Plant-Derived Nanovesicles from Soaked Rice Water: A Novel and Sustainable Platform for the Delivery of Natural Anti-Oxidant γ-Oryzanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PDNV Isolation from Rice Grains (RGs) and Soaked Rice Water (SRW)
2.3. PDNV Characterization for Size, Zetapotential and Morphology
2.4. Total Lipid and Total Protein Determination of PDNVs
2.5. Thin-Layer Chromatography Analysis of PDNV Lipids
2.6. HPLC Analysis of γ-Oryzanol from PDNVs
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Intracellular Uptake Assay and Inhibitor Studies
2.10. Measurement of Intracellular ROS
2.11. Statistical Methods
3. Results and Discussion
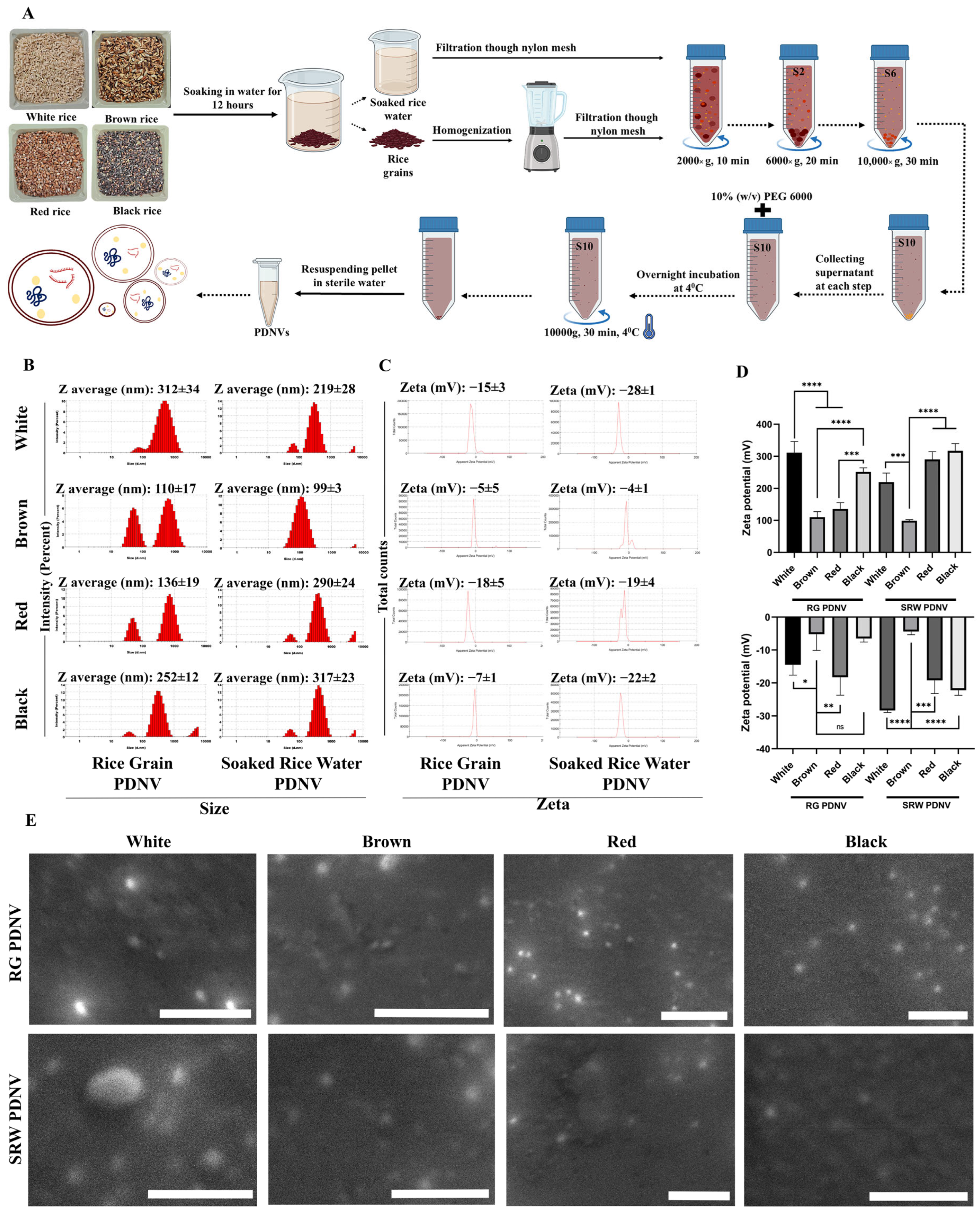
3.1. Isolation of PDNVs from Rice Grain (RG) and Soaked Rice Water (SRW) from Different Rice Varieties
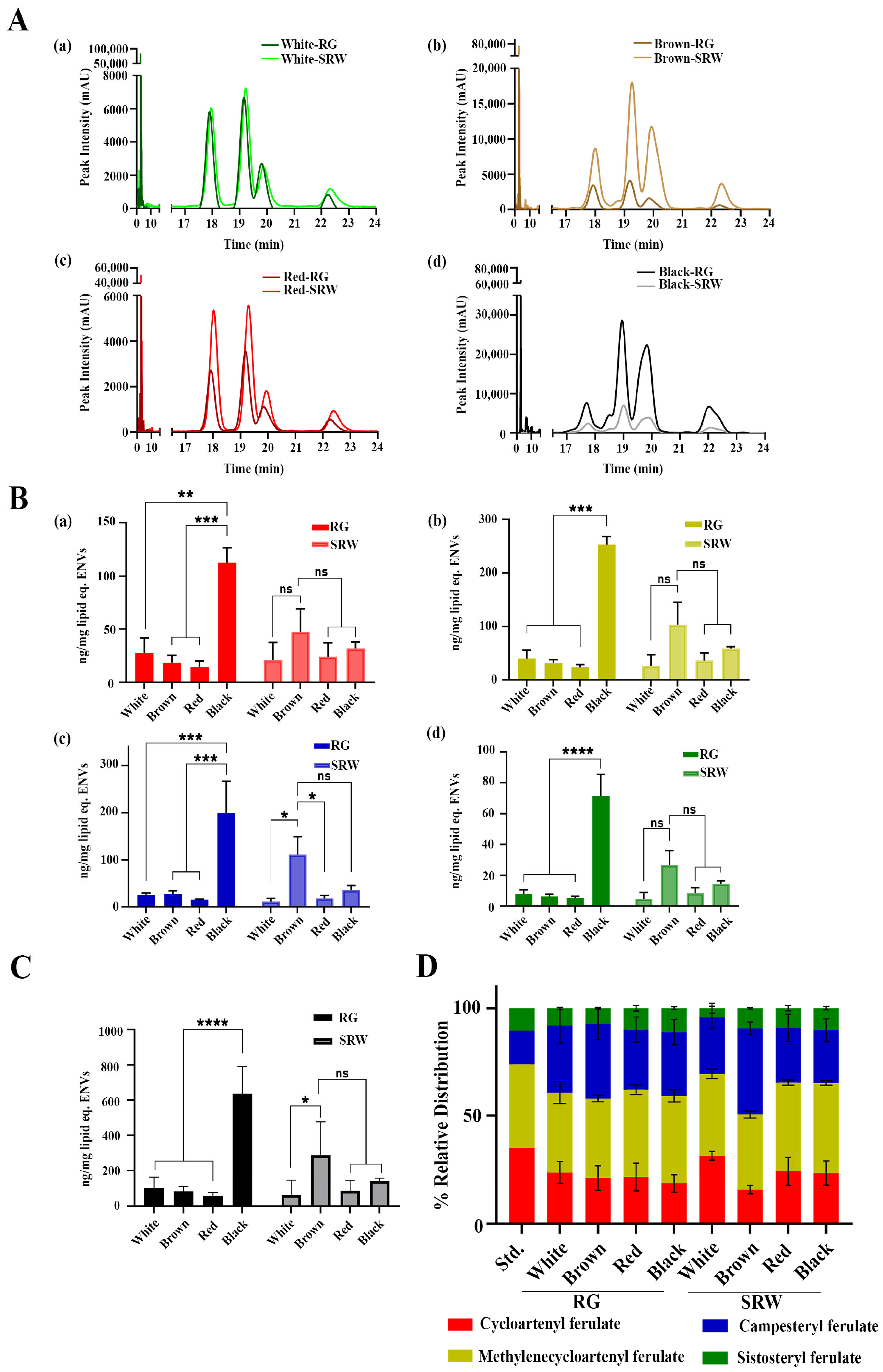
3.2. Both RG and SRW PDNVs Contain GO
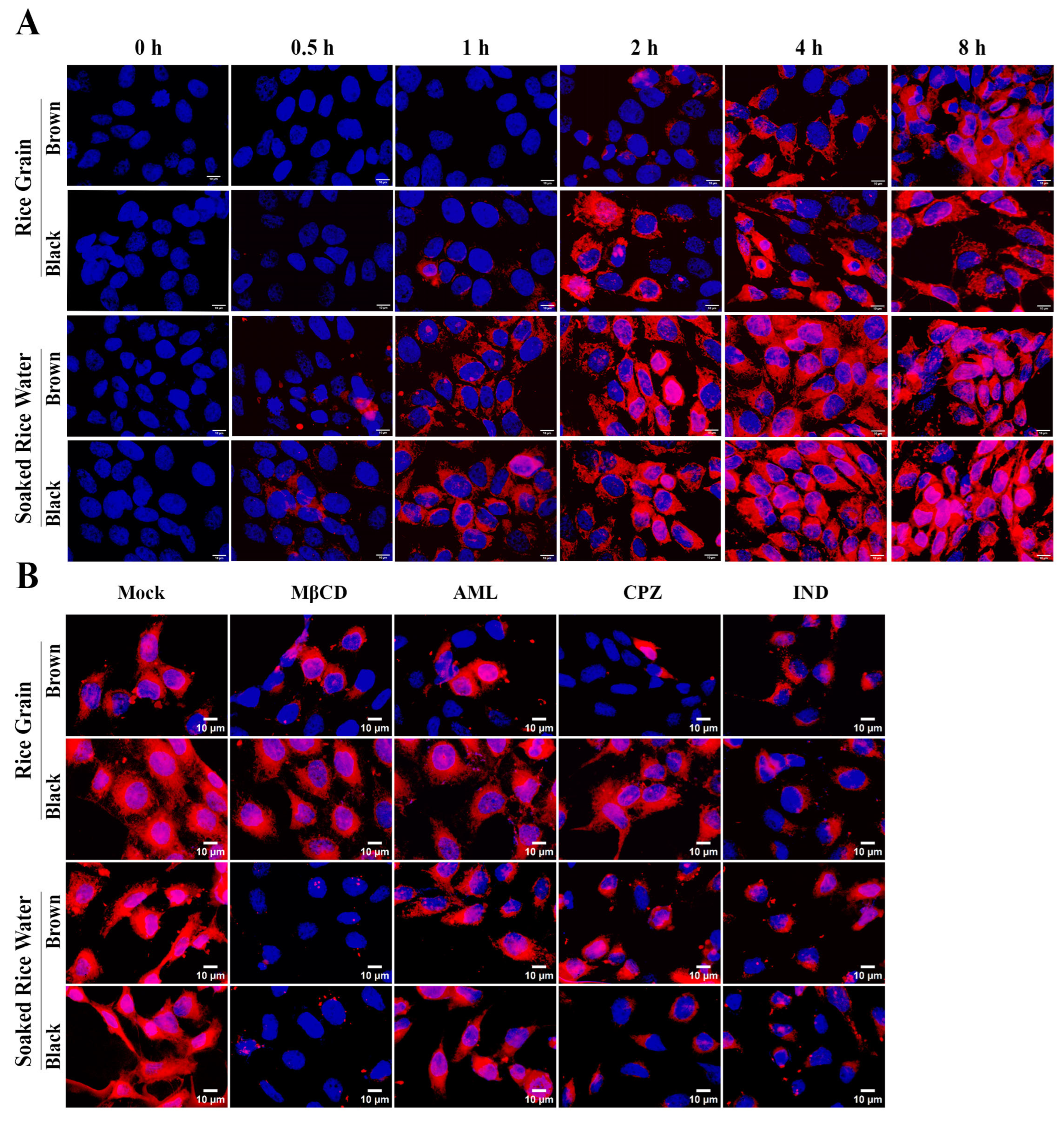
3.3. Both RG and SRW PDNVs Are Non-Toxic to Keratinocytes and Are Taken Up by Keratinocytes
3.4. Brown SRW PDNVs Prevent H2O2-Induced Oxidative Stress in HaCaT Keratinocytes Better than Native GO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P.P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.L.; Chitchumroonchokchai, C.; Ariyapitipun, T.; Adisakwattana, S.; Maekynen, K. Effect of gamma-oryzanol on the bioaccessibility and synthesis of cholesterol. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 49–56. [Google Scholar]
- Emami, S.A.; Tayarani-Najaran, Z.; Akaberi, M.; Ramazani, E. Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms. Curr. Pharm. Des. 2021, 27, 2299–2316. [Google Scholar] [CrossRef]
- Lai, M.C.; Hong, T.-Y.; Liu, Y.-S.; Liu, I.-M. γ-Oryzanol: A nutrient-rich ingredient for promoting wound healing. Heliyon 2025, 11, e42551. [Google Scholar] [CrossRef]
- Pan, Y.; Cai, L.; He, S.; Zhang, Z. Pharmacokinetics study of ferulic acid in rats after oral administration of γ-oryzanol under combined use of Tween 80 by LC/MS/MS. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 143–150. [Google Scholar]
- Saldo, J.; Jaime-Báez, R.; González-Soto, R.A. Comparison of Gamma-Oryzanol Nanoemulsions Fabricated by Different High Energy Techniques. Foods 2024, 13, 2256. [Google Scholar] [CrossRef]
- Ito, J.; Sawada, K.; Hashimoto, H.; Nakagawa, K.; Matsuki, M.; Rahmania, H.; Miyazawa, T. Absorption and Metabolism of γ-Oryzanol, a Characteristic Functional Ingredient in Rice Bran. J. Nutr. Sci. Vitaminol. 2019, 65, S180–S184. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kobayashi, E.; Ito, J.; Shimizu, N.; Kokumai, T.; Hashimoto, H.; Eitsuka, T.; Nakagawa, K. Comparison of Blood Profiles of γ-Oryzanol and Ferulic Acid in Rats after Oral Intake of γ-Oryzanol. Nutrients 2019, 11, 1174. [Google Scholar] [CrossRef]
- Ghaderi, S.; Hamishehkar, H.; Mohammadhassani, Z.; Ghanbarzadeh, S. Formulation of gammaoryzanol-loaded nanoparticles for potential application in fortifying food products. Adv. Pharm. Bull. 2014, 4, 549–554. [Google Scholar] [CrossRef]
- Kumagai, N.; Ito, J.; Shoji, N.; Nakagawa, K.; Suzuki, A.; Takahashi, M.; Parida, I.S. Effect of microemulsion system on water dispersibility and bioavailability of γ-oryzanol. Biosci. Biotechnol. Biochem. 2025, 89, 633–637. [Google Scholar] [CrossRef]
- Panchal, S.; Mishra, N.; Butani, S.; Rawal, T.; Bhatt, A.; Tyagi, R.K.; Jha, A. Chitosan Nanoparticles of Gamma-Oryzanol: Formulation, Optimization, and In vivo Evaluation of Anti-hyperlipidemic Activity. AAPS PharmSciTech 2018, 19, 1894–1907. [Google Scholar] [CrossRef]
- Al-Karagoly, H.; Albukhaty, S.; Abomughaid, M.M.; Jasim, A.J.; Sulaiman, G.M.; Mohammed, H.A.; Jabir, M.S.; Abomughayedh, A.M. Liposome Nanocarriers Based on γ Oryzanol: Preparation, Characterization, and In Vivo Assessment of Toxicity and Antioxidant Activity. ACS Omega 2024, 9, 3554–3564. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Munekata, P.E.S.; Pateiro, M.; Sarriés, M.V.; Domínguez, R.; Lorenzo, J.M. Encapsulation techniques to increase lipid stability. In Food Lipids; Lorenzo, J.M., Munekata, P.E.S., Pateiro, M., Barba, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 413–459. [Google Scholar]
- Melwita, E.; Ju, Y.-H.; Zullaikah, S. Isolation of oryzanol from crude rice bran oil. Bioresour. Technol. 2009, 100, 299–302. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Serra, A.T.; Bento-Silva, A.; Fernández, N.; Cardeira, M.; Baixinho, J.P.; Bronze, M.D.R.; Partidário, A.M.C. Optimization of Supercritical Fluid Extraction for the Recovery of γ-Oryzanol-Rich Extracts with Improved Bioactivity from Rice Bran. Antioxidants 2025, 14, 206. [Google Scholar] [CrossRef]
- Ruiz-Mercado, C.A.; Rocha-Uribe, J.A.; Novelo-Pérez, J.I. Cost estimation for CO2 supercritical extraction systems and manufacturing cost for habanero chili. J. Supercrit. Fluids 2014, 93, 38–41. [Google Scholar] [CrossRef]
- Merlin, D.; Srinivasan, S.; Prasad, M.; Xu, C.; Zhang, Y.; Han, M.K.; Zhang, M.; Wang, L.; Xiao, B.; Zhang, Z.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Innes, R.W.; Rutter, B.D. Growing pains: Addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020, 228, 1505–1510. [Google Scholar] [CrossRef]
- Han, H.S.; Park, J.H.; Choi, K.Y.; Kim, M.; Ly, N.P. Plant-derived nanovesicles: Current understanding and applications for cancer therapy. Bioact. Mater. 2023, 22, 365–383. [Google Scholar] [CrossRef]
- Suttles, J.; Zhuang, X.; Teng, Y.; Zhang, L.; Tseng, M.; Mu, J.; Deng, Z.; Zhang, H.-G.; Miller, D.; Yan, J.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, E.; Zini, N.; Perut, F.; Baldini, N.; Avnet, S.; Roncuzzi, L. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, J.; Li, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2021, 17, 53–69. [Google Scholar] [CrossRef]
- Kasiappan, R.; Chaudhari, S.R.; Kalarikkal, S.P.; Prasad, D.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef]
- Merlin, D.; Zhang, M.; Yang, C. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Sundaram, G.M.; Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef]
- Nawas, Z.Y.; Zamil, D.H.; Khan, R.M.; Braun, T.L. Dermatological uses of rice products: Trend or true? J. Cosmet. Dermatol. 2022, 21, 6056–6060. [Google Scholar] [CrossRef]
- Marto, J.; Neves, Â.; Gonçalves, L.M.; Pinto, P.; Almeida, C.; Simões, S. Rice Water: A Traditional Ingredient with Anti-Aging Efficacy. Cosmetics 2018, 5, 26. [Google Scholar] [CrossRef]
- de la Canal, L.; Pinedo, M.; Lousa, C.d.M. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef]
- RLogozzi, M.; Spada, M.; Dolo, V.; Fais, S.; Di Raimo, R.; Mizzoni, D. Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H2O2-Treated Mice. Antioxidants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Frings, C.S.; Dunn, R.T. A Colorimetric Method for Determination of Total Serum Lipids Based on the Sulfo-phospho-vanillin Reaction. Am. J. Clin. Pathol. 1970, 53, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Varga, Z.; Vukman, K.V.; Mihály, J.; Visnovitz, T.; Koncz, A.; Sódar, B.W.; Székács, I.; Tóth, E.Á.; Buzás, E.I.; Lőrincz, P.; et al. An improved 96 well plate format lipid quantification assay for standardisation of experiments with extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565263. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 85, 76–85. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Vidallon, M.L.P.; Villar, M.A.L. Food Bioscience Nanostructured lipid carrier for bioactive rice bran gamma-oryzanol. Food Biosci. 2022, 50, 102064. [Google Scholar] [CrossRef]
- Kim, H.; Jang, H. c-Oryzanol-Rich Black Rice Bran Extract Enhances the Innate Immune Response. J. Med. Food 2017, 20, 855–863. [Google Scholar] [CrossRef]
- Soga, T.; Sugito, N.; Morikawa, K.; Heishima, K.; Kuranaga, Y.; Ito, Y.; Ito, T.; Akao, Y. Plant hvu-MIR168-3p enhances expression of glucose transporter 1 (SLC2A1) in human cells by silencing genes related to mitochondrial electron transport chain complex I. J. Nutr. Biochem. 2022, 101, 108922. [Google Scholar] [CrossRef]
- Jiao, R.; Jin, Z.; Huang, Y.; Lin, X.; Na, J.; Liu, X. Plant-derived exosome-like nanovesicles: A novel nanotool for disease therapy. Heliyon 2024, 10, e30630. [Google Scholar] [CrossRef]
- Kopanchuk, S.; Rinken, T.; Viil, J.; Lättekivi, F.; Ord, J.; Godakumara, K.; Dissanayake, K.; Midekessa, G.; Rinken, A.; Fazeli, A.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Balogh, A.; Szabó-Taylor, K.; Németh, A.; Szabó, T.G.; Pálóczi, K.; Sódar, B.; Kittel, Á.; György, B.; Pállinger, É.; et al. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS ONE 2015, 10, e0121184. [Google Scholar] [CrossRef]
- Thakur, B.K.; Patnam, S.; Singh, A.D.; Sarwareddy, K.K.; Ponamgi, S.; Sesuraj, B.A.; Manda, V.S. Harnessing tomato-derived small extracellular vesicles as drug delivery system for cancer therapy. Future Sci. OA 2025, 11, 2461956. [Google Scholar] [CrossRef]
- Kumar, M.N.; Kalarikkal, S.P.; Bethi, C.M.S.; Sundaram, G.M.; Narayanan, J.; Singh, S.N. An eco-friendly one-pot extraction process for curcumin and its bioenhancer, piperine, from edible plants in exosome-like nanovesicles. Green Chem. 2023, 25, 6472–6488. [Google Scholar] [CrossRef]
- Narayanan, J.; Kalarikkal, S.P.; Sundaram, G.M.; Jayaram, Y.; Kumar, M.N. Protocol to produce plant-based hybrid nanovesicles from fresh turmeric and pepper using polyethylene glycol. STAR Protoc. 2024, 5, 102924. [Google Scholar] [CrossRef]
- Imam, M.U.; Zamri, N.D.M.; Ghafar, S.A.A.; Ismail, M. Antioxidative Effects of Germinated Brown Rice-Derived Extracts on H2O2-Induced Oxidative Stress in HepG2 Cells. Evid. Based Complement. Alternat. Med. 2014, 2014, 371907. [Google Scholar] [CrossRef]
- Cros, G.; Yokota, T.; Crozier, A.; Pereira-Caro, G. Phytochemical profiles of black, red, brown, and white rice from the Camargue region of France. J. Agric. Food. Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef]
- Jung, T.-D.; Shin, G.-H.; Kim, J.-M.; Choi, S.-I.; Lee, J.-H.; Lee, O.-H.; Oh, S.K.; Lee, S.J.; Park, S.J.; Woo, K.S. Comparative Analysis of γ-Oryzanol, β-Glucan, Total Phenolic Content and Antioxidant Activity in Fermented Rice Bran of Different Varieties. Nutrients 2017, 9, 571. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Diversity in γ-oryzanol profiles of Japanese black-purple rice varieties. J. Food Sci. Technol. 2019, 56, 2778–2786. [Google Scholar] [CrossRef]
- Zamani, E.; Porteghali, P.; Ghorbani, Z.; Ahmadnia, Z.; Shoaibinobarian, N.; Mahdavi-Roshan, M.; Salari, A. Supplementing the standard diet with brown rice bran powder might effectively improve the metabolic syndrome characteristics and antioxidant status: An open label randomized controlled trial. Food Funct. 2025, 16, 750–762. [Google Scholar] [CrossRef]
- Innes, R.W.; Rutter, B.D. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant. Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Heydari, S.; Ranjkesh, M.; Anoush, B.; Kouhsoltani, M.; Javadzadeh, Y.; Hamishehkar, H. Nanoethosomal formulation of gammaoryzanol for skin-aging protection and wrinkle improvement: A histopathological study. Drug Dev. Ind. Pharm. 2017, 43, 1154–1162. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar] [CrossRef]
- Abuharfeil, N.; Almujally, H.; Sharaireh, A. Novel Exosomal miRNA Expression in Irradiated Human Keratinocytes. Int. J. Mol. Sci. 2024, 25, 12477. [Google Scholar] [CrossRef]
- Licastro, D.; Zanotti, F.; Trentini, M.; Camponogara, F.; Tiengo, E.; Degasperi, M.; Zavan, B.; Lovatti, L. An Apple a Day Keeps the Doctor Away: Potential Role of miRNA 146 on Macrophages Treated with Exosomes Derived from Apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H. Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef]
- Bordi, F.; Mura, F.; Sarra, A.; D’oria, V.; Fratantonio, D.; Postorino, P.; De Robertis, M. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, J.; Zhang, M.; Wang, T.; Zhao, J.; Li, Q.-Y.; Yang, H.-Q.; Li, M.; Duan, R.; Yang, X.-X. Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation. Int. J. Mol. Sci. 2025, 26, 2253. [Google Scholar] [CrossRef]
- Oshima, T.; Kamei, I.; Yamasaki, Y.; Sonoda, H.; Nishiyama, K.; Furusho, R.; Kimura, H.; Ogawa, K.; Ikeda, M.; Yamasaki, M. Onion (Allium cepa L.)-Derived Nanoparticles Inhibited LPS-Induced Nitrate Production, However, Their Intracellular Incorporation by Endocytosis Was Not Involved in This Effect on RAW264 Cells. Molecules 2021, 26, 2763. [Google Scholar] [CrossRef]
- Arai, M.; Komori, H.; Fujita, D.; Tamai, I. Uptake Pathway of Apple-derived Nanoparticle by Intestinal Cells to Deliver its Cargo. Pharm. Res. 2021, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Sasaki, D.; Itakura, S.; Todo, H.; Kusamori, K.; Nishikawa, M.; Takayama, Y. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci. Rep. 2021, 11, 22818. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhou, Y.; Vong, C.T.; Li, H.; Xiao, Y.; Gao, C.; Chen, Z.; Hao, W.; Zhu, Y.; Wang, S.; et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics 2022, 12, 5596–5614. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Catanzaro, M.; Memo, M.; Marziano, M.; Rungratanawanich, W.; Guarienti, M.; Lanni, C.; Abate, G.; Uberti, D.; Serafini, M.M. Characterization of the Antioxidant Effects of γ-Oryzanol: Involvement of the Nrf2 Pathway. Oxid. Med. Cell Longev. 2018, 2018, 2987249. [Google Scholar] [CrossRef]
- Wen, L.; Ma, C.; Zhu, L.; Luo, C.; Yi, J.; Ou, Z.; Jiang, W.; Tan, Z.; Wu, J.; Huang, L. γ-Oryzanol suppresses cell apoptosis by inhibiting reactive oxygen species-mediated mitochondrial signaling pathway in H(2)O(2)-stimulated L02 cells. Biomed. Pharmacother. 2020, 121, 109554. [Google Scholar] [CrossRef]
- Manno, M.; Pistocchi, A.; Conigliaro, A.; Raccosta, S.; Gasparro, R.; Cafora, M.; Alessandro, R.; Ganji, N.R.; Urzì, O.; Tinnirello, V.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; He, Q.; Xie, F.; Pan, X.; Zhang, S.; Karrar, E.; Wu, W.; Li, J.; Gu, R.; et al. Hybrid nanovesicles derived from grapes and tomatoes with synergistic antioxidative activity. Biomater. Sci. 2024, 12, 5631–5643. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravilla, J.; Rajendran, S.; Basavaraj, V.M.; Satheeshan, G.; Narayanan, J.; Venkatesh, T.; Sundaram, G.M. Plant-Derived Nanovesicles from Soaked Rice Water: A Novel and Sustainable Platform for the Delivery of Natural Anti-Oxidant γ-Oryzanol. Antioxidants 2025, 14, 717. https://doi.org/10.3390/antiox14060717
Ravilla J, Rajendran S, Basavaraj VM, Satheeshan G, Narayanan J, Venkatesh T, Sundaram GM. Plant-Derived Nanovesicles from Soaked Rice Water: A Novel and Sustainable Platform for the Delivery of Natural Anti-Oxidant γ-Oryzanol. Antioxidants. 2025; 14(6):717. https://doi.org/10.3390/antiox14060717
Chicago/Turabian StyleRavilla, Jahnavi, Soundaram Rajendran, Vidya M. Basavaraj, Greeshma Satheeshan, Janakiraman Narayanan, Thejaswini Venkatesh, and Gopinath M. Sundaram. 2025. "Plant-Derived Nanovesicles from Soaked Rice Water: A Novel and Sustainable Platform for the Delivery of Natural Anti-Oxidant γ-Oryzanol" Antioxidants 14, no. 6: 717. https://doi.org/10.3390/antiox14060717
APA StyleRavilla, J., Rajendran, S., Basavaraj, V. M., Satheeshan, G., Narayanan, J., Venkatesh, T., & Sundaram, G. M. (2025). Plant-Derived Nanovesicles from Soaked Rice Water: A Novel and Sustainable Platform for the Delivery of Natural Anti-Oxidant γ-Oryzanol. Antioxidants, 14(6), 717. https://doi.org/10.3390/antiox14060717








