Inulin Improves the Redox Response in Rats Fed a Diet Containing Recommended Copper Nanoparticle (CuNPs) Levels, While Pectin or Psyllium in Rats Receive Excessive CuNPs Levels in the Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials’ Characterisation: Copper Nanoparticles and Fibre Types
2.2. Animal Study Protocol and Diet Composition
| C | CH | CN | CNH | PN | PNH | JN | JNH | SN | SNH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Casein 1 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 |
| DL-methionine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Cellulose 2 | 8.0 | 8.0 | 8.0 | 8.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Pectin | 6 | 6 | ||||||||
| Inulin | 6 | 6 | ||||||||
| Psyllium | 6 | 6 | ||||||||
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Rapeseed oil | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Cholesterol | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin mix 3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mix 4 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Maize starch 5 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 |
| Calculation: | ||||||||||
| Cu from, mg/kg | ||||||||||
| CuCO3 | 6.5 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CuNPs | 0 | 0 | 6.5 | 13 | 6.5 | 13 | 6.5 | 13 | 6.5 | 13 |
2.3. Blood and Tissue Analyses
2.4. Data Analysis and Statistics
3. Results
3.1. One-Way ANOVA
3.1.1. C vs. CN, PN, JN i SN
3.1.2. CH vs. CNH, PNH, JNH i SNH
3.2. Two-Way ANOVA
3.2.1. Effect of CuNP Dose
3.2.2. Effect of Fibre Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| APE-1 | Apurinic/apyrimidinic endonuclease 1 |
| Casp3 | Caspase 3 |
| Casp8 | Caspase 8 |
| CAT | Catalase |
| Cp | Ceruloplasmin |
| CuNPs | Copper nanoparticles |
| EDTA | Ethylenediaminetetraacetic acid |
| Group C | Rats fed a control diet with standard Cu content in the mineral mixture (6.5 mg/kg from CuCO3), with 8% of cellulose as a dietary fibre source |
| Group CH | Rats fed a control diet with enhanced Cu content in the mineral mixture (13 mg/kg from CuCO3), with 8% of cellulose as dietary fibre source |
| Group CN | Rats fed diets with supplementation of CuNPs (6.5 from Cu-nanoparticles), with 8% of cellulose dietary fibre source |
| Group CNH | Rats fed diets with supplementation of CuNPs (13 mg/kg from Cu-nanoparticles), with 8% of cellulose dietary fibre source |
| Group JN | Rats fed diets with supplementation of CuNPs (6.5 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of inulin dietary fibre source |
| Group JNH | Rats fed diets with supplementation of CuNPs (13 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of inulin dietary fibre source |
| Group PN | Rats fed diets with supplementation of CuNPs (6.5 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of pectin dietary fibre source |
| Group PNH | Rats fed diets with supplementation of CuNPs (13 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of pectin dietary fibre source |
| Group SN | Rats fed diets with supplementation of CuNPs (6.5 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of psyllium dietary fibre source |
| Group SNH | Rats fed diets with supplementation of CuNPs (13 mg/kg from Cu-nanoparticles), with 2% of cellulose and 6% of psyllium dietary fibre source |
| MDA | Malondialdehyde |
| OGG1 | 8-oxoguanine DNA glycosylase |
| PBS | Phosphate-buffered saline |
| PC | Protein carbonyl derivative |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| TAS | Total antioxidant capacity |
References
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.B.; Farag, H.A.M.; Salih, T.H.; Awlqadr, F.H.; Al-Manhel, A.J.A.; Vieira, I.R.S.; Conte-Junior, C.A. Application of Nanoparticles in Human Nutrition: A Review. Nutrients 2024, 16, 636. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Stępniowska, A.; Cholewińska, E.; Kozłowski, K. The effect of administration of copper nanoparticles to chickens in drinking water on estimated intestinal absorption of iron, zinc, and calcium. Poult. Sci. 2016, 96, 2045–2051. [Google Scholar] [CrossRef]
- Cholewińska, E.; Ognik, K.; Fotschki, B.; Zduńczyk, Z.; Juśkiewicz, J. Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS ONE 2018, 18, e0197083. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Shin, N.R.; Shin, I.S.; Moon, C.; Kim, J.H.; Kim, H.C.; Kim, J.C. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part. Fibre Toxicol. 2016, 13, 56. [Google Scholar] [CrossRef]
- Marzec, A.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Stepniowska, A.; Ognik, K. Are the biodistribution and metabolic effects of copper nanoparticles dependent on differences in the physiological functions of dietary fibre? Ann. Anim. Sci. 2025, 25, 175–187. [Google Scholar] [CrossRef]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Lim, J.O.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals toward Sustainable Agriculture and Food Systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef]
- Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Metal Nanoparticles and Supramolecular Macrocycles: A Tale of Synergy. Chem. Eur. J. 2014, 20, 10874–10883. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewińska, E.; Stępniowska, A.; Drażbo, A.; Kozłowski, K.; Jankowski, J. The Effect of Administration of Copper Nanoparticles in Drinking Water on Redox Reactions in the Liver and Breast Muscle of Broiler Chickens. Ann. Anim. Sci. 2019, 19, 663–677. [Google Scholar] [CrossRef]
- Ognik, K.; Cholewińska, E.; Tutaj, K.; Cendrowska-Pinkosz, M.; Dworzański, W.; Dworzańska, A.; Juśkiewicz, J. The effect of the source and dosage of dietary Cu on redox status in rat tissues. J. Anim. Physiol. Anim. Nutr. 2020, 104, 352–361. [Google Scholar] [CrossRef]
- Patel, M.K.; Tanna, B.; Mishra, A.; Jha, B. Physicochemical characterization, antioxidant and anti-proliferative activities of a polysaccharide extracted from psyllium (P. ovata) leaves. Int. J. Biol. Macromol. 2018, 118, 976–987. [Google Scholar] [CrossRef]
- Jabeen, N.; Atif, M. Polysaccharides based biopolymers for biomedical applications: A review. Polym. Adv. Technol. 2024, 35, e6203. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghbi, M.; Sitohy, M.Z. Mineral absorption by albino rats as affected by some types of dietary pectins with different degrees of esterification. Nahrung 2001, 45, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Asvarujanon, P.; Ishizuka, S.; Hara, H. Inhibitory Effects of Psyllium on Rat Mineral Absorption Were Abolished by Reduction of Viscosity with Partial Hydrolysis. Biosci. Biotechnol. Biochem. 2004, 68, 1737–1742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krzysik, M.; Grajeta, H.; Prescha, A. Effect of Pectin and Cellulose on the Content of Minerals in the Femur of Rats. Pol. J. Food Nutr. Sci. 2009, 59, 357–360. [Google Scholar]
- Cholewińska, E.; Sołek, P.; Juśkiewicz, J.; Fotschki, B.; Dworzański, W.; Ognik, K. Chromium nanoparticles improve bone turnover regulation in rats fed a high-fat, low-fibre diet. PLoS ONE 2024, 19, e0300292. [Google Scholar] [CrossRef]
- Marzec, A.; Fotschki, B.; Napiórkowska, D.; Fotschki, J.; Cholewińska, E.; Listos, P.; Juśkiewicz, J.; Ognik, K. The Effect of Copper Nanoparticles on Liver Metabolism Depends on the Type of Dietary Fiber. Nutrients 2024, 16, 3645. [Google Scholar] [CrossRef]
- Cholewińska, E.; Marzec, A.; Sołek, P.; Fotschki, B.; Listos, P.; Ognik, K.; Juśkiewicz, J. The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients 2023, 15, 1588. [Google Scholar] [CrossRef]
- Majewski, M.; Gromadziński, L.; Cholewińska, E.; Ognik, K.; Fotschki, B.; Juśkiewicz, J. The Interaction of Dietary Pectin, Inulin, and Psyllium with Copper Nanoparticle Induced Changes to the Cardiovascular System. Nutrients 2023, 15, 3557. [Google Scholar] [CrossRef]
- OJEU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; OJEU: Luxembourg, 2010; Volume L276, pp. 33–79. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127 (Suppl. S5), 838S–841S. [Google Scholar] [CrossRef]
- Ognik, K.; Wertelecki, T. Effect of different vitamin E sources and levels on selected oxidative status indices in blood and tissues as well as on rearing performance of slaughter turkey hens. J. Appl. Poult. Res. 2012, 21, 259–271. [Google Scholar] [CrossRef]
- Liu, N.; Tong, L.; Li, K.; Dong, Q.; Jing, J. Copper-Nanoparticle-Induced Neurotoxic Effect and Oxidative Stress in the Early Developmental Stage of Zebrafish (Danio rerio). Molecules 2024, 29, 2414. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Eddaikra, A.; Eddaikra, N. Endogenous Enzymatic Antioxidant Defense and Pathologies. In Antioxidants—Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Wijmenga, C.; Klomp, L.W.J. Molecular regulation of copper excretion in the liver. Proc. Nutr. Soc. 2004, 63, 31–39. [Google Scholar] [CrossRef]
- Campbell, J.; Berry, J.; Liang, Y. Anatomy and Physiology of the Small Intestine. In Shackelford’s Surgery of the Alimentary Tract; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 817–841. [Google Scholar]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology, 1st ed.; American Physiological Society: Rockville, MD, USA, 2014; pp. 177–197. [Google Scholar]
- Liu, T.C.; Guo, K.W.; Chu, J.W.; Hsiao, Y.Y. Understanding APE1 cellular functions by the structural preference of exonuclease activities. Comput. Struct. Biotechnol. J. 2021, 19, 3682–3691. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef]
- Rockway, S.W.; Brannon, P.M.; Weber, C.W. Bioavailability of copper bound to dietary fiber in mice and rats. J. Food Sci. 1987, 52, 1423–1427. [Google Scholar] [CrossRef]
- Caballero, B. Nutritional implications of dietary interactions: A review. Food Nutr. Bull. 1988, 10, 1–12. [Google Scholar] [CrossRef]
- Adams, S.; Sello, C.T.; Qin, G.X.; Che, D.; Han, R. Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- McRorie, J.W.; Gibb, R.D.; Sloan, K.J.; McKeown, N.M. Psyllium: The Gel-Forming Nonfermented Isolated Fiber That Delivers Multiple Fiber-Related Health Benefits. Nutr. Today 2021, 56, 169–182. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef]
- Coudray, C.; Feillet-Coudray, C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. Dietary Inulin Intake and Age Can Affect Intestinal Absorption of Zinc and Copper in Rats. J. Nutr. 2006, 136, 117–122. [Google Scholar] [CrossRef]
- Spiller, G.A. (Ed.) CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Lopez, M.J.; Royer, A.; Shah, N.J. Biochemistry, Ceruloplasmin; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes Metab. Syndr. Obes. 2022, 15, 695–711. [Google Scholar] [CrossRef]
- Kim, O.Y.; Shin, M.J.; Moon, J.; Chung, J.H. Plasma ceruloplasmin as a biomarker for obesity: A proteomic approach. Clin. Biochem. 2011, 44, 351–356. [Google Scholar] [CrossRef]
- Ferrer, M.; Buey, B.; Grasa, L.; Mesonero, J.E.; Latorre, E. Protective role of short-chain fatty acids on intestinal oxidative stress induced by TNF-α. Cell Stress Chaperones 2024, 29, 769–776. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Fotschki, B.; Stępniowska, A.; Cholewińska, E.; Napiórkowska, D.; Marzec, A.; Brzuzan, Ł.; Fotschki, J.; Żary-Sikorska, E.; Ognik, K. Dietary Fiber with Functional Properties Counteracts the Thwarting Effects of Copper Nanoparticles on the Microbial Enzymatic Activity and Short-Chain Fatty Acid Production in the Feces of Rats. Pol. J. Food Nutr. Sci. 2024, 74, 363–375. [Google Scholar] [CrossRef]

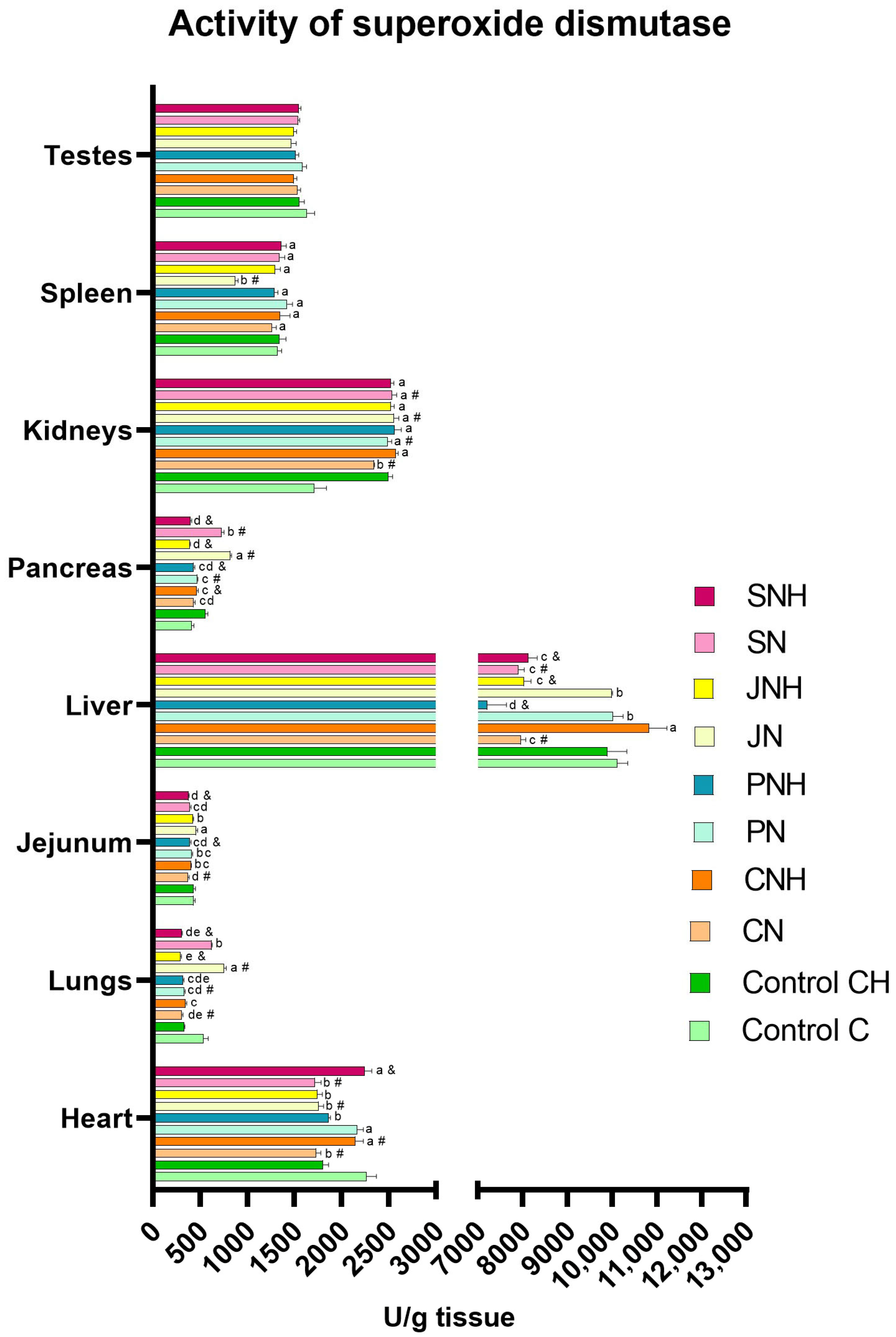
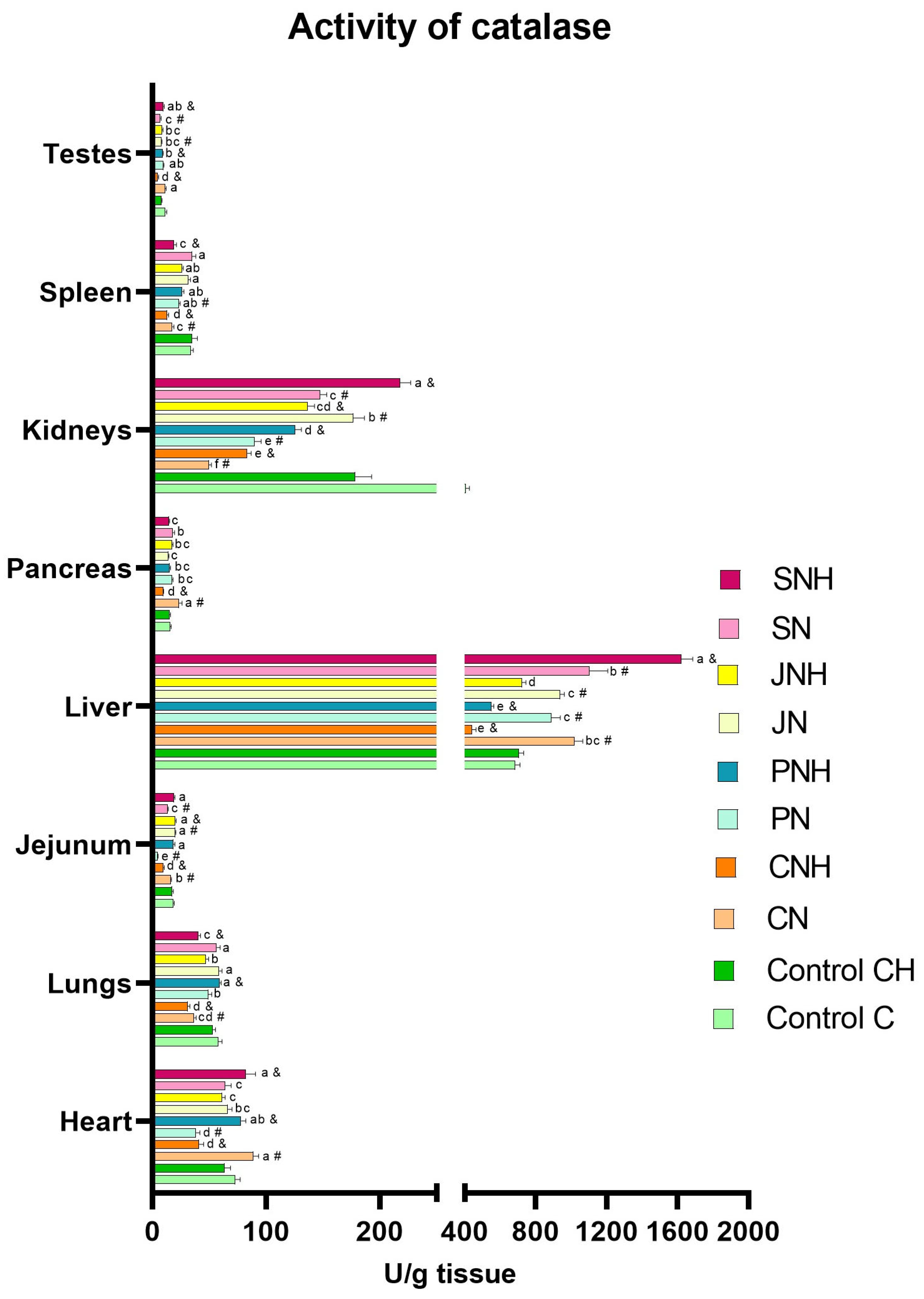
| SOD | CAT | Cp | TAS | MDA | 3-NT | PC | 8-OHdG | DNA Methylation | APE-1 | OGG1 | Casp3 | Casp8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL | ng/mL | U/L | mmol/L | nmol/mL | ng/mL | nmol/mg Protein | ng/mL | % | ng/mL | pg/mL | ng/mL | ng/mL | |
| Control C | 36.6 | 27.1 | 164 | 0.902 | 1.49 | 14.8 | 4.75 | 6.93 | 1.46 | 180 | 493 | 434 | 102 |
| Control CH | 17.2 | 29.7 | 125 | 0.973 | 1.5 6 | 14.7 | 3.36 | 7.80 | 1.16 | 171 | 460 | 441 | 100 |
| 2-way ANOVA: | |||||||||||||
| CN | 17.3 # | 31.7 # | 141 | 0.932 | 1.41 d | 14.6 | 4.82 | 7.93 | 0.853 b# | 167 a# | 454 | 402 a | 99.3 |
| CNH | 18.6 | 31.4 | 148 & | 0.974 | 1.50 cd | 14.6 | 4.20 | 7.76 | 1.52 a | 167 a | 460 | 387 ab& | 97.7 |
| PN | 8.31 # | 33.5 # | 131 # | 1.08 | 1.83 bc# | 15.8 | 3.35 # | 8.07 # | 1.28 a | 169 a | 471 | 411 a | 123 |
| PNH | 10.7 & | 32.2 | 125 | 0.943 | 1.64 cd | 18.4 & | 3.03 | 8.93 & | 0.915 b | 155 ab | 386 & | 335 c& | 101 |
| JN | 5.04 # | 31.9 # | 162 | 0.956 | 1.63 cd | 14.8 | 4.35 | 7.90 # | 1.59 a | 153 ab# | 428 | 347 bc# | 105 |
| JNH | 8.18 & | 29.9 | 158 & | 0.968 | 2.87 a& | 15.2 | 4.75 & | 8.45 | 0.909 b | 152 ab & | 408 | 350 bc& | 93.3 |
| SN | 6.99 # | 31.1 | 138 # | 0.954 | 2.07 b# | 16.8 | 5.29 | 9.05 # | 0.966 b# | 143 b# | 398 # | 330 c# | 77.9 # |
| SNH | 6.56 & | 31.0 | 129 | 1.09 | 1.36 d& | 17.2 | 5.01 & | 8.62 | 0.845 b | 162 a | 399 & | 350 bc& | 83.7 |
| SEM | 0.986 | 0.389 | 2.083 | 0.019 | 0.054 | 0.323 | 0.139 | 0.119 | 0.044 | 1.934 | 7.941 | 6.108 | 2.934 |
| CuNPs dose (D) | |||||||||||||
| L (6.5 mg/kg) | 9.42 | 32.1 | 143 | 0.981 | 1.74 | 15.5 | 4.45 | 8.24 | 1.17 | 158 | 438 | 373 | 101 |
| H (13 mg/kg) | 11.0 | 31.1 | 140 | 0.995 | 1.84 | 16.4 | 4.25 | 8.44 | 1.05 | 159 | 413 | 356 | 93.8 |
| p value | 0.065 | 0.242 | 0.239 | 0.724 | 0.197 | 0.268 | 0.480 | 0.406 | 0.110 | 0.737 | 0.137 | 0.132 | 0.309 |
| Fibre type (F) | |||||||||||||
| C (cellulose) | 18.0 a | 31.6 | 144 b | 0.953 | 1.45 | 14.6 b | 4.52 a | 7.84 b | 1.19 | 167 | 457 | 394 | 97.3 ab |
| P (pectin) | 9.53 b | 32.9 | 128 c | 1.01 | 1.74 | 17.1 a | 3.19 b | 8.50 ab | 1.10 | 162 | 428 | 373 | 112 a |
| J (inulin) | 6.61 c | 30.9 | 160 a | 0.962 | 2.25 | 15.0 ab | 4.55 a | 8.17 ab | 1.25 | 153 | 418 | 349 | 98.9 ab |
| S (psyllium) | 6.77 c | 31.0 | 133 c | 1.02 | 1.71 | 17.0 a | 5.15 a | 8.84 a | 0.906 | 153 | 399 | 340 | 80.8 b |
| p value | <0.001 | 0.309 | <0.001 | 0.513 | <0.001 | 0.033 | <0.001 | 0.028 | 0.015 | 0.024 | 0.090 | 0.004 | 0.017 |
| Interaction D × F | |||||||||||||
| p value | 0.488 | 0.837 | 0.196 | 0.117 | <0.001 | 0.607 | 0.649 | 0.192 | <0.001 | 0.037 | 0.192 | 0.019 | 0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, A.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Ognik, K. Inulin Improves the Redox Response in Rats Fed a Diet Containing Recommended Copper Nanoparticle (CuNPs) Levels, While Pectin or Psyllium in Rats Receive Excessive CuNPs Levels in the Diet. Antioxidants 2025, 14, 695. https://doi.org/10.3390/antiox14060695
Marzec A, Cholewińska E, Fotschki B, Juśkiewicz J, Ognik K. Inulin Improves the Redox Response in Rats Fed a Diet Containing Recommended Copper Nanoparticle (CuNPs) Levels, While Pectin or Psyllium in Rats Receive Excessive CuNPs Levels in the Diet. Antioxidants. 2025; 14(6):695. https://doi.org/10.3390/antiox14060695
Chicago/Turabian StyleMarzec, Aleksandra, Ewelina Cholewińska, Bartosz Fotschki, Jerzy Juśkiewicz, and Katarzyna Ognik. 2025. "Inulin Improves the Redox Response in Rats Fed a Diet Containing Recommended Copper Nanoparticle (CuNPs) Levels, While Pectin or Psyllium in Rats Receive Excessive CuNPs Levels in the Diet" Antioxidants 14, no. 6: 695. https://doi.org/10.3390/antiox14060695
APA StyleMarzec, A., Cholewińska, E., Fotschki, B., Juśkiewicz, J., & Ognik, K. (2025). Inulin Improves the Redox Response in Rats Fed a Diet Containing Recommended Copper Nanoparticle (CuNPs) Levels, While Pectin or Psyllium in Rats Receive Excessive CuNPs Levels in the Diet. Antioxidants, 14(6), 695. https://doi.org/10.3390/antiox14060695







