Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Extraction
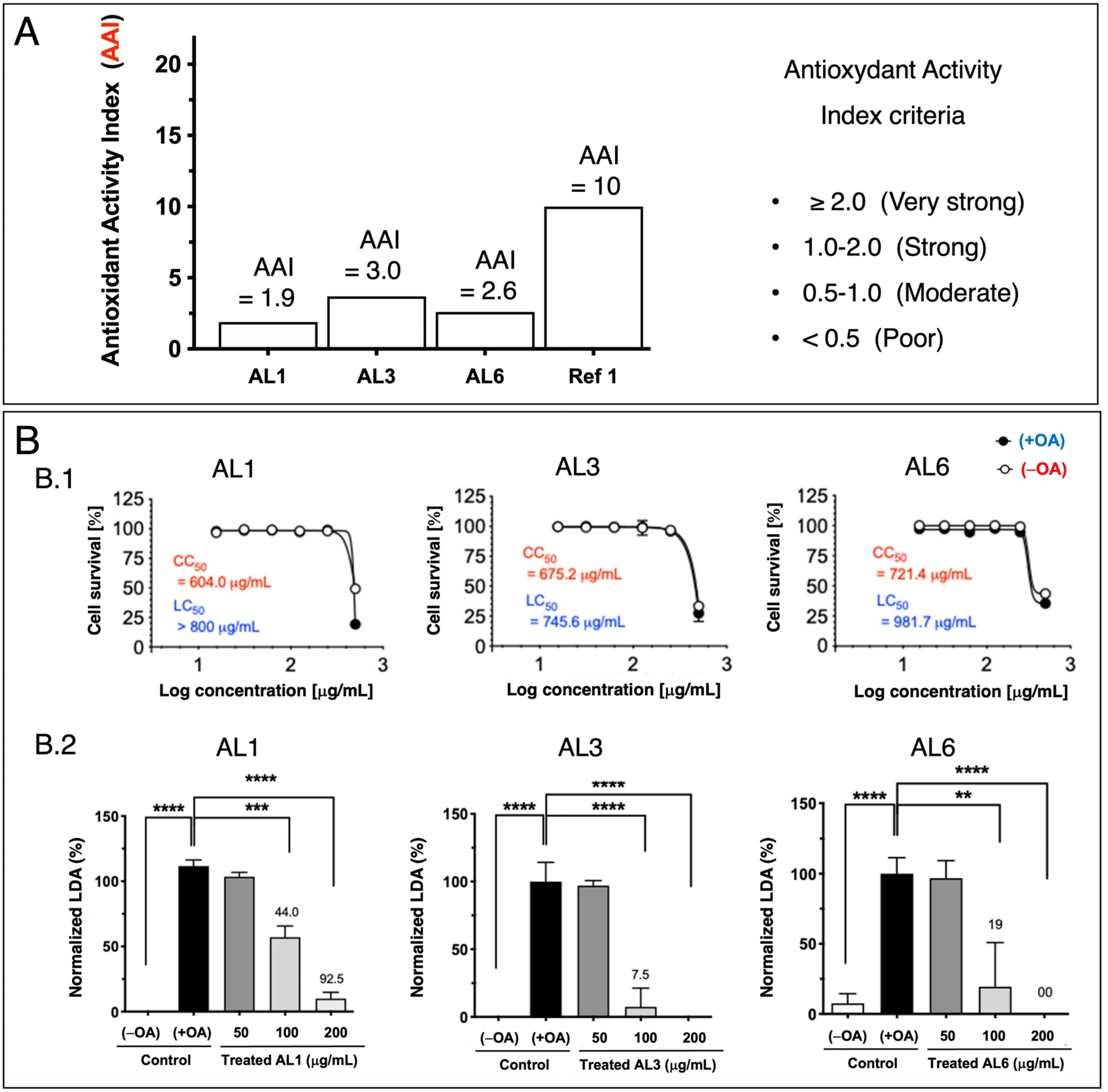
2.2. Antioxidant Activity Index, Evaluation of Cell Viability, Lipid Droplet Accumulation Inhibition Assay, and TG Assay
2.3. Lipidomic Analysis of Neutral Lipids: Analysis of the Accumulation of Triacylglycerols and Oxidized Hydroperoxide Species Using LC-MS
2.4. Metabolite Profiles of AL1, AL3, and AL6 Based on NMR and LC-MS/MS Analysis
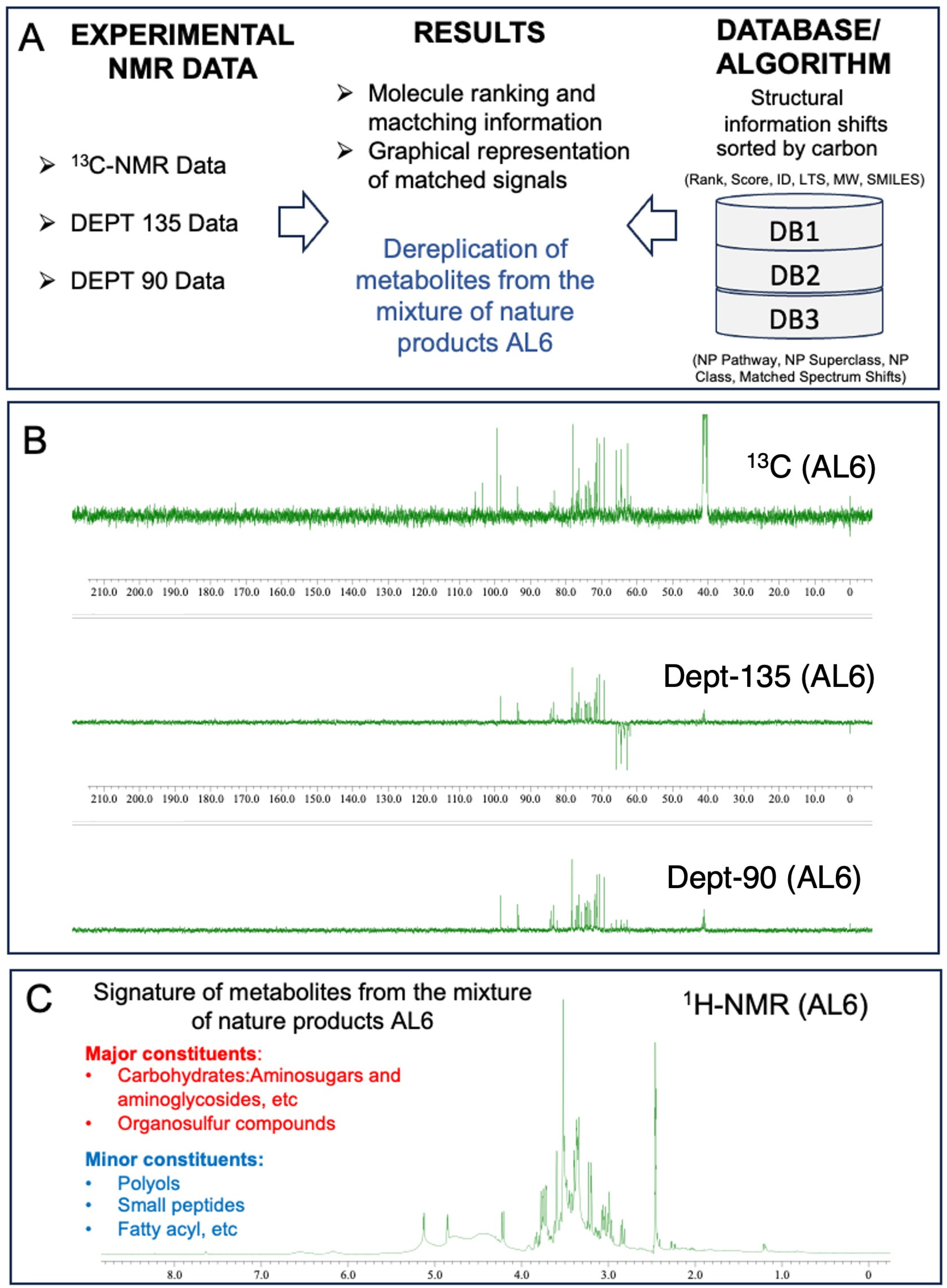
2.5. Rapid Dereplication of AL1 and AL6 Using 1D-NMR
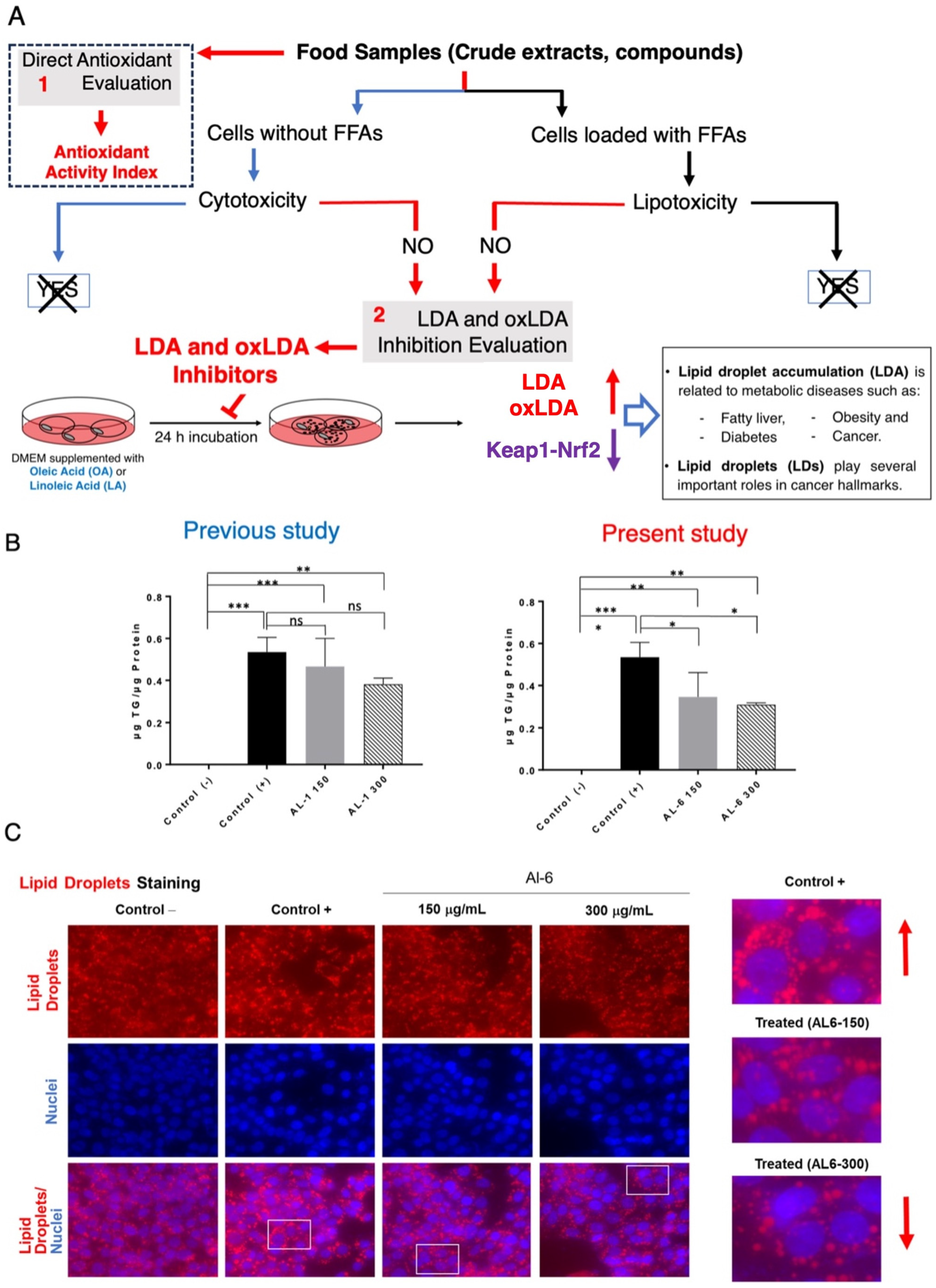
3. Results and Discussion
3.1. Bioactive Lipid Droplet Accumulation Inhibitor (LDAI) Allium Extracts
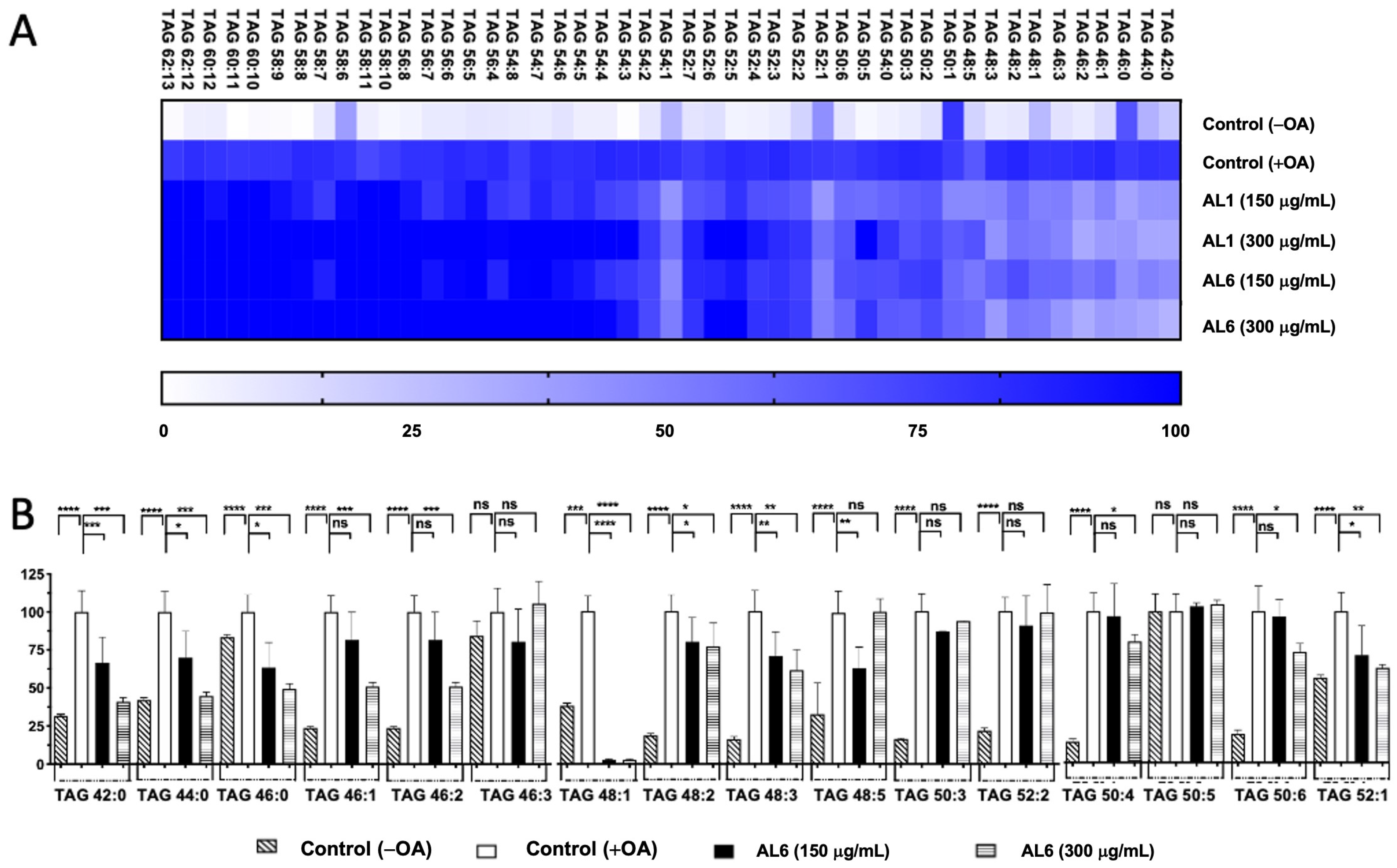
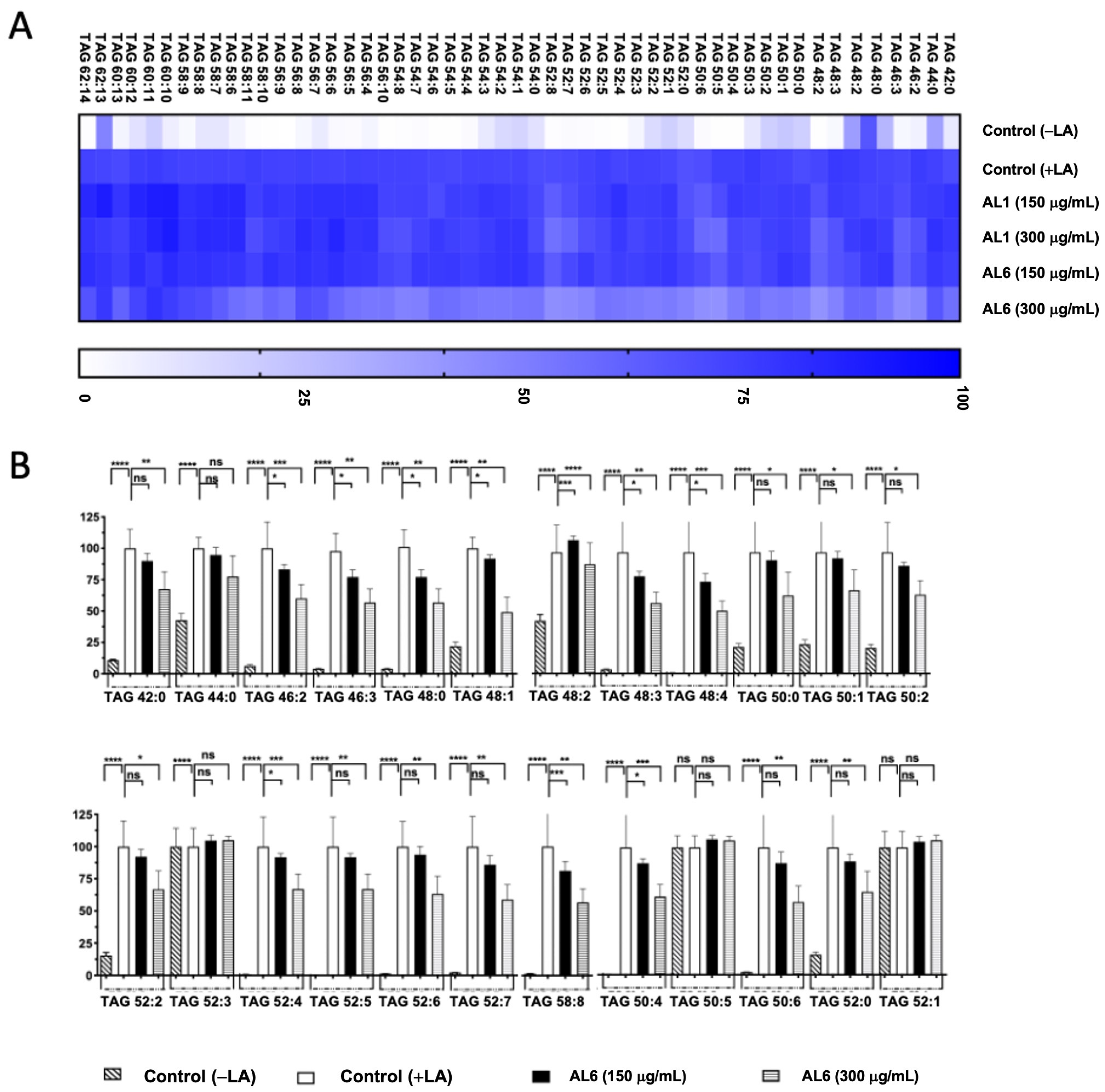
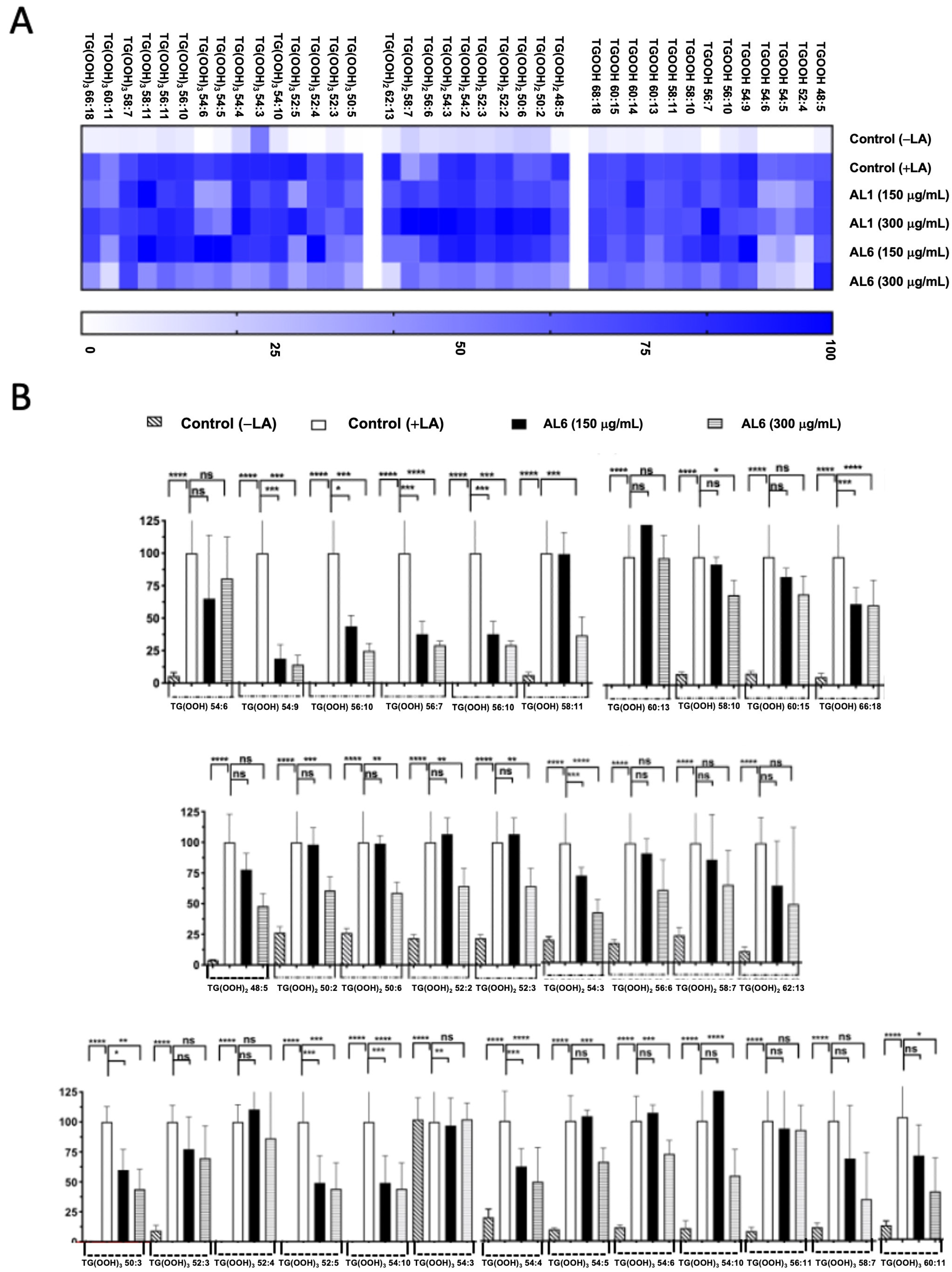
3.2. Quantification of the Effects of AL1 and AL6 on Inhibition of the Accumulation of TAGs and TGOOH Species
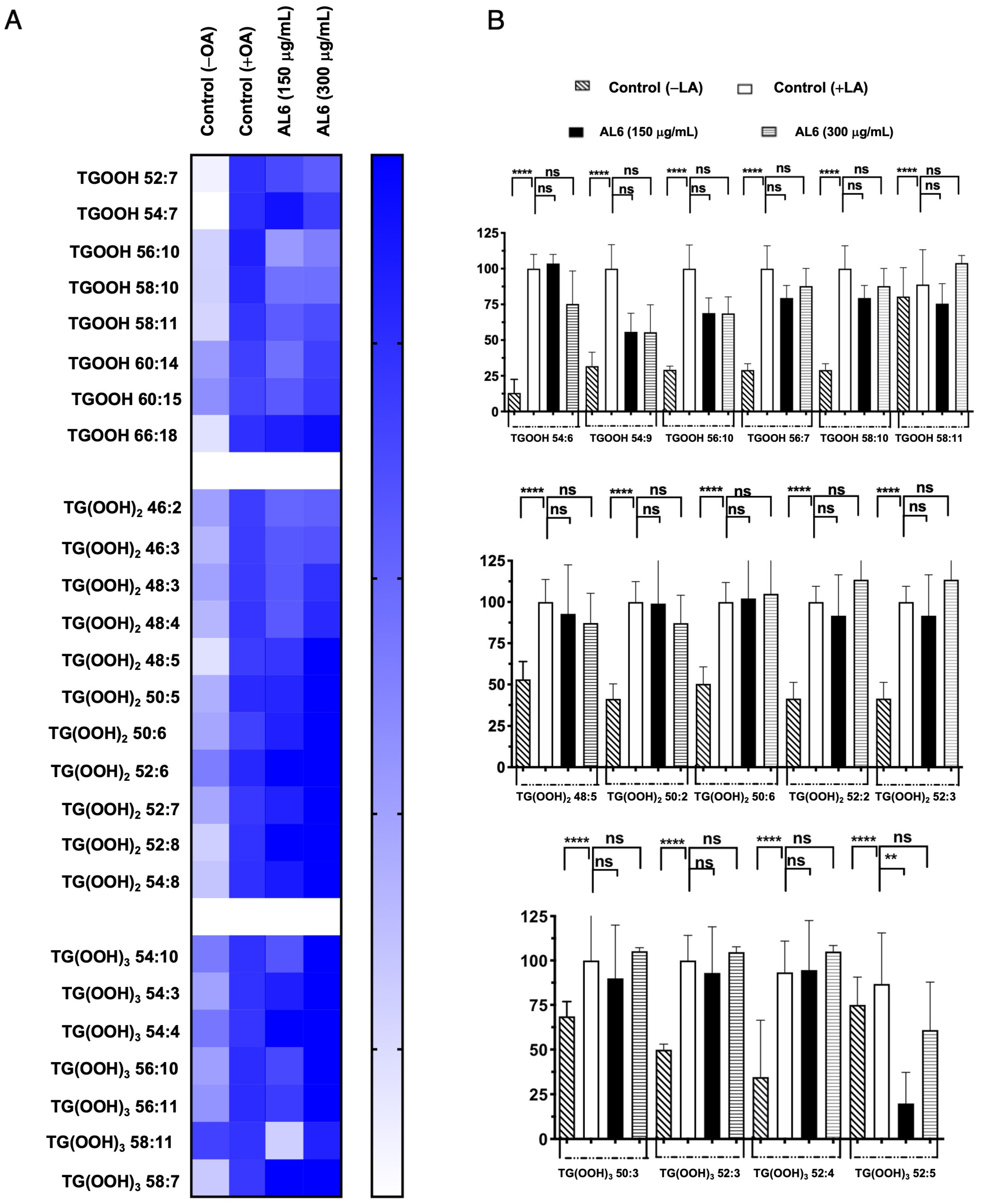
3.2.1. Comparative Analysis of AL1 and AL6 Inhibition of Accumulated TAGs and TGOOH Species Induced by Oleic Acid in Hepatocytes
3.2.2. Comparative Analysis of AL1 and AL6 Inhibition of Accumulated TAG and TGOOH Species Induced by Linoleic Acid in Hepatocytes
3.2.3. Comparative Analysis of Hydroperoxide Lipid Species Inhibited in OA and LA by AL6
3.3. Metabolite Fingerprinting and Rapid Dereplication of Key Compounds in Bioactive Extracts
3.3.1. LC-MS/MS Analysis and Global Natural Product Social-Assisted Dereplication of AL1, AL3, and AL6 Constituents
3.3.2. Comparison of 1D Nuclear Magnetic Resonance Profiling and Fingerprinting of AL1 and AL6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 161–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petschnigg, J.; Wolinski, H.; Kolb, D.; Zellnig, G.; Kurat, C.F.; Natter, K.; Kohlwein, S.D. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J. Biol. Chem. 2009, 284, 30981–30993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadi, E.; Hossein, F.; Hadith, T.; Ali, S.T.; Hossein, P. Association of allium vegetables intake and non-alcoholic fatty liver disease risk: A case-control study. Nutr. Food Sci. 2020, 50, 1075–1083. [Google Scholar]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid Droplets and Liver Disease: From Basic Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Krahmer, N.; Farese, R.V.; Walther, T.C. Balancing the Fat: Lipid Droplets and8 Human Disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Fan, H.; Chen, Y.Y.; Bei, W.J.; Wang, L.Y.; Chen, B.T.; Guo, J. In Vitro Screening for Antihepatic Steatosis Active Components within Coptidis Rhizoma Alkaloids Extract Using Liver Cell Extraction with HPLC Analysis and a Free Fatty Acid-Induced Hepatic Steatosis HepG2 Cell Assay. Evid. Based Complement. Altern. Med. 2013, 2013, 459390. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Cho, Y.Y.; Hsieh, S.C.; Chiang, A.-N. Chinese olive extract ameliorates hepatic lipid accumulation in vitro and in vivo by regulating lipid metabolism. Sci. Rep. 2018, 8, 1057. [Google Scholar] [CrossRef]
- Uehara, K.; Santoleri, D.; Whitlock, A.E.G.; Titchenell, P.M. Insulin Regulation of Hepatic Lipid Homeostasis. Compr. Physiol. 2023, 13, 4785–4809. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Kage, M.; Akiba, J.; Kawaguchi, T.; Yoshio, S.; Kawaguchi, M.; Nakano, D.; Tsutsumi, T.; Hashida, R.; Oshiro, K. Metabolic dysfunction-associated fatty liver disease directly related to liver fibrosis independent of insulin resistance, hyperlipidemia, and alcohol intake in morbidly obese patients. Hepatol. Res. 2022, 52, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Cusi, K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.H.; Abid, M.A.; Abid, M.H.; Suhail, L.; Nazir, A. A New Hope for the Patients of Non-Alcoholic Steatohepatitis: FDA Gives Green Signal for Resmetirom Use. Health Sci. Rep. 2025, 8, e70394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López, P.; del Pilar, R. Pathological Aspects of Fatty Liver Disease. Rev. Colomb. Gastroenterol. 2014, 29, 82–88. [Google Scholar]
- Dolgova, N.; Uhlemann, E.-M.E.; Boniecki, M.T.; Vizeacoumar, F.S.; Ralle, M.; Tonelli, M.; Abbas, S.A.; Patry, J.; Elhasasna, H.; Freywald, A.; et al. MEMO1 is a Metal Containing Regulator of Iron Homeostasis in Cancer Cells. eLife 2024, 13, e86354. [Google Scholar] [CrossRef]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Dibwe, D.F.; Oba, S.; Takeishi, N.; Sakurai, T.; Tsukui, T.; Chiba, H.; Hui, S.P. Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes. Pharmaceuticals 2022, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dibwe, D.F.; Kitayama, E.; Oba, S.; Takeishi, N.; Chiba, H.; Hui, S.-P. Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts. Antioxidants 2024, 13, 513. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Takeishi, N.; Oba, S.; Sakurai, A.; Sakurai, T.; Tsukui, T.; Chiba, H.; Hui, S.P. Identification of a b-carboline alkaloid from chemoselectively derived vanilla bean extract and its prevention of lipid droplet accumulation in human hepatocytes (HepG2). Molecules 2023, 15, 8024. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Oba, S.; Monde, S.; Hui, S.-P. Inhibition of Accumulation of Neutral Lipids and Their Hydroperoxide Species in Hepatocytes by Bioactive Allium sativum Extract. Antioxidants 2024, 13, 1310. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Bruguière, A.; Derbré, S.; Dietsch, J.; Leguy, J.; Rahier, V.; Pottier, Q.; Bréard, D.; Suor-Cherer, S.; Viault, G.; Le Ray, A.-M.; et al. MixONat, a Software for the Dereplication of Mixtures Based on 13C NMR Spectroscopy. Anal. Chem. 2020, 92, 8793–8801. [Google Scholar] [CrossRef]
- Tsukui, T.; Chen, Z.; Fuda, H.; Furukawa, T.; Oura, K.; Sakurai, T.; Hui, S.P.; Chiba, H. Novel Fluorescence-Based Method to Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes. J. Agric. Food Chem. 2019, 67, 9934–9941. [Google Scholar] [CrossRef]
- Del Ángel Benítez, E. Liver steatosis and nonalcoholic steatohepatitis: From pathogenesis to therapy. Medwave 2016, 16, e6535. [Google Scholar]
- Sumida, Y.; Okanoue, T.; Nakajima, A. Phase 3 Drug Pipelines in the Treatment of Non-Alcoholic Steatohepatitis. Hepatol. Res. 2019, 49, 1256–1262. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. BioMed Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef] [PubMed]
- Vitulo, M.; Gnodi, E.; Rosini, G.; Meneveri, R.; Giovannoni, R.; Barisani, D. Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. Int. J. Mol. Sci. 2023, 24, 12748. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Colucci, G.; Ferrigno, A. Innovative Molecular Targets and Therapeutic Approaches in Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis (NAFLD/NASH) 3.0. Int. J. Mol. Sci. 2024, 25, 4010. [Google Scholar] [CrossRef]
- Alonso-Peña, M.; Del Barrio, M.; Peleteiro-Vigil, A.; Jimenez-Gonzalez, C.; Santos-Laso, A.; Arias-Loste, M.T.; Iruzubieta, P.; Crespo, J. Innovative Therapeutic Approaches in Non-Alcoholic Fatty Liver Disease: When Knowing Your Patient Is Key. Int. J. Mol. Sci. 2023, 24, 10718. [Google Scholar] [CrossRef]
| OA | Neutral Lipid Species | |||||||
|---|---|---|---|---|---|---|---|---|
| AL1 | AL6 | AL1 | AL6 | AL1 | AL6 | AL1 | AL6 | |
| TAG | TAG | TGOOH | TGOOH | TG(OOH)2 | TG(OOH)2 | TG(OOH)3 | TG(OOH)3 | |
| Number of accumulated | 51 | 51 | 8 | 8 | 11 | 11 | 7 | 7 |
| Number of inhibited | 9 | 11 | 1 | 3 | 0 | 2 | 2 | 1 |
| LA | Neutral Lipid Species | |||||||
|---|---|---|---|---|---|---|---|---|
| AL1 | AL6 | AL1 | AL6 | AL1 | AL6 | AL1 | AL6 | |
| TAG | TAG | TGOOH | TGOOH | TG(OOH)2 | TG(OOH)2 | TG(OOH)3 | TG(OOH)3 | |
| Number of accumulated | 53 | 53 | (8)13 | (8)13 | (11)10 | (11)10 | (7)15 | (7)15 |
| Number of inhibited | 6 | 20 | 6 | 10 | 1 | 8 | 6 | 9 |
| Databases | Scores | Profile Metabolites | Top Dereplicated | |||
|---|---|---|---|---|---|---|
| 1 | 1–0.90 | 0.89–0.80 | 0.79–0.70 | 1–0.70 | Compounds | |
| DB1 | 6 | 3 | 16 | 3 | 24 | 50 in 1080 |
| DB2 | 6 | 1 | 16 | 6 | 28 | 50 in 2020 |
| DB3 | 23 | 1 | 23 | 20 | 67 | 70 in 6286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibwe, D.F.; Oba, S.; Monde, S.; Hui, S.-P. Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb). Antioxidants 2025, 14, 653. https://doi.org/10.3390/antiox14060653
Dibwe DF, Oba S, Monde S, Hui S-P. Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb). Antioxidants. 2025; 14(6):653. https://doi.org/10.3390/antiox14060653
Chicago/Turabian StyleDibwe, Dya Fita, Saki Oba, Satomi Monde, and Shu-Ping Hui. 2025. "Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb)" Antioxidants 14, no. 6: 653. https://doi.org/10.3390/antiox14060653
APA StyleDibwe, D. F., Oba, S., Monde, S., & Hui, S.-P. (2025). Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb). Antioxidants, 14(6), 653. https://doi.org/10.3390/antiox14060653







