Effects of Mogroside V on Quality and Antioxidant Activity of Boar Frozen–Thawed Sperm
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Preparation
2.2. Animals, Semen Collection, and Sample Preparation
2.3. Experimental Protocols
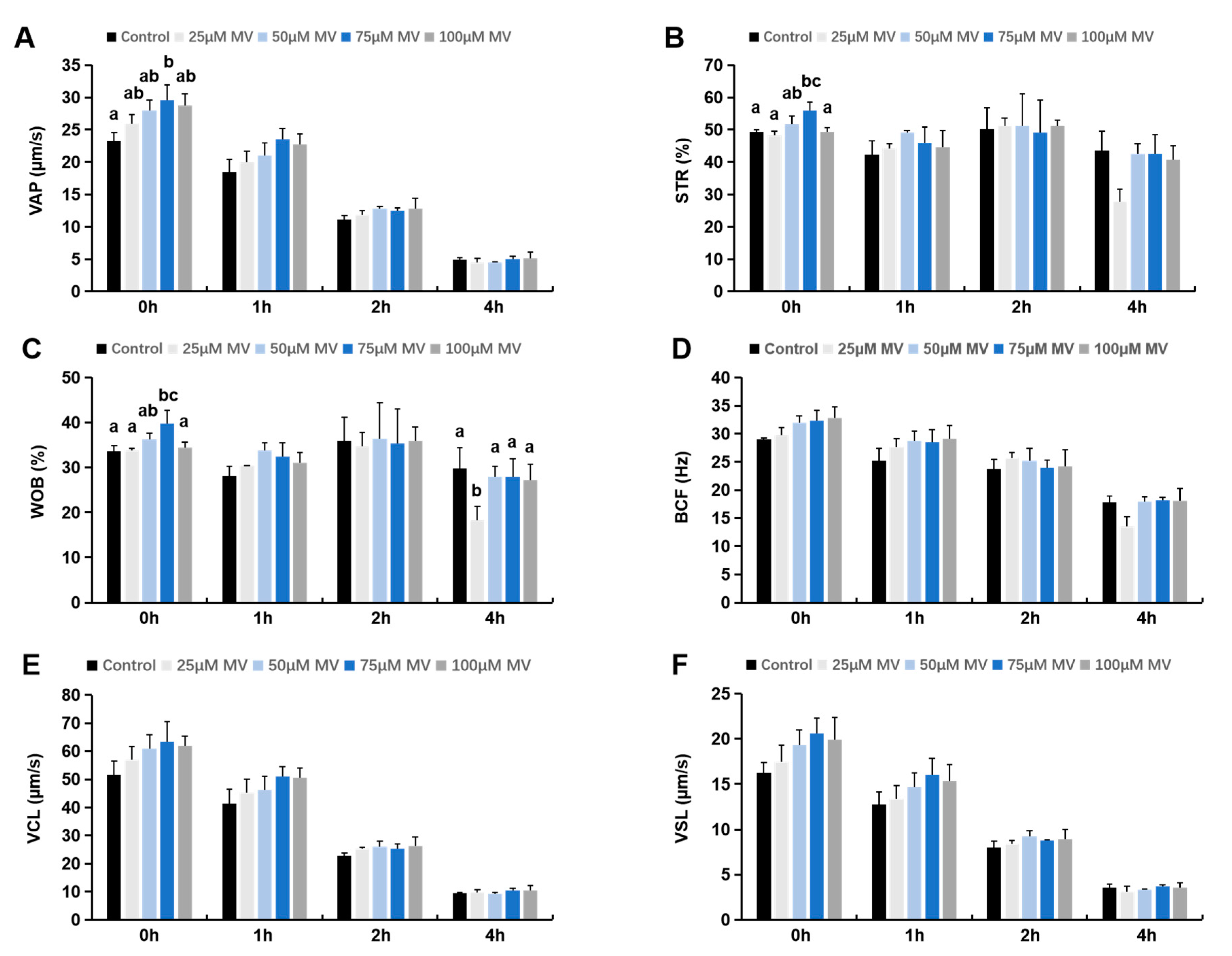
2.4. Detection of Sperm Motility and Kinetic Parameters
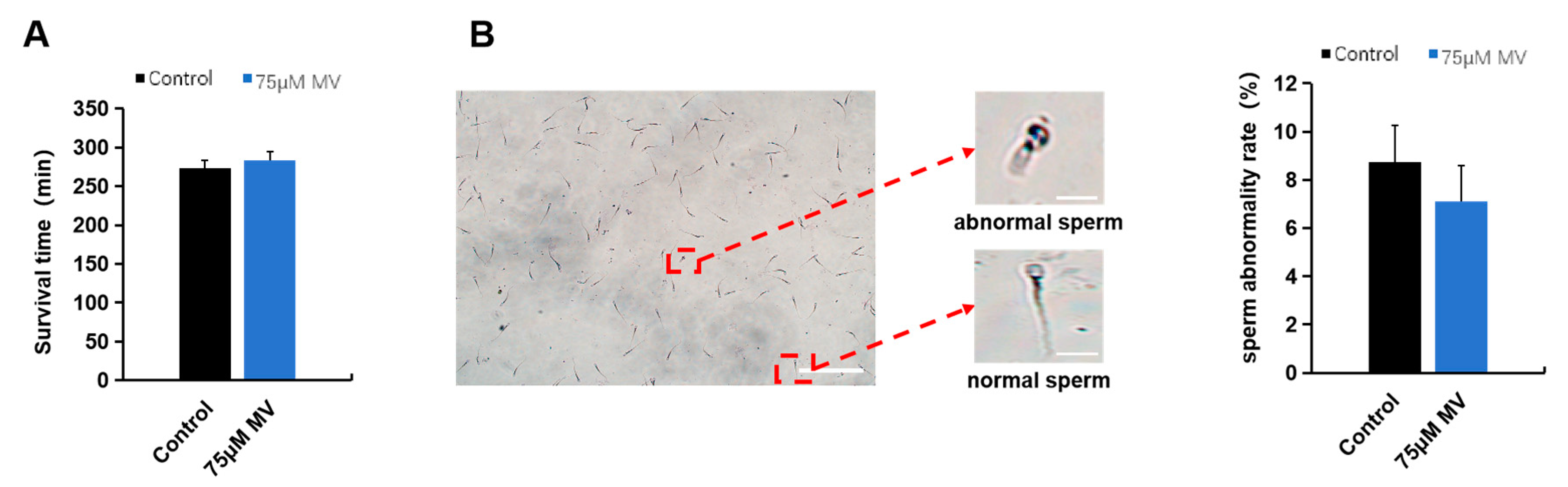
2.5. Measurement of Sperm Survival Time
2.6. Evaluation of Sperm Morphology
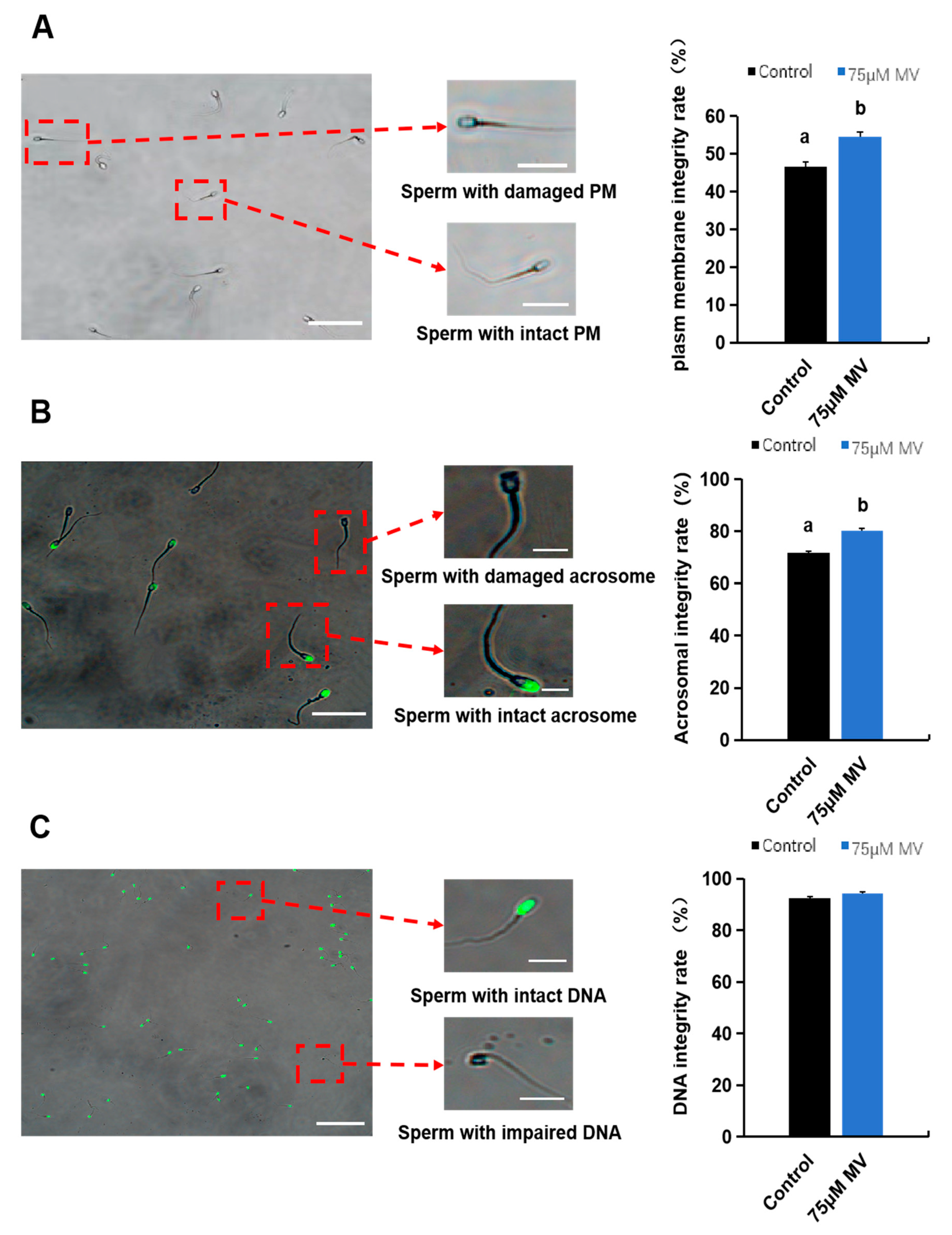
2.7. Analysis of Plasma Membrane Integrity
2.8. Analysis of Acrosome Integrity
2.9. Analysis of DNA Integrity
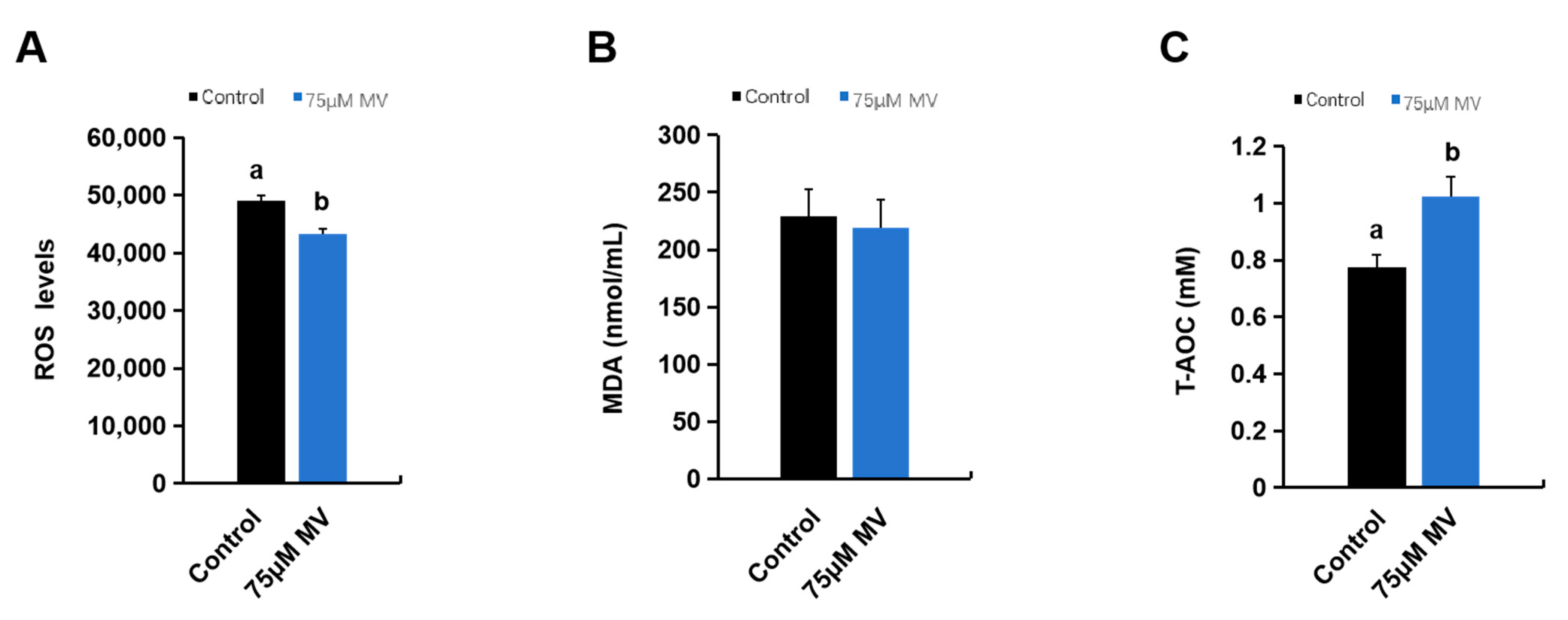
2.10. ROS Content Assay
2.11. MDA Content Assay
2.12. T-AOC Activity Assay
2.13. Statistical Analysis
3. Results
3.1. Effects of MV on Motility and Kinetic Parameters of Frozen–Thawed Sperm
3.2. Effects of MV on Quality Parameters of Frozen–Thawed Sperm
3.3. Effects of MV on Functional Characteristics of Frozen–Thawed Sperm
3.4. Effects of MV on ROS Content, MDA Content, and T-AOC Ability of Frozen–Thawed Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rives, N.; Courbiere, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Sheng, H.; Liang, X.; Wang, Z.; Meng, T.; Li, Y.; Dong, H.; Zhu, W.; Yang, J.; et al. Fertility preservation in males with cancer of trends, region development, and efficacy in mainland China from 16 regions Chinese sperm banks. J. Assist. Reprod. Genet. 2024, 41, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Athurupana, R.; Takahashi, D.; Ioki, S.; Funahashi, H. Trehalose in glycerol-free freezing extender enhances post-thaw survival of boar spermatozoa. J. Reprod. Dev. 2015, 61, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Recent Advances in Boar Sperm Cryopreservation: State of the Art and Current Perspectives. Reprod. Domest. Anim. 2015, 50 (Suppl. S2), 71–79. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- White, I.G. Lipids and calcium uptake of sperm in relation to cold shock and preservation: A review. Reprod. Fertil. Dev. 1993, 5, 639–658. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Fu, J.; Yang, Q.; Li, Y.; Li, P.; Wang, L.; Li, X. A mechanism by which Astragalus polysaccharide protects against ROS toxicity through inhibiting the protein dephosphorylation of boar sperm preserved at 4 degrees C. J. Cell Physiol. 2018, 233, 5267–5280. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, H.; Wang, L.; Hao, Y.Y.; Liang, G.D.; Ma, Y.H.; Yang, G.S.; Hu, J.H. The effects of different levels of superoxide dismutase in Modena on boar semen quality during liquid preservation at 17 degrees C. Anim. Sci. J. 2017, 88, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.G.; Cai, M.M.; Zhang, Y.T.; Liu, Y.; Gao, Z.L.; Song, J.; Liu, Z.H. Effect of Astragalus polysaccharide addition to thawed boar sperm on in vitro fertilization and embryo development. Theriogenology 2018, 121, 21–26. [Google Scholar] [CrossRef]
- Shafiei, M.; Forouzanfar, M.; Hosseini, S.M.; Esfahani, M.H. The effect of superoxide dismutase mimetic and catalase on the quality of postthawed goat semen. Theriogenology 2015, 83, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Tusimova, E.; Kovacik, A.; Paal, D.; Greifova, H.; Abdramanov, A.; Lukac, N. Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Feng, T.; Niu, T.; Yuan, Y.; Liu, Q.; Xiao, J.; Xu, G.; Hu, J. Notoginsenoside R1 protects boar sperm during liquid storage at 17 degrees C. Reprod. Domest. Anim. 2020, 55, 1072–1079. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Zeng, W. Resveratrol Improves Boar Sperm Quality via 5′AMP-Activated Protein Kinase Activation during Cryopreservation. Oxid. Med. Cell Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, L.; Liu, Y.; Dou, D.; Sun, Y.; Ma, L. Pharmacological activities of mogrosides. Future Med. Chem. 2018, 10, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Z.; Lu, F.; Liu, J.; Song, Y.; Li, D. Cucurbitane glycosides derived from mogroside IIE: Structure-taste relationships, antioxidant activity, and acute toxicity. Molecules 2014, 19, 12676–12689. [Google Scholar] [CrossRef]
- Qin, X.; Xiaojian, S.; Ronggan, L.; Yuxian, W.; Zhunian, T.; Shouji, G.; Heimbach, J. Subchronic 90-day oral (Gavage) toxicity study of a Luo Han Guo mogroside extract in dogs. Food Chem. Toxicol. 2006, 44, 2106–2109. [Google Scholar] [CrossRef]
- Shen, J.; Shen, D.; Tang, Q.; Li, Z.; Jin, X.; Li, C. Mogroside V exerts anti-inflammatory effects on fine particulate matter-induced inflammation in porcine alveolar macrophages. Toxicol. In Vitro 2022, 80, 105326. [Google Scholar] [CrossRef]
- Li, Y.; Shen, D.; Wang, K.; Xue, Y.; Liu, J.; Li, S.; Li, X.; Li, C. Mogroside V ameliorates broiler pulmonary inflammation via modulating lung microbiota and rectifying Th17/Treg dysregulation in lipopolysaccharides-induced lung injury. Poult. Sci. 2023, 102, 103138. [Google Scholar] [CrossRef]
- Tan, Y.R.; Shen, S.Y.; Li, X.Y.; Yi, P.F.; Fu, B.D.; Peng, L.Y. Mogroside V reduced the excessive endoplasmic reticulum stress and mitigated the Ulcerative colitis induced by dextran sulfate sodium in mice. J. Transl. Med. 2024, 22, 488. [Google Scholar] [CrossRef]
- Song, J.L.; Qian, B.; Pan, C.; Lv, F.; Wang, H.; Gao, Y.; Zhou, Y. Protective activity of mogroside V against ovalbumin-induced experimental allergic asthma in Kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiao, D.; Li, Y.; Jiang, C.; Tang, X.; Song, J.; Chen, Q. Mogroside V Inhibits Hyperglycemia-induced Lung Cancer Cells Metastasis through Reversing EMT and Damaging Cytoskeleton. Curr. Cancer Drug Targets 2019, 19, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, G.; Peng, Y.; Wang, M.; Li, X. Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J. Ethnopharmacol. 2020, 247, 112273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Ding, Y.; Wang, W.; Liao, L.; Zhong, J.; Sun, P.; Lei, F.; Zhang, Y.; Xie, W. Effects of Mogrosides on High-Fat-Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Molecules 2018, 23, 1894. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sui, L.; Zhang, H.; Zhang, H.; Yan, K.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V protects porcine oocytes from in vitro ageing by reducing oxidative stress through SIRT1 upregulation. Aging 2019, 11, 8362–8373. [Google Scholar] [CrossRef]
- Nie, J.; Yan, K.; Sui, L.; Zhang, H.; Zhang, H.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 2020, 141, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Cui, K.; Li, P.; Liu, X.; Du, Y.; Xu, H.; Yang, X.; Lu, S.; Liang, X. Mogroside V alleviates the heat stress-induced disruption of the porcine oocyte in vitro maturation. Theriogenology 2024, 217, 37–50. [Google Scholar] [CrossRef]
- Pena, A.; Linde-Forsberg, C. Effects of Equex, one- or two-step dilution, and two freezing and thawing rates on post-thaw survival of dog spermatozoa. Theriogenology 2000, 54, 859–875. [Google Scholar] [CrossRef]
- Revell, S.G.; Mrode, R.A. An osmotic resistance test for bovine semen. Anim. Reprod. Sci. 1994, 36, 77–86. [Google Scholar] [CrossRef]
- Avdatek, F.; Yeni, D.; Inanc, M.E.; Cil, B.; Tuncer, B.P.; Turkmen, R.; Tasdemir, U. Supplementation of quercetin for advanced DNA integrity in bull semen cryopreservation. Andrologia 2018, 50, e12975. [Google Scholar] [CrossRef] [PubMed]
- Najafi, L.; Halvaei, I.; Movahedin, M. Canthaxanthin protects human sperm parameters during cryopreservation. Andrologia 2019, 51, e13389. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhao, Y.H.; Hao, H.S.; Wang, H.Y.; Huang, J.M.; Yan, C.L.; Du, W.H.; Pang, Y.W.; Zhang, P.P.; Liu, Y.; et al. Resveratrol significantly improves the fertilisation capacity of bovine sex-sorted semen by inhibiting apoptosis and lipid peroxidation. Sci. Rep. 2018, 8, 7603. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.K.; Tseng, H.C.; Hwu, Y.M.; Fan, C.C.; Lin, M.H.; Yu, J.J.; Yeh, L.Y.; Li, S.H. Expression of cystatin C in the female reproductive tract and its effect on human sperm capacitation. Reprod. Biol. Endocrinol. 2018, 16, 8. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, J.; Hao, Y.Y.; Li, H.; Hu, J.X.; Yang, G.S.; Hu, J.H. Effects of iodine methionine on boar sperm quality during liquid storage at 17 degrees C. Reprod. Domest. Anim. 2017, 52, 1061–1066. [Google Scholar] [CrossRef]
- Chen, W.J.; Wang, J.; Qi, X.Y.; Xie, B.J. The antioxidant activities of natural sweeteners, mogrosides, from fruits of Siraitia grosvenori. Int. J. Food Sci. Nutr. 2007, 58, 548–556. [Google Scholar] [CrossRef]
- Qi, X.Y.; Chen, W.J.; Zhang, L.Q.; Xie, B.J. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr. Res. 2008, 28, 278–284. [Google Scholar] [CrossRef]
- Shi, D.; Zheng, M.; Wang, Y.; Liu, C.; Chen, S. Protective effects and mechanisms of mogroside V on LPS-induced acute lung injury in mice. Pharm. Biol. 2014, 52, 729–734. [Google Scholar] [CrossRef]
- Wang, H.; Meng, G.L.; Zhang, C.T.; Wang, H.; Hu, M.; Long, Y.; Hong, H.; Tang, S.S. Mogrol attenuates lipopolysaccharide (LPS)-induced memory impairment and neuroinflammatory responses in mice. J. Asian Nat. Prod. Res. 2020, 22, 864–878. [Google Scholar] [CrossRef]
- Liang, H.; Cheng, R.; Wang, J.; Xie, H.; Li, R.; Shimizu, K.; Zhang, C. Mogrol, an aglycone of mogrosides, attenuates ulcerative colitis by promoting AMPK activation. Phytomedicine 2021, 81, 153427. [Google Scholar] [CrossRef]
- Comaschi, V.; Lindner, L.; Farruggia, G.; Gesmundo, N.; Colombi, L.; Masotti, L. An investigation on lipoperoxidation mechanisms in boar spermatozoa. Biochem. Biophys. Res. Commun. 1989, 158, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Gil, M.A.; Hernandez, M.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A. Survival and fertility of boar spermatozoa after freeze-thawing in extender supplemented with butylated hydroxytoluene. J. Androl. 2004, 25, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef] [PubMed]
- Beygi, Z.; Forouhari, S.; Mahmoudi, E.; Hayat, S.M.G.; Nourimand, F. Role of Oxidative Stress and Antioxidant Supplementation in Male Fertility. Curr. Mol. Med. 2021, 21, 265–282. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [CrossRef]
- Silva, P.F.; Gadella, B.M. Detection of damage in mammalian sperm cells. Theriogenology 2006, 65, 958–978. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Yanez-Ortiz, I.; Catalan, J.; Rodriguez-Gil, J.E.; Miro, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Silva, P.F.; Gadella, B.M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze-thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Liu, G.; Zhou, P.; Li, P.; Zhao, Z.; Zhang, R.; Tang, X.; Wang, Z.; Wei, Z.; et al. Saponins from Momordica charantia exert hypoglycemic effect in diabetic mice by multiple pathways. Food Sci. Nutr. 2023, 11, 7626–7637. [Google Scholar] [CrossRef]
- Silvestre, G.F.G.; de Lucena, R.P.; da Silva Alves, H. Cucurbitacins and the Immune System: Update in Research on Anti- inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Curr. Med. Chem. 2022, 29, 3774–3789. [Google Scholar] [CrossRef] [PubMed]
| MV (μmol/L) 1 | Conservation Time (hours) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| 0 | 48.00 ± 2.95 a | 40.33 ± 2.28 a | 26.60 ± 2.24 | 12.90 ± 2.06 |
| 25 | 51.30 ± 2.17 ab | 44.03 ± 1.15 ab | 29.57 ± 2.96 | 11.77 ± 2.62 |
| 50 | 55.60 ± 2.70 ab | 47.07 ± 0.91 bc | 31.03 ± 2.89 | 12.53 ± 2.40 |
| 75 | 58.20 ± 2.63 bc | 49.27 ± 2.09 bcd | 30.33 ± 3.47 | 13.53 ± 2.87 |
| 100 | 54.20 ± 1.63 ab | 47.47 ± 2.14 bc | 32.17 ± 5.36 | 13.80 ± 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, H.; Wang, X.; Hu, K.; Zuo, X.; Li, H.; Diao, Z.; Feng, J.; Zhang, Y.; Cao, Z. Effects of Mogroside V on Quality and Antioxidant Activity of Boar Frozen–Thawed Sperm. Antioxidants 2025, 14, 622. https://doi.org/10.3390/antiox14060622
Sui H, Wang X, Hu K, Zuo X, Li H, Diao Z, Feng J, Zhang Y, Cao Z. Effects of Mogroside V on Quality and Antioxidant Activity of Boar Frozen–Thawed Sperm. Antioxidants. 2025; 14(6):622. https://doi.org/10.3390/antiox14060622
Chicago/Turabian StyleSui, Heming, Xin Wang, Kunlong Hu, Xiaoyu Zuo, Haonan Li, Zhengyu Diao, Jiajing Feng, Yunhai Zhang, and Zubing Cao. 2025. "Effects of Mogroside V on Quality and Antioxidant Activity of Boar Frozen–Thawed Sperm" Antioxidants 14, no. 6: 622. https://doi.org/10.3390/antiox14060622
APA StyleSui, H., Wang, X., Hu, K., Zuo, X., Li, H., Diao, Z., Feng, J., Zhang, Y., & Cao, Z. (2025). Effects of Mogroside V on Quality and Antioxidant Activity of Boar Frozen–Thawed Sperm. Antioxidants, 14(6), 622. https://doi.org/10.3390/antiox14060622







