Woo, E.-R.; Lee, D.G. Antifungal effect of amentoflavone derived from Selaginella tamariscina. Arch. Pharmacal Res. 2006, 29, 746–751. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Xia, Z.M.; Tian, Y.; Zhang, D.; Rui, W.J.; Dong, J.X.; Xiao, F.J. Anticancer effects of five biflavonoids from Ginkgo biloba l. male flowers in vitro. Molecules 2019, 24, 1496. [Google Scholar] [CrossRef]
- Chiang, C.H.; Yeh, C.Y.; Chung, J.G.; Chiang, I.T.; Hsu, F.T. Amentoflavone induces apoptosis and reduces expression of anti-apoptotic and metastasis-associated proteins in bladder cancer. Anticancer. Res. 2019, 39, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yang, Y.; Guo, Z.-J.; Cai, R.-L.; Hashida, H.; Li, H.-X. Amentoflavone inhibits colorectal cancer epithelial-mesenchymal transition via the miR-16-5p/HMGA2/β-catenin pathway. Ann. Transl. Med. 2022, 10, 1009. [Google Scholar] [CrossRef]
- Sirimangkalakitti, N.; Juliawaty, L.D.; Hakim, E.H.; Waliana, I.; Saito, N.; Koyama, K.; Kinoshita, K. Naturally occurring biflavonoids with amyloid β aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg. Med. Chem. Lett. 2019, 29, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X. Proceedings of chemistry, pharmacology, pharmacokinetics and synthesis of biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.; Ding, A.; Li, S.F. A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules 2017, 22, 299. [Google Scholar] [CrossRef]
- Tatlı Çankaya Iİ, A.-O.; Devkota, H.A.-O.; Zengin, G.A.-O.; Šamec, D.A.-O. Neuroprotective potential of biflavone ginkgetin: A review. Life 2023, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hoshino, Y.; Matsuyama, H.; Kohari, Y. Efficient synthesis of biflavones having a ring-a ring of two flavone units using suzuki cross-coupling reactions. Heterocycles 2010, 81, 1871–1879. [Google Scholar] [CrossRef]
- Rahman, M.; Riaz, M.; Desai, U.R. Synthesis of biologically relevant biflavanoids—A review. Chem. Biodivers. 2007, 4, 2495–2527. [Google Scholar] [CrossRef]
- Yang, X.; Lim, S.H.M.; Lin, J.; Wu, J.; Tang, H.; Zhao, F.; Liu, F.; Sun, C.; Shi, X.; Kuang, Y.; et al. Oxygen mediated oxidative couplings of flavones in alkaline water. Nat. Commun. 2022, 13, 6424. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Report. 2002, 7, 41–46. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, D.; Li, X.-Z.; Jiang, Z.; Tan, C.; Wei, X.-Y.; Ling, J.; Jing, J.; Liu, F.; Li, N. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Sci. Rep. 2017, 7, 10729. [Google Scholar] [CrossRef]
- Safna Hussan, K.P.; Shahin Thayyil, M.; Rajan, V.K.; Muraleedharan, K. DFT studies on global parameters, antioxidant mechanism and molecular docking of amlodipine besylate. Comput. Biol. Chem. 2019, 80, 46–53. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A comprehensive review of free radicals, oxidative stress, and antioxidants: Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Frimayanti, N.; Yam, M.L.; Lee, H.B.; Othman, R.; Zain, S.M.; Rahman, N.A. Validation of quantitative structure-activity relationship (QSAR) model for photosensitizer activity prediction. Int. J. Mol. Sci. 2011, 12, 8626–8644. [Google Scholar] [CrossRef]
- Teles Fujishima, M.A.; Silva, N.D.S.R.d.; Ramos, R.D.S.; Batista Ferreira, E.F.; Santos, K.L.B.d.; Silva, C.H.T.d.P.d.; Silva, J.O.d.; Campos Rosa, J.M.; Santos, C.B.R.d. An antioxidant potential, quantum-chemical and molecular docking study of the major chemical constituents present in the leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef]
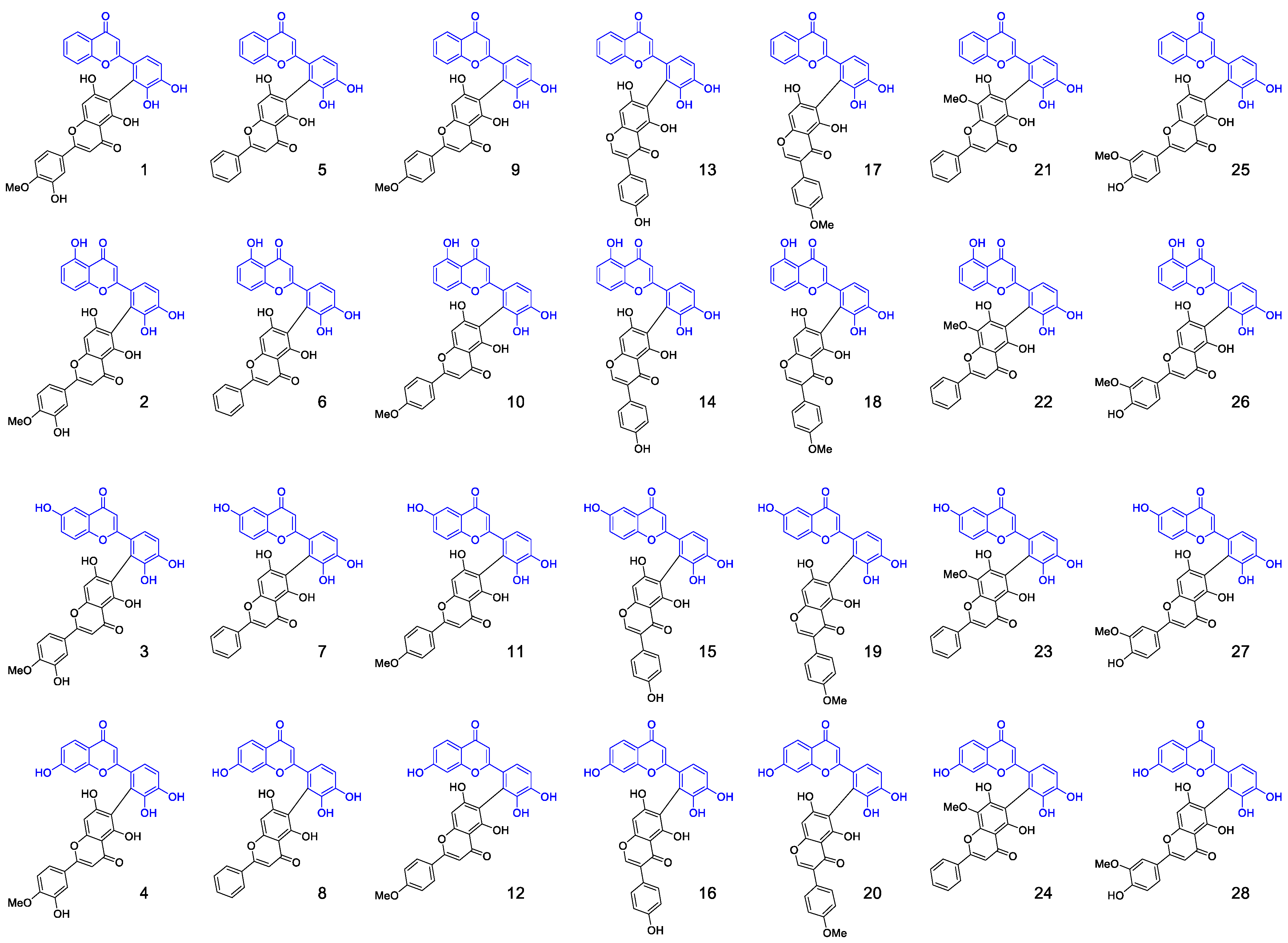


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yang, X.; Zhang, Q.; Toy, J.Y.H.; Huang, D. Food Grade Synthesis of Hetero-Coupled Biflavones and 3D-Quantitative Structure–Activity Relationship (QSAR) Modeling of Antioxidant Activity. Antioxidants 2025, 14, 742. https://doi.org/10.3390/antiox14060742
Zheng H, Yang X, Zhang Q, Toy JYH, Huang D. Food Grade Synthesis of Hetero-Coupled Biflavones and 3D-Quantitative Structure–Activity Relationship (QSAR) Modeling of Antioxidant Activity. Antioxidants. 2025; 14(6):742. https://doi.org/10.3390/antiox14060742
Chicago/Turabian StyleZheng, Hongling, Xin Yang, Qiuyu Zhang, Joanne Yi Hui Toy, and Dejian Huang. 2025. "Food Grade Synthesis of Hetero-Coupled Biflavones and 3D-Quantitative Structure–Activity Relationship (QSAR) Modeling of Antioxidant Activity" Antioxidants 14, no. 6: 742. https://doi.org/10.3390/antiox14060742
APA StyleZheng, H., Yang, X., Zhang, Q., Toy, J. Y. H., & Huang, D. (2025). Food Grade Synthesis of Hetero-Coupled Biflavones and 3D-Quantitative Structure–Activity Relationship (QSAR) Modeling of Antioxidant Activity. Antioxidants, 14(6), 742. https://doi.org/10.3390/antiox14060742








