1. Introduction
Phenolic acids are a ubiquitous class of bioactive molecules that occur in plants in both free and conjugate forms [
1]. Their importance as vital dietary components in the human diet is well recognized by the scientific community, as well as their applicability in drug discovery as simple molecules protecting against oxidative stress, inflammation, and cancer [
2,
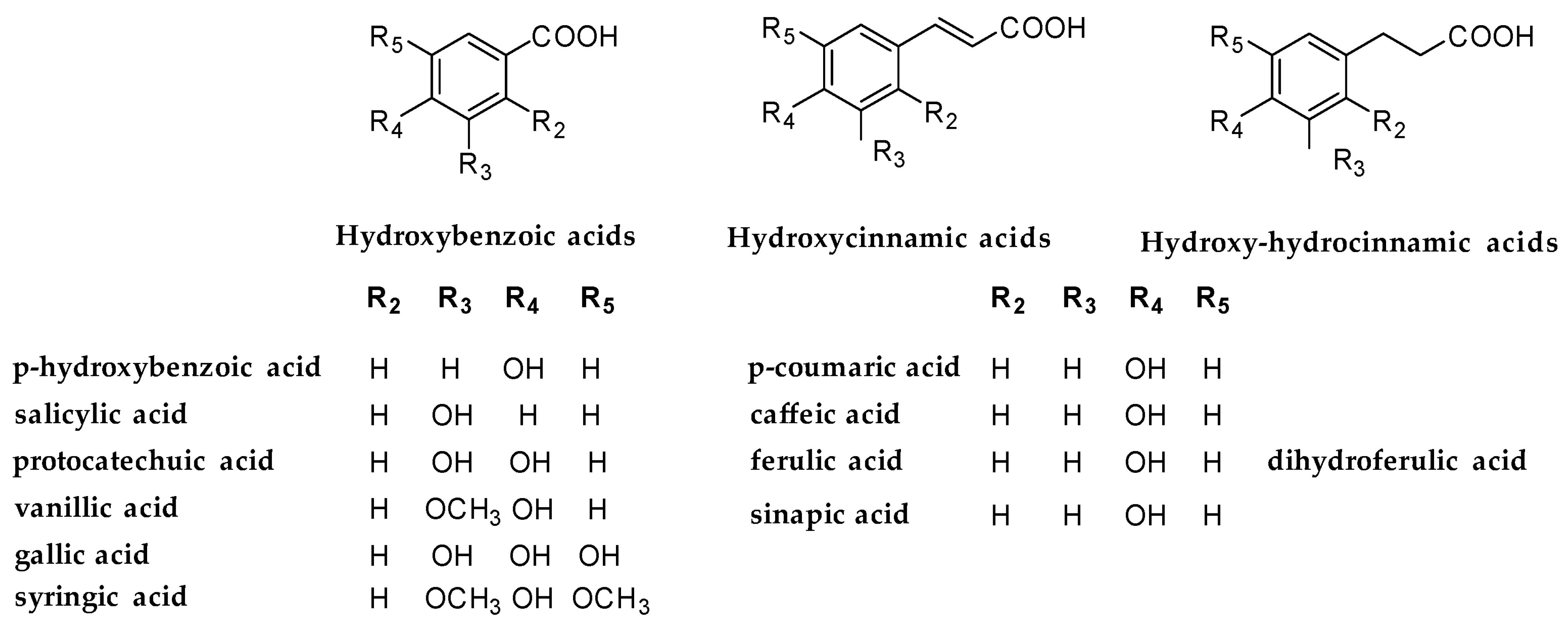
3]. Their activity is mainly due to the presence of a catechol group in a benzoic or a cinnamic acid (
Figure 1) which is responsible for strong antioxidant activity.
We recently demonstrated that natural phenolic alcohols originate from the biosynthetic transformation of the amino acid tyrosine, namely hydroxytyrosol and tyrosol, and can be used as synthetic building blocks for a new generation of donepezil-like analogs that act as neuroprotective agents [
4,
5,
6]. These results inspired the hypothesis that phenolic alcohols, originating from natural phenolic acids upon reduction, could provide new building blocks for the design of new compounds that are potentially active against neurodegeneration.
Grape pomace is an agricultural waste composed of grape skins, seeds, and stems, accumulated in tons during the processes of wine production [
7]. The composition of grape pomace depends, among other factors, on the soil type, the grape variety, and agro-climatic conditions and is characterized by the presence of several classes of phenolic compounds, such as anthocyanins, flavonoids, tannins, and phenolic acids [
7,
8,
9,
10,
11]. The latter are found as conjugated molecules that have been esterified with other hydroxy acids, mono/di-saccharides, and polymers, while a minor percentage are present as free monomers [
1,
7]. The overproduction of grape pomace is a problem for the wine production sector due to the high cost of its disposal. Conversely, the valorization of biomass from wineries can be a valuable solution from both ecological and economic perspectives. In the last 10 years, many extraction procedures have been proposed to recover bioactive molecules from grape pomace [
7] exploiting classical solid–liquid extractions, including using hydroalcoholic solvents both alone [
8,
9,
10] and in combination with alternative heating sources, such as microwaves (MAE) and ultrasound (UAE) [
10,
11,
12,
13], which allows for the enhancement of extraction yields, lowering the energy demands of the process. Most of them have been oriented toward the non-selective recovery of the most bioactive molecules, regardless of their specific chemical structure.
More recently, natural deep eutectic solvents (NADES) have been proposed as non-volatile and sustainable solvents, being compatible with both classical and alternative extraction techniques [
12] in the recovery of phenols from biomass in general [
13,
14,
15,
16,
17,
18,
19], and from grape pomace in particular [
14,
15,
20,
21,
22,
23,
24]. Among the several advantages of using natural deep eutectic solvents, such as being able to work at high temperatures without evaporation and being non-toxic for both users and the environment, NADES allow for the tuning of the physical–chemical properties of the solvent, such as viscosity and pH, in order to selectively extract a specific class of compounds [
16,
17]. Moreover, being composed of a mixture of a hydrogen-bond acceptor (HBA) and a hydrogen-bond donator (HBD), their selectivity can be fine-tuned in the same range of physical–chemical characteristics thanks to the specific intramolecular interactions that occur between the bioactive components to be extracted and the components of the NADES themselves [
18].
Since the beginning of research on the valorization of grape pomace, it has been clear to the scientific community that the first parameter-enhancing phenol extraction method involved the pH [
19]. Indeed, acidic conditions (especially citric acid) were able, at the same time, to easily destroy the cell membrane, to stabilize anthocyanins, and to promote the hydrolysis of phenolic acid conjugated forms.
A pioneering work by Putnik et al. demonstrated that adding 1% mol of HCl to the extraction solvent had a positive influence on the extraction yields of hydroxycinnamic acids (HCAs), more so than increasing the temperature or prolonging processing times [
20,
21]. The use of carboxylic acid-based NADES, particularly those with low pH, has been reported to effectively enhance the extraction of phenolic compounds, as these conditions favor the recovery of biomolecules in their neutral form [
21]. Recently, acidic NADES formulated with lactic and oxalic acids have shown superior performance compared to their non-acidic counterparts in the extraction of phenolics (anthocyanins, flavonoids, and phenolic acids) particularly when coupled with ultrasound-assisted techniques [
22,
23].
It is worth noting that the pHs of carboxylic-acid based NADES decrease with increasing temperature [
24]. Such an aspect was relatively underestimated in the extraction protocols using NADES as an extraction medium for grape pomace, which have been mainly performed between 45 °C and 65 °C to avoid the degradation of thermolabile compounds such as anthocyanins [
22,
23].
Usually, a high temperature, typically around 90 °C, is needed to obtain phenolic acids in their free form [
25]. It is likely that phenolic acids are stable until 150 °C [
26]. On the other hand, it has been demonstrated that carboxylic acid-based DES are stable until temperatures of up to 100 °C, where a small proportion of esterification occurs [
27].
In this work, we hypothesized and demonstrated that the combination of high-temperature extraction techniques (90–100 °C) with acidic NADES improves the hydrolysis of phenolic acids from their ester forms and optimizes their selective extraction to obtain them as purified molecules. We made a comparative analysis of five acidic–hydroalcoholic mixtures, with five acidic deep eutectic solvents, under three different extraction techniques, namely solid–liquid, microwave-assisted, and ultrasound-assisted extraction. We evaluated the total phenolic content (TPC), hydroxycinnamic acid content (HCA), and total anthocyanin content (TA) of all samples. A UHPLC-ESI-HRMS quantitative analysis of the phenolic profile, obtained after solvent purification for the best records, was also performed to provide a more reliable solution to exploiting grape pomace as a source of pure phenolic acids. Finally, an ORAC test on the best extraction mixture compared to the purified phytocomplex was performed to clarify the effect of the extraction medium on the antioxidant power against ROS (radical oxygen species). The extract enriched in free phenolic acids could represent a feedstock of active molecules that, after isolation, can serve as building block for the synthesis of pharmaceutical active ingredients against neurodegeneration.
2. Materials and Methods
2.1. Grape Pomace
Dried grape pomace (residual water content, 7.3%) composed of a blend of Nerello, Merlot, and Cabernet Sauvignon grapes, seeds, and stems was provided by Azienda Agricola De Fazio (Calabria, Italy). Grape pomace was stored under vacuum and used without any selection or pre-treatment.
Figure S1 (Supplementary Materials) shows the dimension of the unmodified grape pericarps used for extraction. The calculation of the yields of phenolic compounds was based on grams of dry grape pomace.
2.2. Chemicals and Reagents
All chemicals and reagents, namely ethanol, citric acid, oxalic acid, (±) lactic acid, HCl, choline chloride, potassium chloride, sodium acetate, sodium carbonate, Folin–Ciocalteu reagent, AAPH (2,2′-Azobis(2-methylpropionamidine) dihydrochloride), fluorescein sodium salt, phosphate buffer solution, and Trolox were purchased from Merk Italy (Milan, Italy). Purified water was obtained through a Milli-Q Integral 5 system (Millipore, Merck KGaA, Darmstadt, Germany).
Analytical standards of caffeic acid, gallic acid, protocatechuic acid, and trans-ferulic acid were purchased from Merck Italy (Milan, Italy). Working solutions were prepared daily.
2.3. Preparation of Hydroalcoholic Solutions
Hydroalcoholic solutions for extraction were prepared by mixing 60% of ethanol with 40% of acidified water. HCl, citric acid, and oxalic acid were used to acidify the water and obtain five solutions (HA) at five different values of final pH: 2.5%
v/
v HCl (HA1), 3 g/L citric acid (HA2), 15 g/L citric acid (HA3), 30 g/L citric acid (HA4), 1.4 g/L oxalic acid (HA5). For the pH values of the final solutions, please refer to
Table 1 [
7].
2.4. Preparation of Carboxylic-Acid-Based NADES
The NADES used as extraction solvents were prepared by mixing ChCl and carboxylic acids in the proportions reported in
Table 1, under stirring at 80 °C for 2 h. Five different NADES were prepared: ChCl/citric acid 1:1 mol (DES1), ChCl/citric acid 1:2 mol (DES2), ChCl/oxalic acid 1:1 mol (DES3), ChCl/oxalic acid 1:2 mol (DES4), ChCl/lactic acid 1:2 mol (DES5). IR and
1H-NMR measurements on the NADES were compared with those reported in the literature (See
Supplementary Materials Figures S8–S19) [
28,
29,
30]. A measurement of the pH was performed according to the method reported by Jurić et al. [
31]. A total of 20%
w/
w of H
2O was added to freshly prepared NADES before using them as an extraction solvent.
Table 1.
Chemical composition and pH value of the extraction solvents at 25 °C.
Table 1.
Chemical composition and pH value of the extraction solvents at 25 °C.
| Entry | Solvent | Chemical Composition | Final pH |
|---|
| 1 | HA1 | 60%wt EtOH/40% H2O with 2.5% HCl | 1.5 |
| 2 | HA2 | 60%wt EtOH/40% H2O with 3 g L−1 citric acid | 3.4 |
| 3 | HA3 | 60%wt EtOH/40% H2O with 15 g L−1 citric acid | 2.8 |
| 4 | HA4 | 60%wt EtOH/40% H2O with 30 g L−1 citric acid | 2.5 |
| 5 | HA5 | 60%wt EtOH/40% H2O with 1.4 g L−1 oxalic acid | 2.8 |
| 6 | DES1 | ChCl/citric acid 1:1 mol | 1.5 1 |
| 7 | DES2 | ChCl/citric acid 1:2 mol | 1.0 1 |
| 8 | DES3 | ChCl/oxalic acid 1:1 mol | 1.1 1 |
| 9 | DES4 | ChCl/oxalic acid 1:2 mol | 1.2 1 |
| 10 | DES5 | ChCl/lactic acid 1:2 mol | 1.8 1 |
2.5. Solid–Liquid Extraction of Grape Pomace with HA Solutions
Hydroalcoholic solutions (HAs) were added to dry grape pomace at a 1:10 solid–liquid ratio, in a Soxhlet apparatus, under stirring at reflux temperature (100 °C) for 2 h. The solid matrix was then removed by filtration under vacuum, washed with water until reaching a final dilution of 1:10 v/v, and the solution was used without any other purification for spectrophotometric measurements.
2.6. Solid–Liquid Extraction of Grape Pomace with NADES Solutions
NADES with 20% w/w of H2O were mixed with dry solid grape pomace with a proportion of 1:10 w/w and heated at 100 °C under stirring for 2 h. Then, the suspension was cooled to r.t. and centrifuged to separate the solid fraction from the supernatant. The separated solution was then diluted 1:10 v/v with water, to break the NADES supramolecular interactions, and used for spectrophotometric measurements.
2.7. Microwave-Assisted Extraction (MAE) of Grape Pomace
Microwave-assisted extraction (MAE) of grape pomace was realized in a CEM Discover SP oven (Matthews, NC, USA). A total of 1 g of dry pomace was suspended in 10 g of DES3 or DES5 (with the addition of 20% v/v of H2O) in a 30 mL glass tube sealed with a Teflon cup. Microwave heating was performed in dynamic mode at a power of 80 W at temperatures of 65 °C and 100 °C for 10, 30 and 45 min. The extraction solution was then filtered, diluted with water (1:10 v/v), and used for spectrophotometric measurements.
2.8. Ultrasound-Assisted Extraction (UAE) of Grape Pomace
Ultrasound-assisted extraction (UAE) of grape pomace was performed using a titanium high-power US horn by Danacamerini (Turin, Italy). In a glass round-bottomed flask, 1 g of dry pomace was added to 10 g of DES3 or DES5 (with the addition of 20% v/v of H2O) and the flask was immersed in an ice bath to control the temperature in the range of 45–65 °C during extraction. The horn was immersed in the solution and the US was activated (214 KHz, 50 W) for 10, 30, and 45 min. At the end, the extraction mixture was filtered, diluted with water (1:10 v/v), and used for spectrophotometric measurements.
2.9. Total Phenolic Content (TPC)
The total phenolic content (TPC) was measured by Folin–Ciocalteu spectrophotometric assay [
32]. Briefly, 1 mL of the extraction solution was combined with 5 mL of Folin–Ciocalteu reagent (diluted 1:10) in a 50 mL graduated flask. After 4 min, 4 mL of Na
2CO
3 solution at 7.5%
w/
v (15%
w/
v for NADES extracts) was added and stirred for 30 min in the dark. The absorbance at 765 nm was recorded in a UV–visible spectrophotometer (UV/VIS Spectrometer Lambda 35, PerkinElmer, Waltham, MA, USA). A calibration curve of gallic acid at concentrations of 50, 100, 150, 250, and 500 mg L
−1 was used (R
2 = 0.995, see
Figure S2). The total phenolic content was determined as mg of gallic acid equivalents per g (mg GAE g
−1) of dry pomace.
2.10. Hydroxycinnamic Acid Content (HCA)
The total content of hydroxycinnamic acids was determined as previously reported in the literature by Howard et al. [
33]. Briefly, 0.25 mL of the extraction solution was mixed with 0.25 mL of a 1 g L
−1 solution of HCl in aqueous ethanol (96%
v/
v) and 4.5 mL of a 2 g L
−1 solution of HCl (1 M). The absorbance at 320 nm was recorded in a UV–visible spectrophotometer (UV/VIS Spectrometer Lambda 35, PerkinElmer, Waltham, MA, USA). A calibration curve of caffeic acid at concentrations of 1, 2.5, 5, 10, and 25 mg L
−1 in aqueous methanol (80%
v/
v) was used (R
2 = 0.991, see
Figure S3). The HCA content was determined as mg of caffeic acid equivalents per g (mg CAE g
−1) of dry pomace.
2.11. Total Anthocyanin Content (TA)
The total anthocyanin content was determined as previously reported in the literature by Lee et al. [
34]. The absorbance of the extraction solutions was determined at two different wavelengths, namely 510 nm and 700 nm, and two different pHs, namely 1.0 and 4.5. Briefly, 1 mL of the extraction solutions was mixed with 9 mL of either a pH 1.0 buffer (0.02 M KCl adjusted with HCl) or a pH 4.5 buffer (0.2 M sodium acetate). The absorbance at both 510 nm and 700 nm was recorded in a UV–visible spectrophotometer (UV/VIS Spectrometer Lambda 35, PerkinElmer, Waltham, MA, USA), and the anthocyanin content was expressed as CGE-cyanidin-3-glucoside equivalent (mg CGE g
−1 dry pomace) according to the following equation [
34]:
with
where
Mw—molecular weight of cyanidine-3-glucoside (449.2 g mol−1);
DF—dilution factor;
V—volume of the extracting solution (L);
M—mass of the solid material (g);
ε—molar extinction coefficient of cyanidine-3-glucoside (26,900 L cm−1 mol−1);
L—path length (1 cm).
2.12. Purification from NADES
DES3 and DES5 extracts obtained by MAE were selected as preferred samples for characterization and phenolic acid quantification by UHPLC-ESI-HRMS, in comparison to the HA2 extract. Before analysis, DES samples were purified from NADES by elution through a Sepabeds Dion HP20 styrere/polyvinylbenzyl resin. Briefly, 10 mL of extract was eluted on a 10 g resin column, activated with 50 mL of ethanol followed by 50 mL of water. ChCl was removed by washing the column with 125 mL of water, then the phenolic acids were recovered washing with 150 mL of ethanol. The ethanolic phase was collected, evaporated under reduced pressure and suspended in 1 mL of EtOH for analysis. In contrast, the HA2 solution was used without any other purification for analysis.
2.13. UHPLC-ESI-HRMS Analysis
UHPLC-ESI-HRMS analysis was performed as reported by Roppolo et al. [
35]. Briefly, separation was performed by a Dionex Ultimate 3000 RS (Thermo Scientific, Rodano, MI, Italy) equipped with a Hypersil Gold C18 column (100 × 2.1 mm, 1.9 µm particle size). The chromatographic column, maintained at a temperature of 30 °C, was equilibrated in 98% solvent A (0.1% formic acid in ultrapure water) and 2% solvent B (methanol). The concentration of solvent B was linearly increased from 2% to 23% in 6 min, remained in isocratic conditions for 6 min, and finally returned to 2% in 6 min, remaining in isocratic conditions for 3 min. The flow rate was maintained at 300 µL min
−1. The volume of the injected sample was 5 µL. The total run time, including column wash and equilibration, was 38 min.
A high-resolution Q-Exactive orbitrap mass spectrometer (Thermo Scientific, Rodano, MI, Italy) with an electrospray ionization source, operating in negative mode, was used for detection with the following operating conditions: 70,000 resolving power (defined as FWHM at m/z 200), IT 100 ms, ACG target = 1 × 106, scan range (100–900 m/z). MS/MS analysis were performed according to the following operating conditions: resolution, 35.000; AGC target = 1 × 105; maximum IT, 200 ms; collision energy (stepped NCE), 20, 30, 40. The quadrupole isolation window was set to 2.0 m/z. High-purity nitrogen was used as the sheath gas (30 arb units) and auxiliary gas (10 arb units).
Compounds were characterized according to the corresponding HRMS spectra, retention times, accurate masses, and characteristic fragmentations. Xcalibur software (version 4.1) was used for instrument control, data acquisition, and data analysis.
Individual concentrations of extracted phenolic acids were derived by the external calibration curves of the respective commercial analytical standards. In particular, the concentrations of gallic acid (
m/
z 169.0133), syringic acid (
m/
z 197.0808), and shikimic acid (
m/
z 173.0807) were obtained with respect to a calibration curve of gallic acid (r
2 = 0.9934, see
Figure S4a) in the range between 0.5 and 100 mg L
−1. The concentrations of protocatechuic acid (
m/
z 153.0184), salicylic acid (
m/
z 137.023), and benzoic acid (
m/
z 121.0284) were obtained with respect to a calibration curve of protocatechuic acid (r
2 = 0.9984, see
Figure S4c) in the range between 0.05 and 100 mg L
−1. A preset caffeic acid standard calibration curve (r
2 = 1, See
Figure S4b) in the concentration range of 0.01–100 mg L
−1 was used to determine the content of coumaric acid (
m/
z 163.0387). Finally, the dihydroferulic acid (
m/
z 195.0287) content was determined using a trans-ferulic acid calibration curve (r
2 = 0.9955, see
Figure S4d) in the range of 0.5–100 mg L
−1 and 0.5–10 mg L
−1.
2.14. Statistical Analysis
A statistical evaluation for differences was performed on data coming from spectrophotometric assays.
Data were expressed as the mean ± standard deviation (SD) on three repetitions processed by one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons (GraphPad Prism 10.4.0 scientific software)
Tables S1–S3 report the
p-value for all data statistically evaluated (
Supplementary Materials).
2.15. ORAC Test
The oxygen radical absorbance capacity (ORAC) test was performed on a microplate fluorometer Varioskan LUX (Thermo Scientific™), controlled by Thermo Scientific™ Skanlt™ Software (Waltham, MA, USA) for microplate readers. The test was performed following the procedure previously published by Nardi et al. [
36] for NADES mixtures, on 1 mg mL
−1 concentrated samples. The final ORAC
FL values were expressed as Trolox equivalents (μmol g
−1 of dry grape pomace) as a mean of three different measurements.
4. Discussion
Phenolic acids are present in grape pomace in a conjugate form; their extraction needs acidic hydrolysis to be efficient [
24,
25]. In our work, we explored different acidic extraction media, both hydroalcoholic solutions and NADES, in combination with alternative energy sources, for the in situ hydrolysis and extraction of grape pomace, with the aim of optimizing the yield of phenolic acids. In the preliminary part of the work, a comparative study was performed to understand the dependence of phenolic acid extraction efficiency on pH and on the nature of the acid. Our results clearly indicate that, although strongly acidic conditions are essential to hydrolyze and extract phenolic acids, the chemical nature of the acid showed an even greater influence than pH alone in determining the selectivity and efficiency of extraction. Specifically, it was found that carboxylic acids are more efficient than HCl at the same pH values, with citric acid proven most effective for the extraction of anthocyanins, while lactic and oxalic acid showed a superior performance for phenolic acid recovery. Indeed, we found that oxalic- and lactic-acid-based DES, in combination with microwave irradiation, were able to efficiently hydrolyze and extract phenolic acids. For instance, the extraction of grape pomace with ChCl/oxalic acid 1:1 DES, heated for 10 min at 100 °C, under microwave irradiation, produced a phytocomplex containing, in addition to other phenols, 1.15 mg g
−1 of phenolic acids. This result is significantly higher than those reported in the literature. Indeed, classic solid–liquid extractions with hydroalcoholic solutions, acidified with both organic or inorganic acids, have produced an amount of phenolic acids two orders of magnitude lower than our method [
20]; on the other hand, both US- and MW-assisted methods using non-acidic DES have extracted phenolic acids in the range of 2.74–160 μg g
−1 [
14,
15]. Among the few comparable examples, the work of Alrugah et al. [
25] employing a non-acidic ternary DES under US assistance (1 h, 65 °C) also reported high percentages of phenolic acids from grape pomace. However, their quantification relied on HPLC-UV following sample pre-treatment consisting of acidic hydrolysis, which increased the amount of free phenolic acids. No data were reported on the amount of free phenolic acids after extraction.
Regarding acidic NADES, lactic-acid-based DES under US assistance have been reported in the literature as the best solvents for phenolic acid recovery [
23], ranging between 0.7 and 1.0 mg g
−1. Nevertheless, our study was the first report employing UHPLC-ESI-HRMS as a quantification method to support spectrophotometric assays. We reported here that lactic acid NADES underwent the spontaneous polymerization of lactic acid and such polymers cannot be separated by the natural extract using the styrene/polyvinyl resin purification, as suggested for the separation of natural extracts from NADES media [
22]. Indeed, UHPLC-ESI-HRMS analysis revealed that the final phytocomplex is dirty, due to the presence of such polymers, which had a non-zero absorbing power at 320–330 nm, thus interfering with spectrophotometric determination. Moreover, HPLC-UV methods working at different wavelengths (254, 370, 560 nm), often reported in the literature [
22,
23] for phenolic acid quantification, were not able to detect such polymers, thus inducing an error in the interpretation of the experimental data referred of lactic-acid-based NADES. Some studies reported the temperature instability of lactic acid based NADES, even for low temperatures [
27]. Despite these findings, our work demonstrated that, independently from the heating source and the temperature of extraction, lactic acid based NADES are not the most suitable media for natural matrix extraction, due to their thermal instability; moreover, spectrophotometric assays and HPLC-UV quantifications are not the most reliable and exhaustive methods to characterize such extracts.
Concerning the alternative heating sources explored in this work, microwave irradiation (MAE), at a high temperature (100 °C) for a short time (10 min) was the most efficient within our scope. Indeed, MAE allowed us to reach comparable yields with respect to classical solid–liquid extraction in shorter time, thus resulting energetically more sustainable. Nevertheless, prolonged time of exposure was detrimental for the phytocomplex. In our study, UAE performed slightly less well than MAE as a technique to obtain free phenolic acids after acid hydrolysis. Indeed, UAE was often reported in the literature as the softest technique to extract temperature-labile molecules without breaking their structure [
22,
23].
Finally, it is necessary to briefly discuss the ecotoxicity and human toxicity of such NADES. According to the literature, the eco-compatibility of NADES against organic solvents is due to their low volatility, but the final disposal of NADES and the toxicity of their single components is controversial. It has been established that ChCl itself is non-toxic [
52], while ChCl/carboxylic acid NADES have moderate toxicity against Gram-negative and Gram-positive bacteria [
53,
54], marine bacteria [
55], and eukaryotic cells [
49]. Such low toxicity is mainly correlated with the carboxylic acid, with EC
50 values comparable to those of the single component and dependent on the acid content. In more detail, considering the carboxylic acids used in this study, the order of toxicity was lactic acid < citric acid < oxalic acid. The acid character is responsible for protein denaturation, and consequent activation of enzymatic pathways leading to cell death [
49,
52,
53,
54,
55]. On the other hand, low-to-negligible phytotoxicity was reported for all carboxylic-acid-based NADES [
49].
Concerning the specific phenolic acids present in the grape pomace extract, dihydro-3-coumaric acid (420 μg g−1) was the most representative, followed by gallic acid (308 μg g−1). More generally, we found that hydroxybenzoic acids were more representative than hydrocinnamic acids. Further studies to obtain such phenolic acids as isolated molecules are needed.
On the other hand, the possibility of using the DES3 extract as a “ready-to-use” antioxidant formulation was demonstrated by the ORAC test, performed on the phenolic extract with and without NADES. This is a standardized test usually applied to measure the ROS chain-breaking activity of food and beverages, by H atom transfer. Catecholic compounds, such as phenolic acids, are particularly reactive, as they can originate resonant stable radicals after hydrogen transfer, due to the intramolecular hydrogen interaction between the
o-diphenolic compounds [
56].
Indeed, our results confirmed that, even if the DES3 itself has no activity against ROS, the formulation of phenols in the DES had an antioxidant activity higher than hydroalcoholic extracts with comparable TPC values, previously reported in the literature [
50]. Although, after dilution, our extraction mixture cannot still be considered a NADES, this result revealed a role of NADES components in the antioxidant power of the mixture. Potential applications of ChCl/oxalic acid 1:1 NADES as a food preservative or antioxidant active ingredient in pharmaceutical formulations have been investigated in the literature [
49]. As mentioned in a previous study, it showed medium toxicity against HeLa and MCF-7 cells (EC
50 of 330.90 ± 29.75 and 558.98 ± 54.32 mg L
−1, respectively), while negligible toxicity was found in normal HEK293Tcells (EC
50 > 2.000 mg L
−1). Moreover, antimicrobial and antibacterial activity against several Gram-positive and Gram-negative bacteria and yeast was found, due to the presence of oxalic acid [
49]. Based on these preliminary results, the potential applications of the DES3 extraction mixture as a “ready-to-use” preparation in food preservation, especially for those applications exploiting its antibacterial power, need to be further explored. Other antioxidant activity assays, both chemical (DPPH, ABTS, and FRAP assays) and cellular (H
2O
2-induced ROS inhibition in cell culture), need to be performed.
In addition, we demonstrated that the method provides, after purification from NADES, an extract enriched in phenolic acids with improved antioxidant activity relative to the simple formulation. Future work will focus on optimizing separation techniques to obtain the single components of the extract. Single phenolic acids will be used as chemical building blocks for the synthesis of nature-inspired active ingredients against neurodegeneration.