CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.1.1. Cell Culture and Treatment
2.1.2. Cell Viability Assay
2.1.3. Detection of Intracellular ROS, Mitochondrial ROS, and Mitochondrial Membrane Potential
2.1.4. Cellular Ferroptosis Marker Assays
2.1.5. Real-Time Quantitative PCR
2.1.6. Western Blot Analysis
2.2. In Vivo Experimental Procedures
2.2.1. Animal Housing and Administration
2.2.2. Body Weight Measurement
2.2.3. Liver Weight and Organ Index
2.2.4. Hematoxylin and Eosin (H&E) Staining
2.2.5. Prussian Blue Staining
2.2.6. Fluorescence In Situ Hybridization (FISH) Experiment
2.2.7. Immunofluorescence Staining of Frozen Sections
2.2.8. Tissue Ferroptosis Marker Assays
2.2.9. Transmission Electron Microscopy
2.3. Statistical Analysis
3. Results
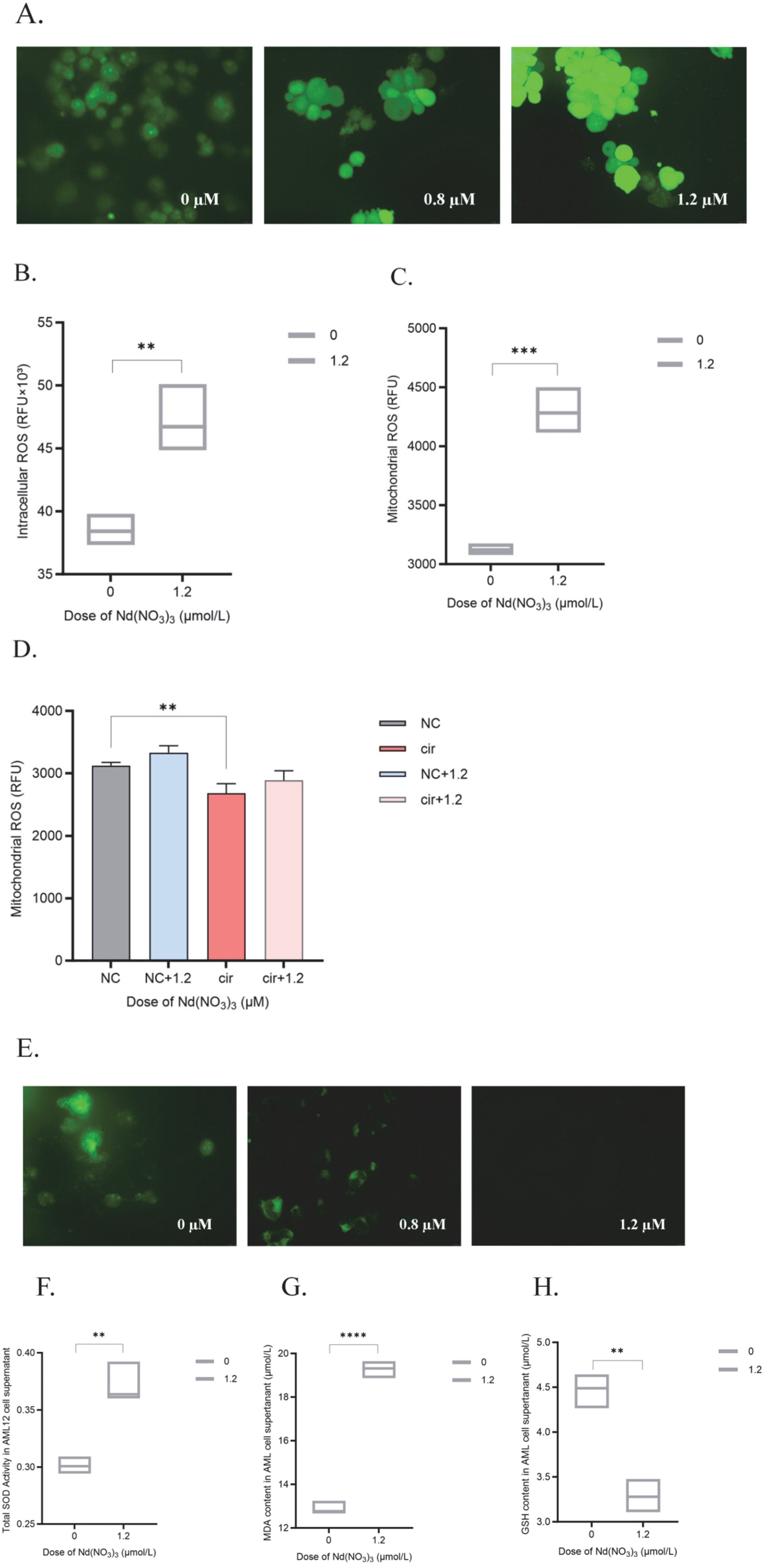
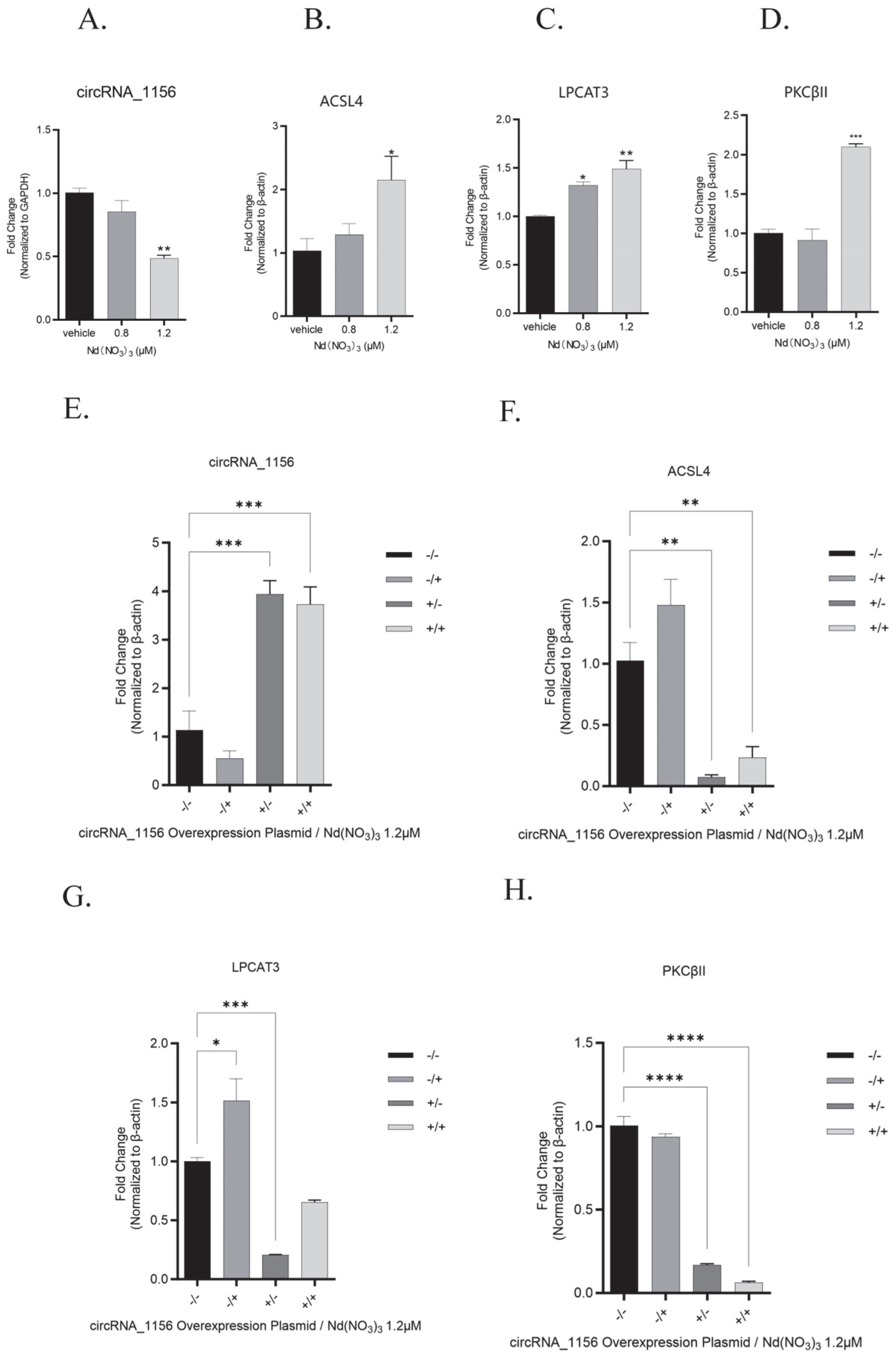
3.1. In Vitro Experimental Results
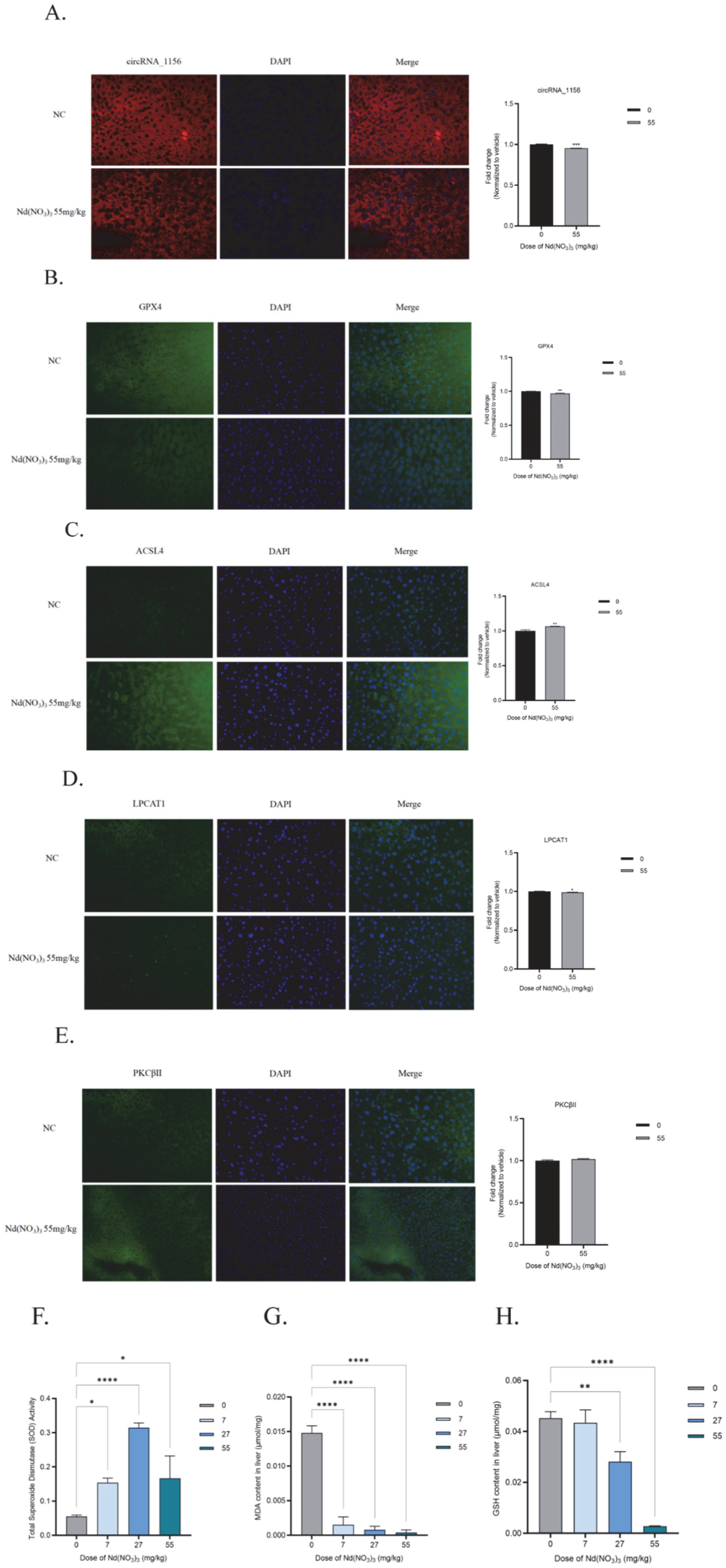
3.2. In Vivo Experimental Results
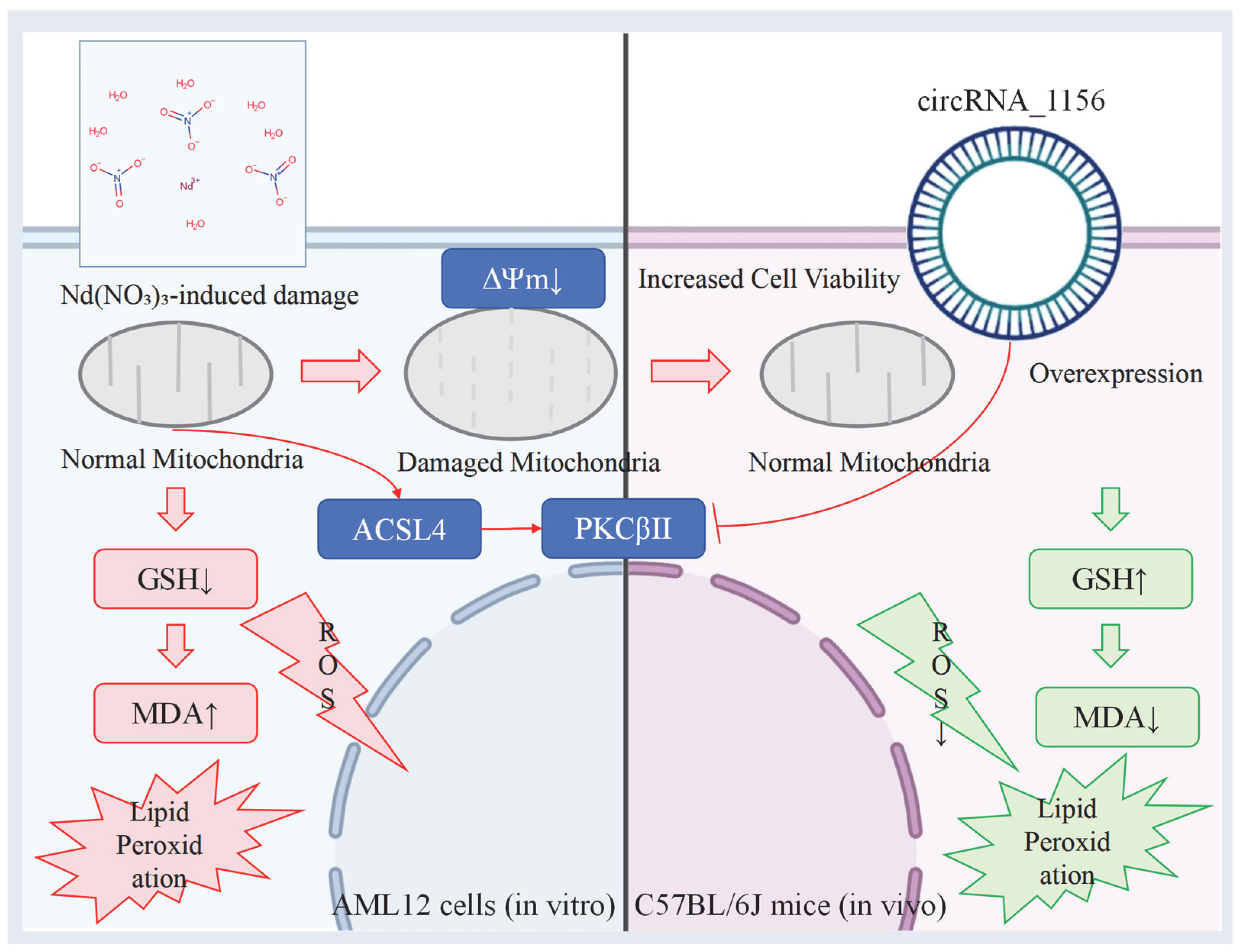
3.3. Comprehensive Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.F.; Jiang, J. Ferroptosis and cancer: Interlinkages and potential applications. Clin. Transl. Rep. 2023, 1, 12–20. [Google Scholar] [CrossRef]
- Fang, X.L.; Ding, S.Y.; Du, X.Z.; Wang, J.H.; Li, X.L. Ferroptosis—A Novel Mechanism with Multifaceted Actions on Stroke. Front. Neurol. 2022, 13, 881809. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.Y.; Liu, C.B.; Li, L.; Yang, M.; Jiang, N.; Luo, S.L.; Sun, L. Acyl-CoA synthase ACSL4: An essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. 2023, 136, 2521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, D.; Liu, J.B.; Tang, D.L.; Chen, X. Protein modification and degradation in ferroptosis. Redox Biol. 2024, 75, 103259. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xia, S.Y. Relationship Between ACSL4-Mediated Ferroptosis and Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 99–111. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Wang, X.; Xiong, F.; Hu, Z.; Qiao, X.; Yuan, X.; Wang, D. ACSL3 and ACSL4, Distinct Roles in Ferroptosis and Cancers. Cancers 2022, 14, 5896. [Google Scholar] [CrossRef]
- Mu¨ller, T.; Dewitz, C.; Schmitz, J.; Schro¨der, A.S.; Bra¨sen, J.H.; Stockwell, B.R.; Murphy, J.M.; Kunzendorf, U.; Krautwald, S. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell. Mol. Life Sci. 2017, 74, 3631–3645. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Song, S.J.; Fan, Z.W.; Li, W.T.; Jin, X.; Jiang, W.; Bai, J.; Shi, Z.Z. PKCiota Inhibits the Ferroptosis of Esophageal Cancer Cells via Suppressing USP14Mediated Autophagic Degradation of GPX4. Antioxidants 2024, 13, 114. [Google Scholar] [CrossRef]
- Tao, W.H.; Shan, X.S.; Zhang, J.X.; Liu, H.Y.; Wang, B.Y.; Wei, X.; Zhang, M.; Peng, K.; Ding, J.; Xu, S.X.; et al. Dexmedetomidine Attenuates Ferroptosis-Mediated Renal Ischemia/Reperfusion Injury and Inflammation by Inhibiting ACSL4 via α2-AR. Front. Pharmacol. 2022, 13, 782466. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, L.; Mäkitie, A.A.; Florek, E.; Czarnywojtek, A.; Saba, N.F.; Ferlito, A. Iron, Ferroptosis, and Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 15127. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, J.; Liao, Z.; Qiao, J.; Chen, C.; Ling, C.; Wang, H. An update on the therapeutic implications of long-chain acyl-coenzyme A synthetases in nervous system diseases. Front. Neurosci. 2022, 16, 1030512. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhang Huan Tian, J.F.; Hao, P.; Liu, L.; Zhou, Y.Q.; Zhang, J.; Song, X.T.; Ge, C.J. The Chains of Ferroptosis Interact in the Whole Progression of Atherosclerosis. J. Inflamm. Res. 2023, 16, 4575–4592. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F. Targeting ferroptosis as a prospective therapeutic approach for diabetic nephropathy. Ann. Med. 2024, 56, 2346543. [Google Scholar] [CrossRef]
- Resende, F.; Araújo, S.D.; Tavares, L.P.; Teixeira, M.M.; Costa, V.V. The Multifaceted Role of Annexin A1 in Viral Infections. Cells 2023, 12, 1131. [Google Scholar] [CrossRef]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Jiang, Y.; Bu, J.; Gu, X. Targeting ferroptosis, the achilles’ heel of breast cancer: A review. Front. Pharmacol. 2022, 13, 1036140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Chen, J.Q.; Lu, Y.; Bawcom, A.R.; Wu, J.J.; Ou, J.H.; Asara, J.M.; Armstrong, A.J.; Wang, Q.B.; Li, L.; et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J. Clin. Investig. 2023, 133, e166647. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhou, Y.; Feng, Y.T.; Hou, W.L.; Chen, Y.F.; Xing, Z.Z.; Zhang, Y.F.; Wei, Q.; Yin, Y.; Guo, J.; et al. Cyclin-dependent kinase 12 deficiency reprogrammes cellular metabolism to alleviate ferroptosis potential and promote the progression ofcastration-resistant prostate cancer. Clin. Transl. Med. 2024, 14, e1678. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, Y.Q.; Liu, B.H.; Zhou, H.; Wang, J.M.; Chen, Q.X. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 2020, 43, 147–158. [Google Scholar] [CrossRef]
- Hong, Z.H.; Liu, F.; Zhang, Z.G. Ubiquitin modification in the regulation of tumor immunotherapy resistance mechanisms and potential therapeutic targets. Exp. Hematol. Oncol. 2024, 13, 91. [Google Scholar] [CrossRef]
- Huang, L.L.; Zhang, L.; Zhang, Z.; Tan, F.Y.; Ma, Y.X.; Zeng, X.J.; Cao, D.; Deng, L.L.; Liu, Q.; Sun, H.; et al. Loss of nephric augmenter of liver regeneration facilitates acute kidney injury via ACSL4-mediated ferroptosis. J. Cell. Mol. Med. 2024, 28, e18076. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Tian, W.; Ye, Y.F.; Gu, W.; Bao, Z.Y.; Xu, L.; Sun, G.C.; Li, C.; Tu, Y.M.; Chao, H.L.; et al. Hsp90 induces ACSL4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.H.; Zhao, T.M.; Song, Y.; Lin, L.; Fan, X.F.; Cui, B.X.; Feng, H.J.; Wang, X.Y.; Yu, Q.X.; Zhang, J.; et al. The emerging role of ferroptosis in non-cancer liver diseases: Hype or increasing hope? Cell Death Dis. 2020, 11, 518. [Google Scholar] [CrossRef]
- Wang, W.X.; Wang, T.T.; Zhang, Y.; Deng, T.; Zhang, H.Y.; Ba, Y. Gastric cancer secreted miR-214-3p inhibits the anti-angiogenesis effect of apatinib by suppressing ferroptosis in vascular endothelial cells. Oncol. Res. 2024, 32, 489–502. [Google Scholar] [CrossRef]
- Tian, X.Y.; Li, S.Y.; Ge, G.Y. Apatinib Promotes Ferroptosis in Colorectal Cancer Cells by Targeting ELOVL6/ACSL4 Signaling. Cancer Manag. Res. 2021, 13, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Hui, D.C.; Pan, Z.Y.; Yu, Y.X.; Liu, L.; Yu, X.F.; Wu, C.; Sun, M.Y. Curcumin promotes ferroptosis in hepatocellular carcinoma via upregulation of ACSL4. J. Cancer Res. Clin. Oncol. 2024, 150, 429. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, S.Y.; Li, F.Z.; Lv, C.S.; Yang, Q.K. High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma. Biol. Direct 2021, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Song, J.B.; Yung, B.C.; Zhou, Z.J.; Wu, A.G.; Chen, X.Y. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, e1704007. [Google Scholar] [CrossRef]
- Cai, H.; Ren, Y.; Chen, S.; Wang, Y.; Chu, L. Ferroptosis and tumor immunotherapy: A promising combination therapy for tumors. Front. Oncol. 2023, 13, 1119369. [Google Scholar] [CrossRef]
- Jia, B.; Li, J.; Song, Y.; Luo, C. ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries. Int. J. Mol. Sci. 2023, 24, 10021. [Google Scholar] [CrossRef]
- Jo’zsef, L.; Zouki, C.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-κB and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef]
- Xia, L.P.; Yang, M.; Liu, Y. Portulaca oleracea L. polysaccharide inhibits ovarian cancer via inducing ACSL4-dependent ferroptosis. Aging 2024, 16, 5108. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, L.; Yang, R.; Xu, T. The recent advancements of ferroptosis in the diagnosis, treatment and prognosis of ovarian cancer. Front. Genet. 2023, 14, 1275154. [Google Scholar] [CrossRef] [PubMed]
- Richmond, G.S.; Smith, T.K. Phospholipases A1. Int. J. Mol. Sci. 2011, 12, 588–612. [Google Scholar] [CrossRef]
- Guo, N. Identification of ACSL4 as a biomarker and contributor of ferropotosis in clear cell renal cell carcinoma. Transl. Cancer Res. 2022, 11, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Torres-Velarde, J.M.; Allen, K.N.; Pascual, A.S.; Leija, R.G.; Luong, D.; Santillán, D.D.; Ensminger, D.C.; Medina, J.P. Peroxiredoxin 6 suppresses ferroptosis in lung endothelial cells. Free Radic. Biol. Med. 2024, 218, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J. Ferroptosis: A new strategy for cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1241282. [Google Scholar] [CrossRef]



| Target Gene | GenBank®Accession Number | Primer Sequences (5′–3′) Forward (Fw) and Reverse (Rw) | Length (bp) | Annealing T (°C) | Fragment Size (bp) |
|---|---|---|---|---|---|
| circRNA_1156 | NC_000077.7 | F: GTAAGGTGTGGCTTCTGGCT | 20 | 60 | 113 |
| R: CAGTTGGAAAGGTGCAAGGC | 20 | ||||
| ACSL4 | NM_207625 | F: CCTTTGGCTCATGTGCTGGAAC | 22 | 60 | 5280 |
| R: GCCATAAGTGTGGGTTTCAGTAC | 23 | ||||
| LPCAT3 | NM_145130 | F: CCATCTCTTCCACACCTTCACG | 22 | 60 | 1932 |
| R: GGATGAGGAACTGAAGCACGAC | 22 | ||||
| PKCβII | NM_002738 | F: CCAAGATGACGATGTGGAGTGC | 22 | 60 | 8010 |
| R: CTCCATCACAAAGTACAGGCGG | 22 | ||||
| GAPDH | NM_008084 | F: CATCACTGCCACCCAGAAGACTG | 23 | 60 | 1257 |
| R: ATGCCAGTGAGCTTCCCGTTCAG | 23 | ||||
| β-actin | NM_007393 | F: CATTGCTGACAGGATGCAGAAGG | 23 | 60 | 1935 |
| R: TGCTGGAAGGTGGACAGTGAGG | 23 |
| Antibody Name | Molecular Weight Size |
|---|---|
| ACSL4 | 79 kDa |
| LPCAT1 | 59 kDa |
| PKCβII | 71 kDa |
| GPX4 | 20/22 kDa |
| SLC7A11 | 37 kDa |
| β-actin | 43 kDa |
| p53 | 53 kDa |
| COX2 | 20 kDa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Leng, J.; Xu, J.; Qian, K.; Zheng, Z.; Tao, G.; Xiao, P.; Hong, X. CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway. Antioxidants 2025, 14, 700. https://doi.org/10.3390/antiox14060700
Wang N, Leng J, Xu J, Qian K, Zheng Z, Tao G, Xiao P, Hong X. CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway. Antioxidants. 2025; 14(6):700. https://doi.org/10.3390/antiox14060700
Chicago/Turabian StyleWang, Ning, Jing Leng, Jing Xu, Kelei Qian, Zhiqing Zheng, Gonghua Tao, Ping Xiao, and Xinyu Hong. 2025. "CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway" Antioxidants 14, no. 6: 700. https://doi.org/10.3390/antiox14060700
APA StyleWang, N., Leng, J., Xu, J., Qian, K., Zheng, Z., Tao, G., Xiao, P., & Hong, X. (2025). CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway. Antioxidants, 14(6), 700. https://doi.org/10.3390/antiox14060700






