Obtainment of Flavonoid-Enriched Fractions from Maqui (Aristotelia chilensis) and Murta (Ugni molinae) Extracts via Preparative HPLC and Evaluation of Their Anti-Inflammatory Effects in Cell-Based Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Reagents
2.3. Methods
2.3.1. Preparation of Maqui and Murta Extracts and HPLC Quantification of Anthocyanins and Quercetin Glycosides
2.3.2. Preparative Chromatography Conditions (Prep-HPLC)
2.3.3. Experimental Designs
2.3.4. Determination of Optimal Maqui/Murta Mixture Obtained by Preparative HPLC
2.3.5. Coating of Mixtures of Extracts with Chitosan
2.3.6. Cell Assays
2.3.7. Statistical Analysis
3. Results and Discussion
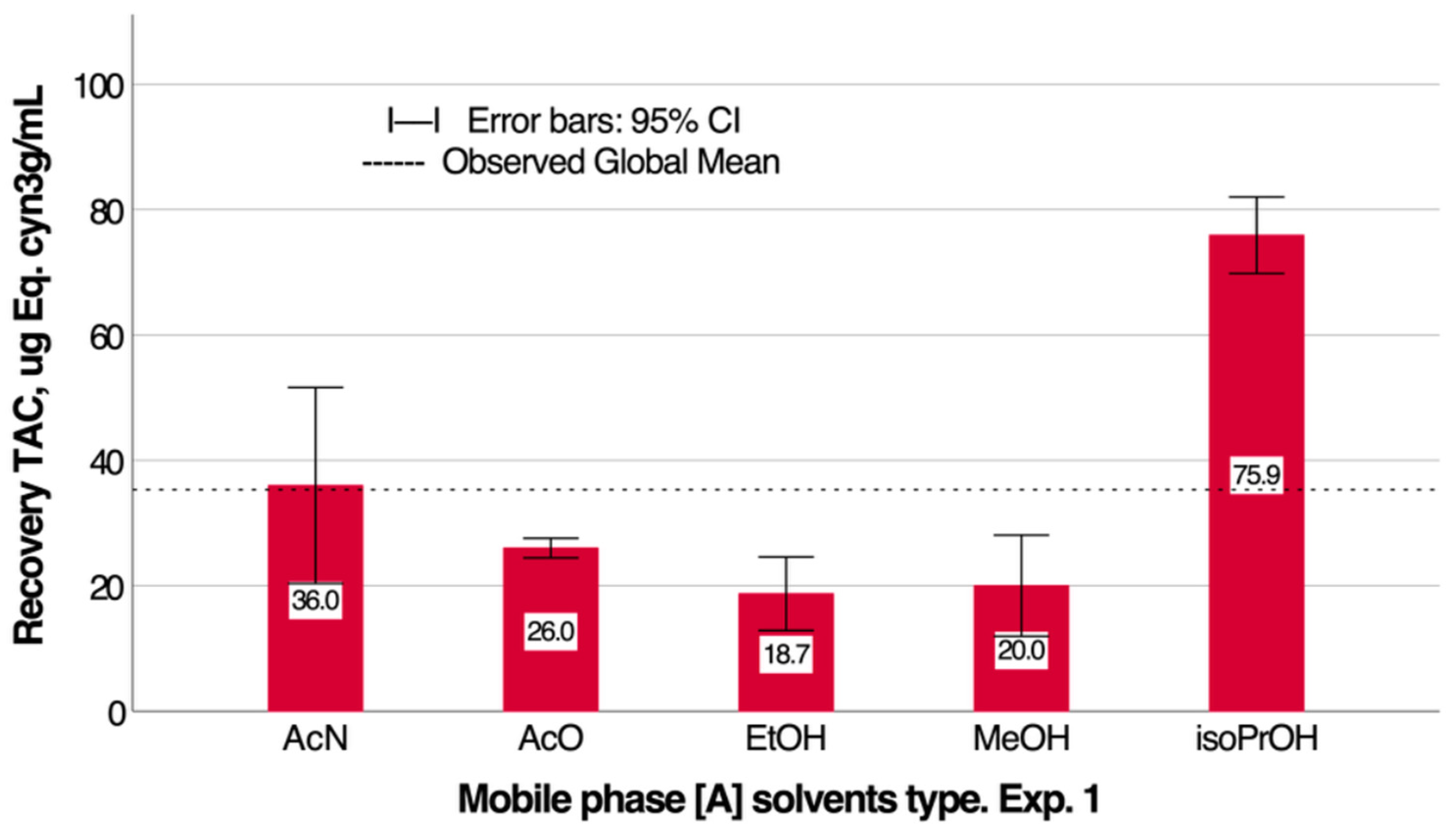
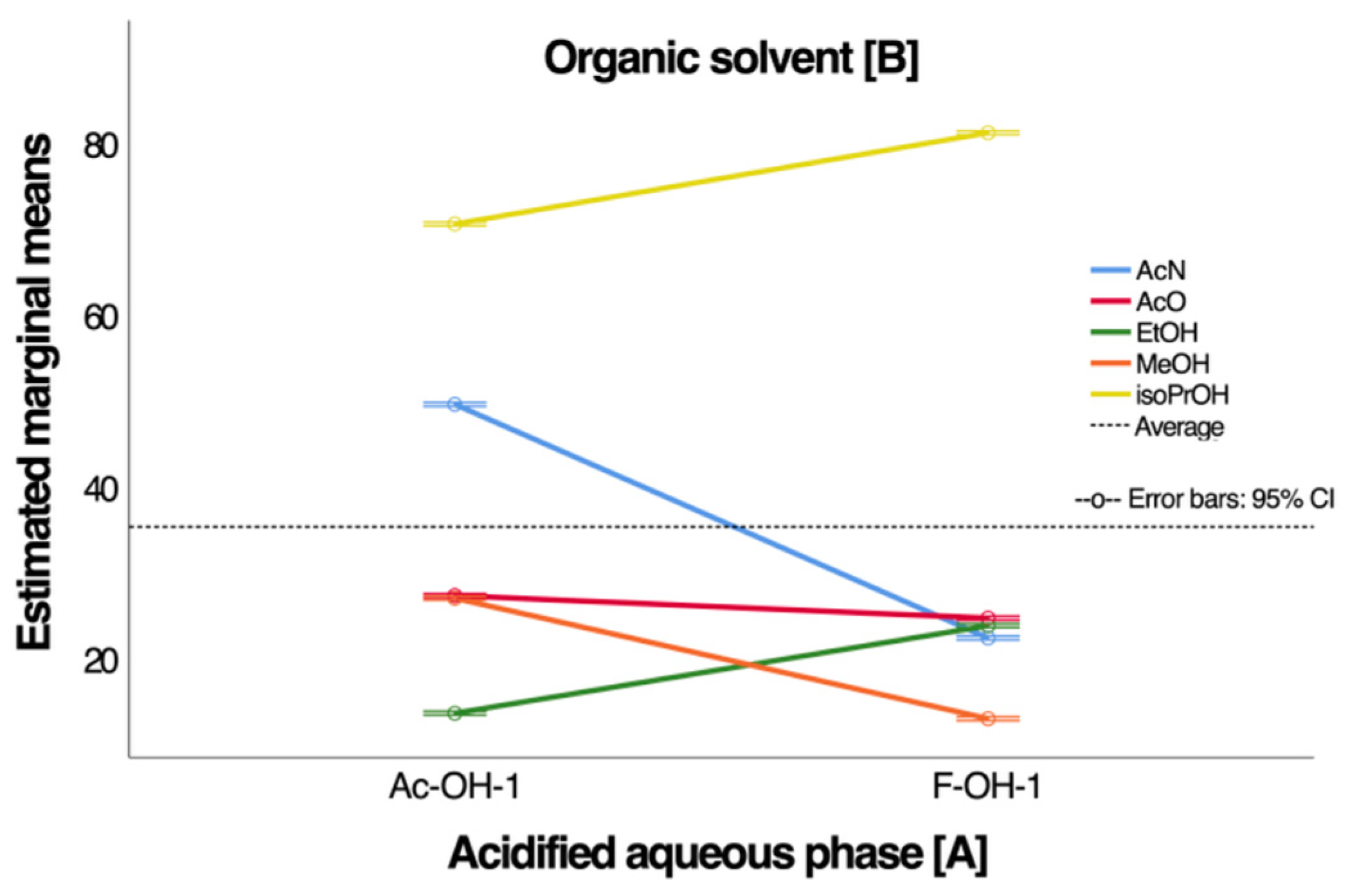
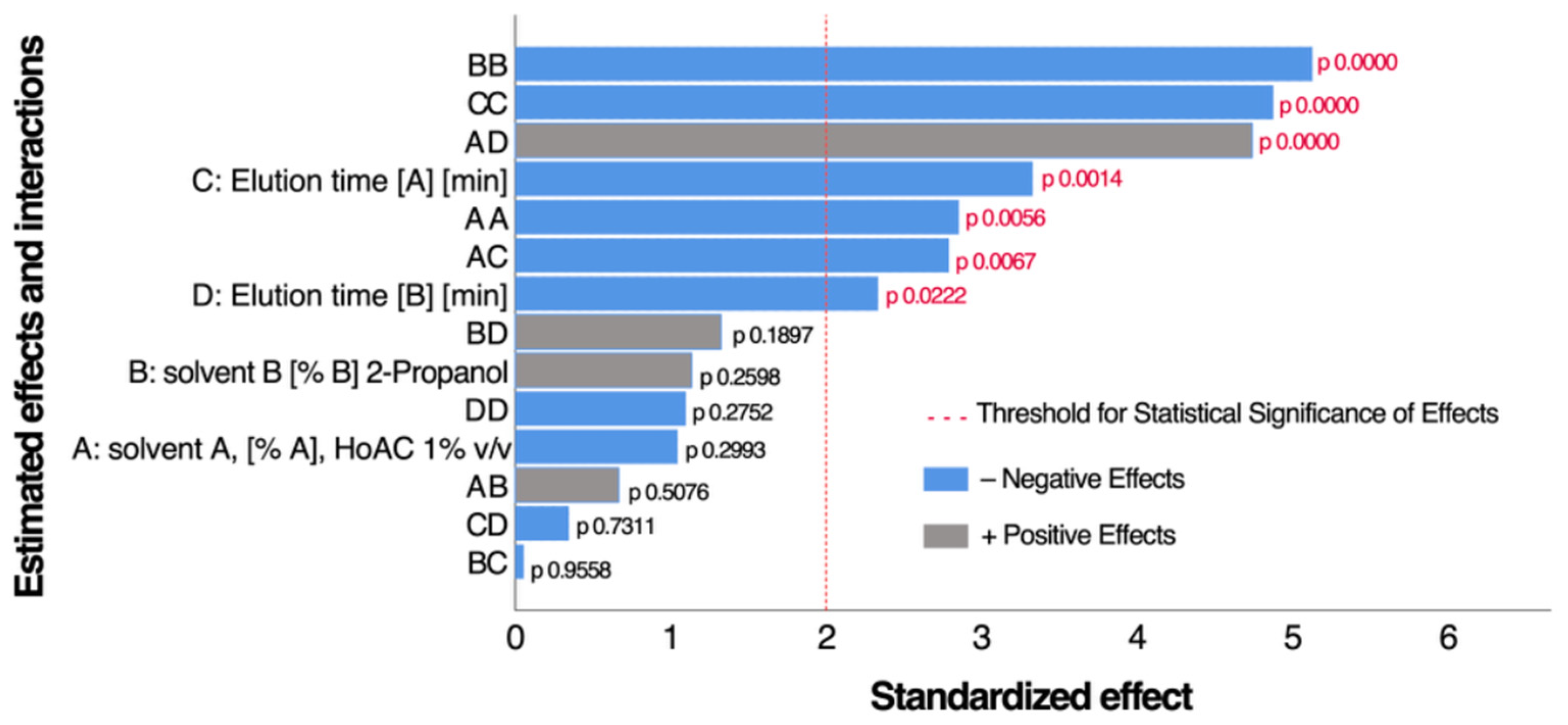
3.1. Determination and Optimization of Anthocyanin Recovery Conditions for Maqui Extract Using Preparative HPLC
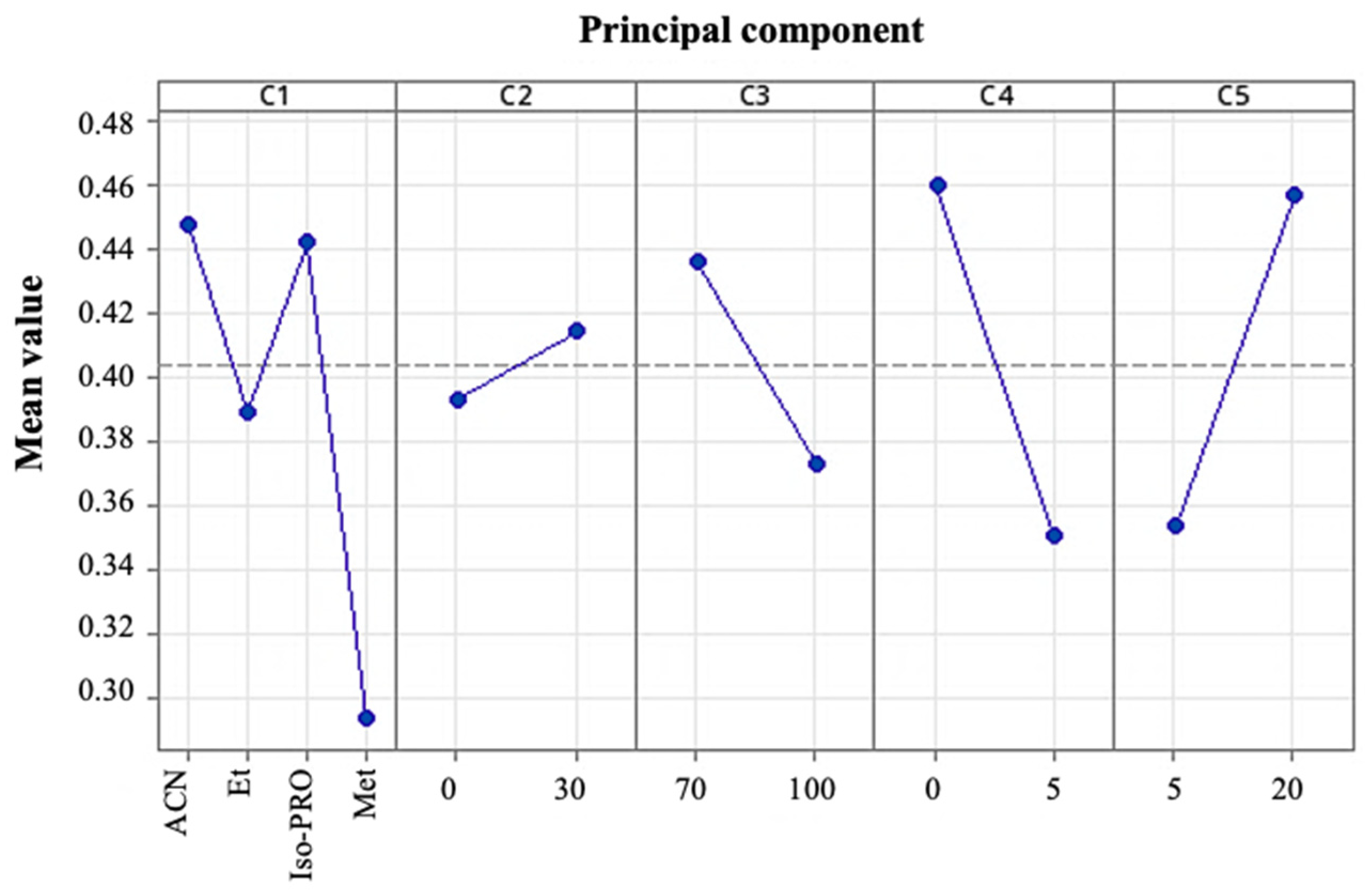
3.2. Determination and Optimization of TQG Recovery Conditions for Murta Extract Using Preparative HPLC
3.3. Determination of Optimal Maqui/Murta Concentrate Mixture Obtained via Preparative HPLC
3.4. Cell Assays
3.4.1. Cytotoxicity Assays
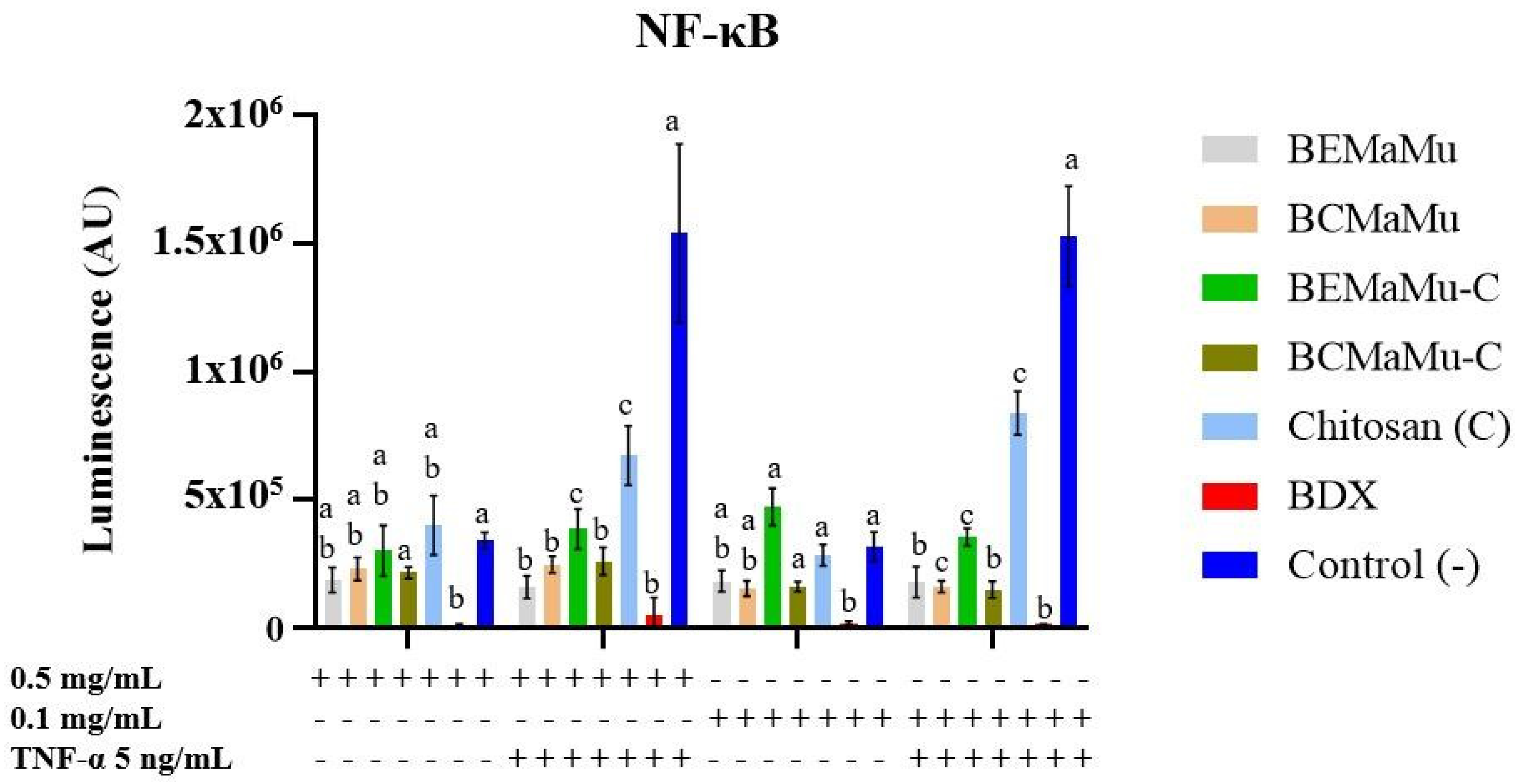
3.4.2. Anti-Inflammatory and Antioxidant Effect of the Extracts in NF-κB- and Nrf2-Transfected Caco-2 Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ma | freeze-dried maqui from Isla Natura de Chiloé, Chile. |
| Mu | freeze-dried murta from Isla Natura de Chiloé, Chile. |
| C | low-molecular-weight chitosan (Sigma-Aldrich, USA). |
| EMa | maqui extract obtained with EtOH/H2O (0.1% HCl, v/v) in an 80/20 v/v ratio. |
| EMu | murta extract obtained with EtOH/H2O (0.1% HCl, v/v) in an 80/20 v/v ratio. |
| CMa | maqui concentrate obtained by pre-HPLC. |
| CMu | murta concentrate obtained by pre-HPLC. |
| BMaMu | blend of maqui and murta freeze-dried powder in an 80/20 w/w ratio. |
| BEMaMu | blend of maqui and murta extract in an 80/20 w/w ratio. |
| BCMaMu | blend of maqui and murta concentrate obtained by pre-HPLC in a 94.5/5.5 w/w ratio. |
| BEMaMu-C | blend of maqui and murta extract in an 80/20 w/w ratio coated with a 1% w/v chitosan solution of acetic acid at a 1.25:1 ratio. |
| BCMaMu-C | blend of maqui and murta concentrate obtained by pre-HPLC in a 94.5/5.5 w/w ratio. coated with a 1% w/v chitosan solution of acetic acid at a 1.25:1 ratio. |
References
- Wu, T.; Cheng, H.; Zhuang, J.; Liu, X.; Ouyang, Z.; Qian, R. Risk factors for inflammatory bowel disease: An umbrella review. Front. Cell. Infect. Microbiol. 2025, 14, 1410506. [Google Scholar] [CrossRef]
- Jamieson, P.E.; Carbonero, F.; Stevens, J.F. Dietary (poly)phenols mitigate inflammatory bowel disease: Therapeutic targets, mechanisms of action, and clinical observations. Curr. Res. Food Sci. 2023, 6, 100521. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7563–7588. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Sun, Y.; Yao, X.; Zhu, Y.; Jafari, S.M.; Sun, B.; Wang, J. Stabilization of anthocyanins by simultaneous encapsulation-copigmentation via protein-polysaccharide polyelectrolyte complexes. Food Chem. 2023, 416, 135732. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Andrade, D.; Maldonado-Bravo, F.; Alburquerque, A.; Pérez, C.; Gamboa, A.; Caro, N.; Díaz-Dosque, M.; Gotelland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of Maqui (Aristotelia chilensis) extract in chitosan–tripolyphosphate and chenopodin-based systems. Antioxidants 2024, 13, 273. [Google Scholar] [CrossRef]
- Rezaie, M.; Farhoosh, R.; Iranshahi, M.; Sharif, A.; Golmohamadzadeh, S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015, 173, 577–583. [Google Scholar] [CrossRef]
- Hauser, C.; Peñaloza, A.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Promising antimicrobial and antioxidant extracts of Murta leaves (Ugni molinae Turcz): Shelf-life extension and food safety. Food Packag. Shelf Life 2014, 1, 77–85. [Google Scholar] [CrossRef]
- Liberal, Â.; Molina, A.K.; Pereira, C.; Dias, M.I.; Ferreira, I.C.F.R.; Barros, L. Solid-liquid extraction of polyphenols. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–112. [Google Scholar] [CrossRef]
- Lucas Tenório, C.J.; Assunção Ferreira, M.R.; Lira Soares, L.A. Recent advances on preparative LC approaches for polyphenol separation and purification: Their sources and main activities. Trends Food Sci. Technol. 2022, 128, 129–146. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the South Andes region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.A.; Fuentealba-Sandoval, V.; López, M.D.; Peña-Rojas, K.; Fischer, S. Accumulation of delphinidin derivatives and other bioactive compounds in wild maqui under different environmental conditions and fruit ripening stages. Ind. Crops Prod. 2022, 184, 115064. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Hassimotto, N.M.A.; Lajolo, F.M.; Fabi, J.P. Nanotechnology as a tool to mitigate the effects of intestinal microbiota on metabolization of anthocyanins. Antioxidants 2022, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Rosales, T.K.O.; Fabi, J.P. Nanoencapsulated anthocyanin as a functional ingredient: Technological application and future perspectives. Colloids Surf. B Biointerfaces 2022, 218, 112707. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Ng, K. Recent development in fabrication and evaluation of phenolic-dietary fiber composites for potential treatment of colonic diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 6860–6884. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Gómez, M.; Robert, P. Polyphenol content and antioxidant activity of maqui (Aristotelia chilensis [Molina] Stuntz) during fruit development and maturation in central Chile. Chil. J. Agric. Res. 2012, 72, 582–589. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.A.; Muñoz, L.A.; Fernández-López, J. Evaluation of protective effect of different dietary fibers on polyphenolic profile stability of maqui berry during in vitro gastrointestinal digestion. Food Funct. 2018, 9, 573–584. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Anthocyanin profiles in south Patagonian wild berries by HPLC–DAD–ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Garrido Makinistian, F.; Sette, P.; Gallo, L.; Bucalá, V.; Salvatori, D. Optimized aqueous extracts of maqui (Aristotelia chilensis) suitable for powder production. J. Food Sci. Technol. 2019, 56, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD–ESI/MSn and NMR-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Ortiz, T.; Argüelles-Arias, F.; Begines, B.; García-Montes, J.M.; Pereira, A.; Victoriano, M.; Vázquez-Román, V.; Pérez Bernal, J.L.; Callejón, R.M.; De-Miguel, M.; et al. Native Chilean berries preservation and in vitro studies of a polyphenol highly antioxidant extract from maqui as a potential agent against inflammatory diseases. Antioxidants 2021, 10, 843. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef]
- Sheikh, A.A.; Qazi, A.K.; Shah, A.M.; Mahajan, G.; Wani, N.A.; Hamid, A.; Moondhe, D.M. Chemoprevention of NCI-H322 lung cancer cells proliferation and tumor regression in murine ascites model by dietary flavonoid quercetin. Biointerface Res. Appl. Chem. 2022, 12, 6950–6959. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospects of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- López, J.; Vera, C.; Bustos, R.; Florez-Mendez, J. Native berries of Chile: A comprehensive review on nutritional aspects, functional properties, and potential health benefits. J. Food Meas. Charact. 2021, 15, 1139–1160. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and characterization of phenolic compounds and anthocyanins from murta (Ugni molinae Turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef]
- Plantas Medicinales Aromáticas. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2009, 8, 195–203. Available online: http://www.redalyc.org/articulo.oa?id=85617461004 (accessed on 20 March 2025).
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Molina, S.; Diaz, A.; Abugoch, L.; Diaz-Dosque, M.; Valenzuela, F.; Yazdani-Pedram, M. The effect of chitosan as internal or external coating on the 5-ASA release from calcium alginate microparticles. AAPS PharmSciTech. 2010, 11, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Protection by apple peel polyphenols against indometacin-induced oxidative stress, mitochondrial damage and cytotoxicity in Caco-2 cells. J. Pharm. Pharmacol. 2010, 62, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Apple peel polyphenol extract protects against indomethacin-induced damage in Caco-2 cells by preventing mitochondrial complex I inhibition. J. Agric. Food Chem. 2011, 59, 11501–11508. [Google Scholar] [CrossRef]
- Yang, J.L.; Sun, M.Y.; Yuan, Q.; Tang, S.; Dong, M.-J.; Zhang, R.-D.; Liu, Y.-Y.; Mao, L. Keap1-Nrf2 signaling activation by Bardoxolone-methyl ameliorates high glucose-induced oxidative injury in human umbilical vein endothelial cells. Aging 2020, 12, 10370–10380. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). PubChem Database: Octanol–Water Partition Coefficient [Internet]; U.S. National Library of Medicine: Bethesda, MD, USA, 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 4 May 2025).
- Food Database. Delphinidin 3-Sambubioside 5-Glucoside [Internet]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/44256891 (accessed on 4 May 2025).
- INRAE. Wageningen University & Plant & Food Research. PhytoHub: Cyanidin 3,5-O-Diglucoside [Internet]. Available online: https://phytohub.eu/entries/PHUB000498 (accessed on 4 May 2025).
- Rossetto, M.; Vanzani, P.; Mattivi, F.; Lunelli, M.; Scarpa, M.; Rigo, A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch. Biochem. Biophys. 2002, 408, 239–245. [Google Scholar] [CrossRef]
- Dai, F.; Chen, W.F.; Zhou, B. Antioxidant synergism of green tea polyphenols with α-tocopherol and l-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505. [Google Scholar] [CrossRef]
- Wang, K.; Hu, S. The synergistic effects of polyphenols and intestinal microbiota on osteoporosis. Front. Immunol. 2023, 12, 1285621. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Deng, Z.; Chen, F.; Zheng, L.; Pan, Y.; Xing, Q.; Tsao, R.; Li, H. Synergistic antioxidant effects of petunidin and lycopene in H9c2 cells submitted to hydrogen peroxide: Role of Akt/Nrf2 pathway. J. Food Sci. 2020, 85, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, Z.Y.; Chen, X.; Zhang, B.; Fan, Y.; Li, H. Synergistic antioxidant effects of phenolic acids and carotenes on H2O2-induced H9c2 cells: Role of cell membrane transporters. Food Chem. 2021, 341 Pt 2, 128000. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Jimenez, P.; Garcia-Diaz, D.F. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Bustos, F.; Valenzuela, X.; López-Carballo, G.; Vilariño, J.M.; Galotto, M.J. Chilean berry Ugni molinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res. Int. 2017, 102, 119–128. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, S.; Gao, X.; Huang, H.; Lao, F.; Dai, X. Protective Effect of Chitosan Oligosaccharide against Hydrogen Peroxide-Mediated Oxidative Damage and Cell Apoptosis via Activating Nrf2/ARE Signaling Pathway. Neurotox. Res. 2021, 39, 1708–1720. [Google Scholar] [CrossRef]
- Umarudin, U.; Widyarti, S.; Warsito, W.; Rahayu, S. In silico approach of bioactive molecule chitosan 501.1 kDa from snail shell as antioxidant and inhibitor of the keap1-nrf2 protein-protein interaction. Pharm. Educ. 2023, 23, 5–10. Available online: https://pharmacyeducation.fip.org/pharmacyeducation/article/view/2225 (accessed on 29 April 2025). [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-Glucosidase/α-Amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Castillo, R.L.; Beltrán, C.; Miranda, A.; Fuentes, J.; Gotteland, M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: Role of NF-κB and Nrf2. J. Nutr. Biochem. 2016, 27, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]

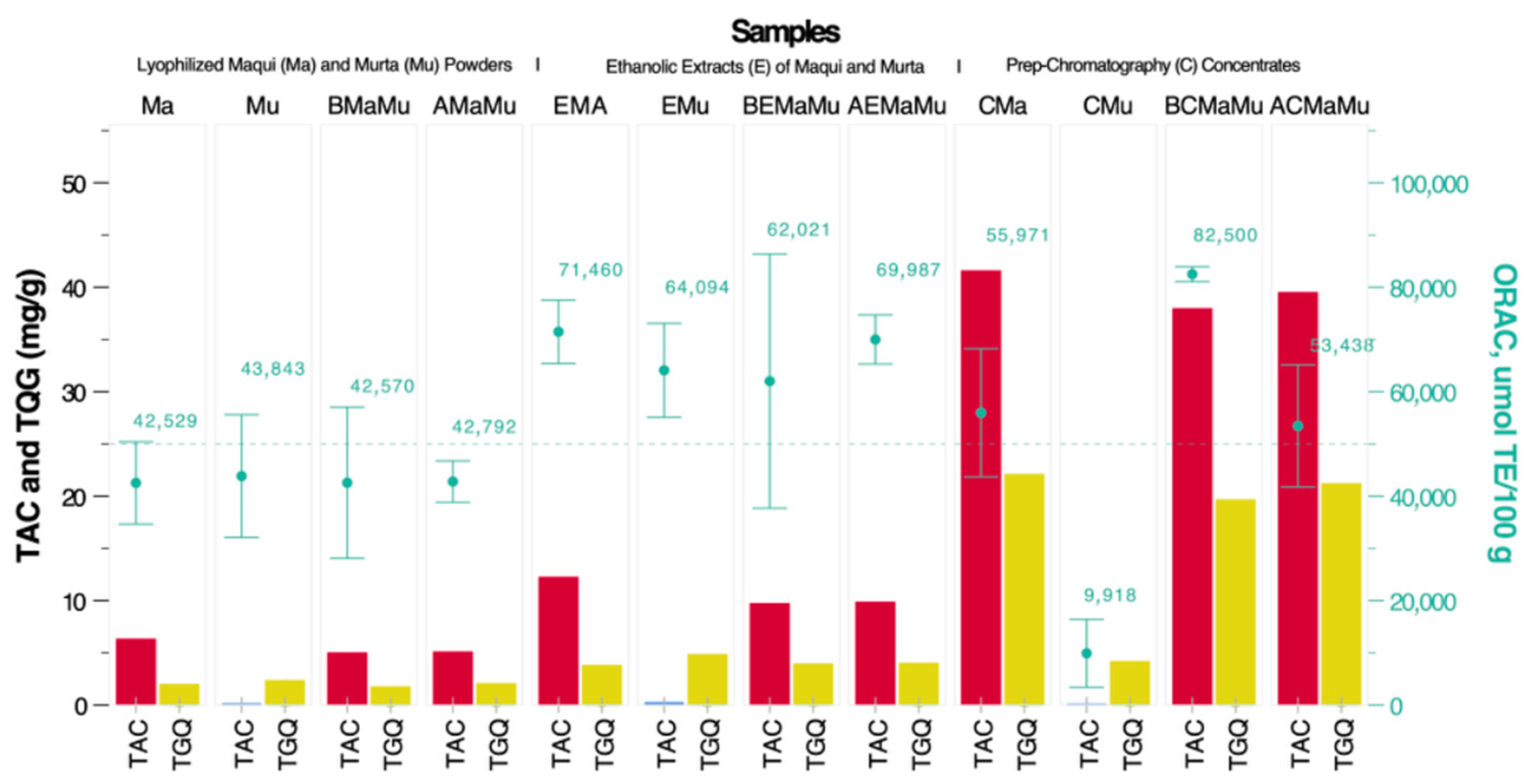
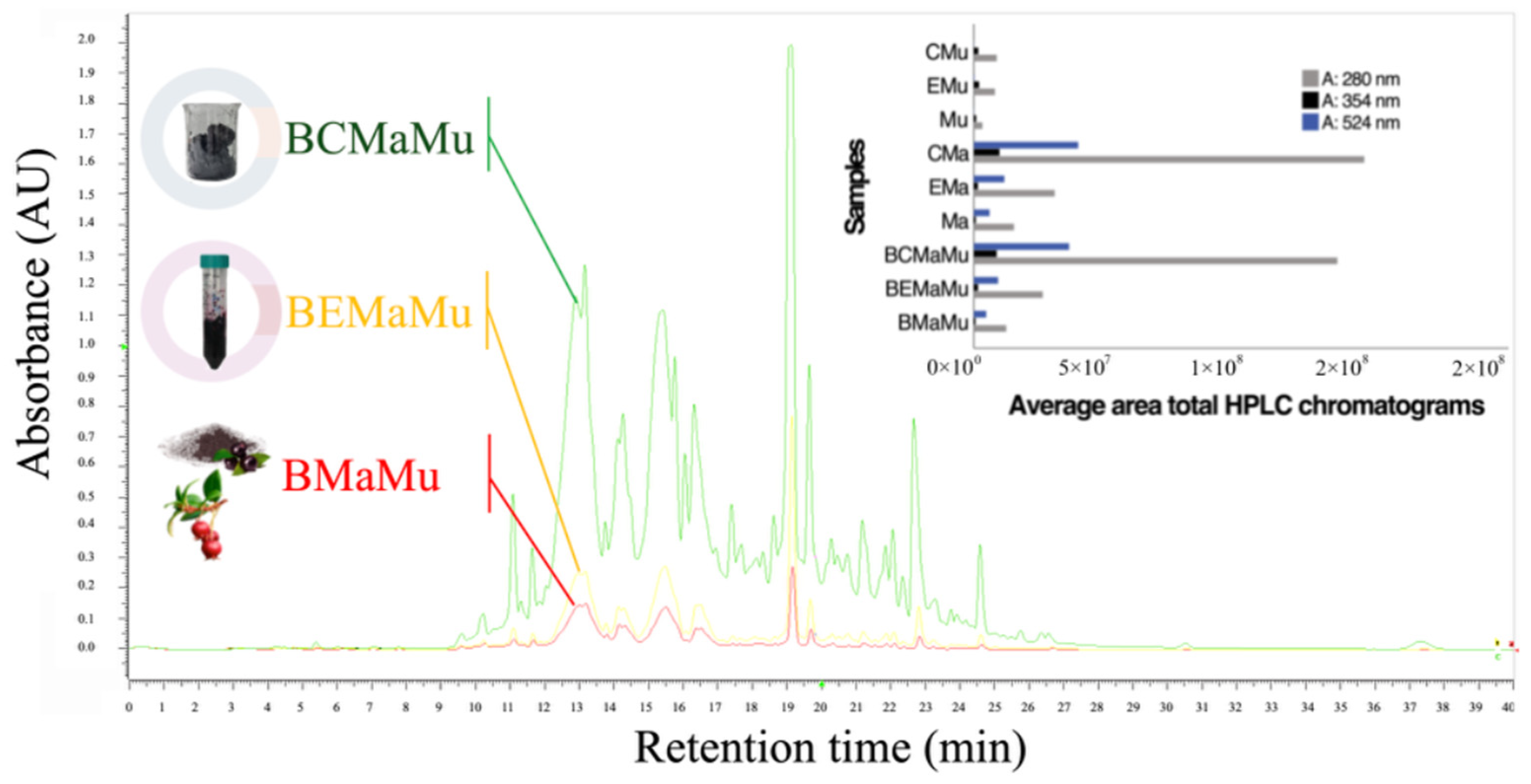




| Samples | N | Average ORAC, µmol ET/100 g | SD | p |
|---|---|---|---|---|
| Exp1. CMa 100% + CMu 0% | 3 | 55,971 a | 4940 | 0.000 |
| Exp2: CMa 50% + CMu 50% | 3 | 53,927 | 7951 | 0.999 |
| Exp3: CMa 0% + CMu 100% | 3 | 9918 b | 2622 | 0.000 |
| Exp4: CMa 75% + CMu 25% | 3 | 71,328 c | 6221 | 0.036 |
| Exp5: CMa 50% + CMu 50% | 3 | 36,488 d | 6441 | 0.006 |
| Exp6: CMa 25% + CMu 75% | 3 | 30,438 e | 4020 | 0.001 |
| Optimal mix: CMa 94.5% + CMu 5.5% | 3 | 82,500 f | 578 | 0.000 |
| Total | 21 | 48,653 | 24,027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alburquenque, A.; Busch, C.; Gómez-Lillo, G.; Gamboa, A.; Perez, C.; Fuentes, N.C.; Gotteland, M.; Abugoch, L.; Tapia, C. Obtainment of Flavonoid-Enriched Fractions from Maqui (Aristotelia chilensis) and Murta (Ugni molinae) Extracts via Preparative HPLC and Evaluation of Their Anti-Inflammatory Effects in Cell-Based Assays. Antioxidants 2025, 14, 600. https://doi.org/10.3390/antiox14050600
Alburquenque A, Busch C, Gómez-Lillo G, Gamboa A, Perez C, Fuentes NC, Gotteland M, Abugoch L, Tapia C. Obtainment of Flavonoid-Enriched Fractions from Maqui (Aristotelia chilensis) and Murta (Ugni molinae) Extracts via Preparative HPLC and Evaluation of Their Anti-Inflammatory Effects in Cell-Based Assays. Antioxidants. 2025; 14(5):600. https://doi.org/10.3390/antiox14050600
Chicago/Turabian StyleAlburquenque, Amador, Carolina Busch, Gabriela Gómez-Lillo, Alexander Gamboa, Camilo Perez, Nelson Caro Fuentes, Martin Gotteland, Lilian Abugoch, and Cristian Tapia. 2025. "Obtainment of Flavonoid-Enriched Fractions from Maqui (Aristotelia chilensis) and Murta (Ugni molinae) Extracts via Preparative HPLC and Evaluation of Their Anti-Inflammatory Effects in Cell-Based Assays" Antioxidants 14, no. 5: 600. https://doi.org/10.3390/antiox14050600
APA StyleAlburquenque, A., Busch, C., Gómez-Lillo, G., Gamboa, A., Perez, C., Fuentes, N. C., Gotteland, M., Abugoch, L., & Tapia, C. (2025). Obtainment of Flavonoid-Enriched Fractions from Maqui (Aristotelia chilensis) and Murta (Ugni molinae) Extracts via Preparative HPLC and Evaluation of Their Anti-Inflammatory Effects in Cell-Based Assays. Antioxidants, 14(5), 600. https://doi.org/10.3390/antiox14050600






