Vitamin C Inhibits Scale Drop Disease Virus Infectivity by Targeting Nrf2 to Reduce Ferroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Viral Strain
2.2. Artificial Infection
2.3. VC Treatment and Grouping Strategy in MFF-1
2.4. ML-385 Treatment and Grouping Strategy in MFF-1
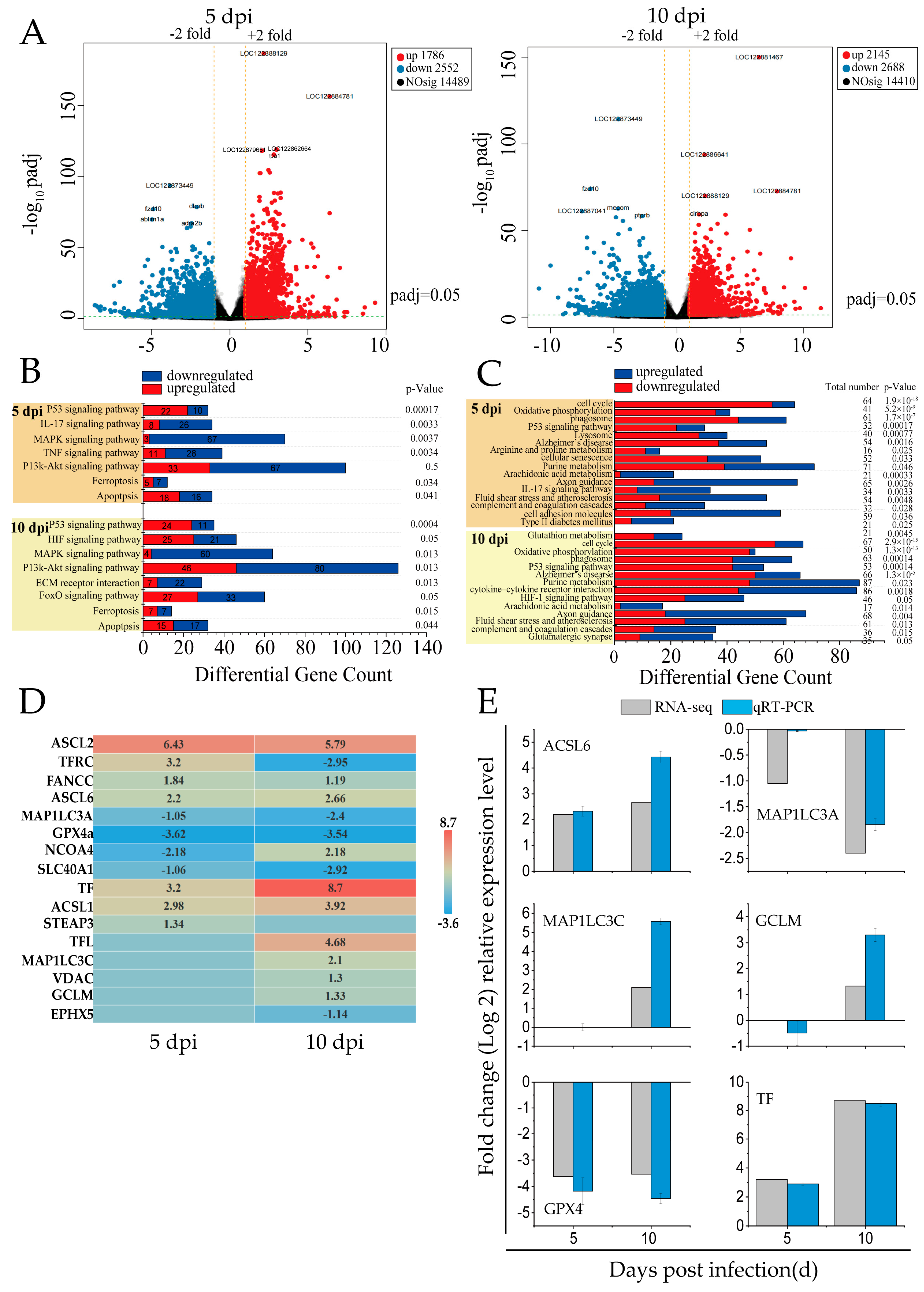
2.5. Transcriptome Sequencing (RNA-Seq)
2.6. Quantitative Reverse Transcription PCR (qRT-PCR)
2.7. SDDV Copy Number Detection
2.8. Reactive Oxygen Species (ROS) Assay
2.9. Lipid Peroxidation (LPO) Assay
2.10. Cell Viability Assay
2.11. Western Blot Analysis
2.12. Immunofluorescence Assay (IFA)
2.13. Statistical Analysis
3. Results
3.1. SDDV-Induced Ferroptosis in the Spleen Tissues of Mandarin Fish
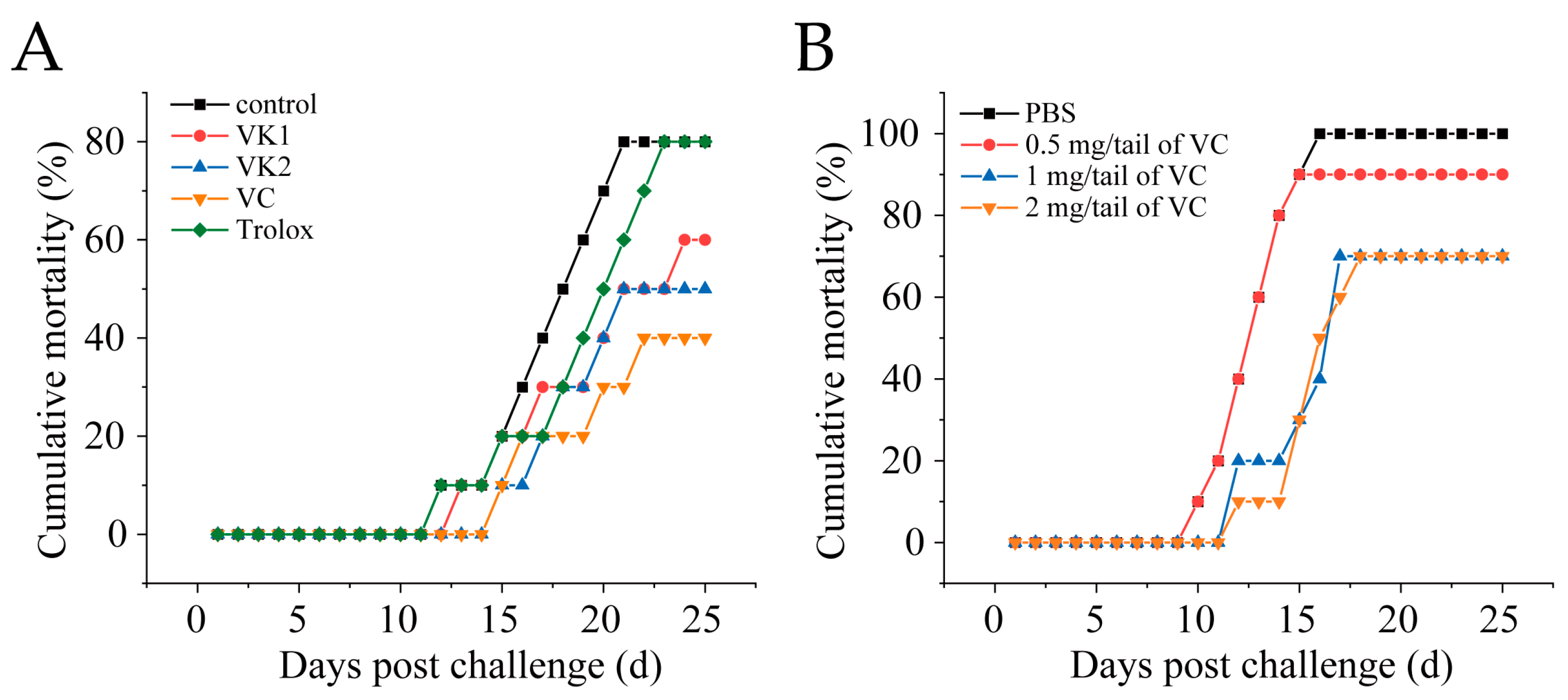
3.2. VC Reduces Mortality in Mandarin Fish Infected with SDDV
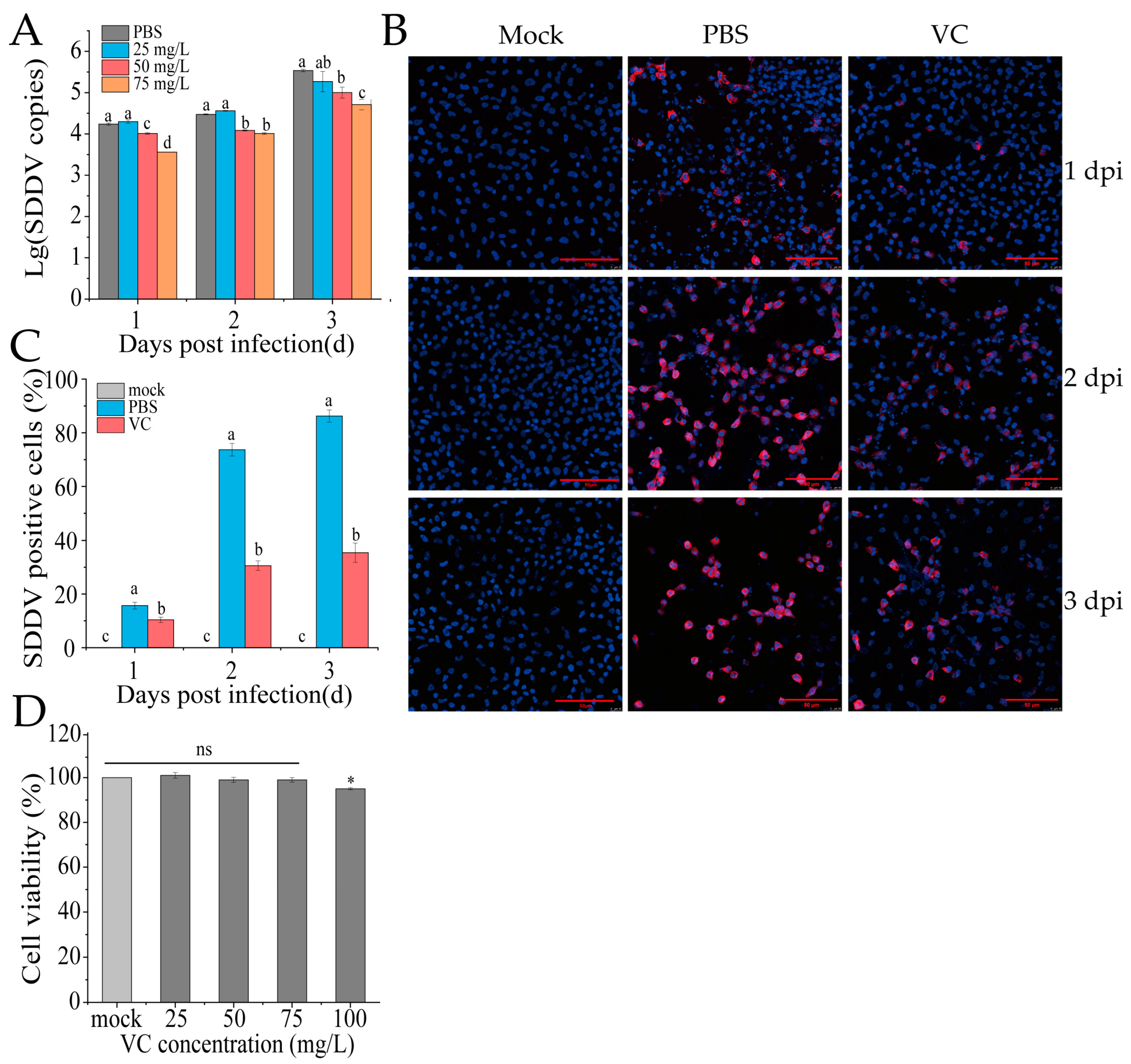
3.3. VC Inhibits SDDV Infection in MFF-1 Cells
3.4. VC Inhibits SDDV-Induced Ferroptosis
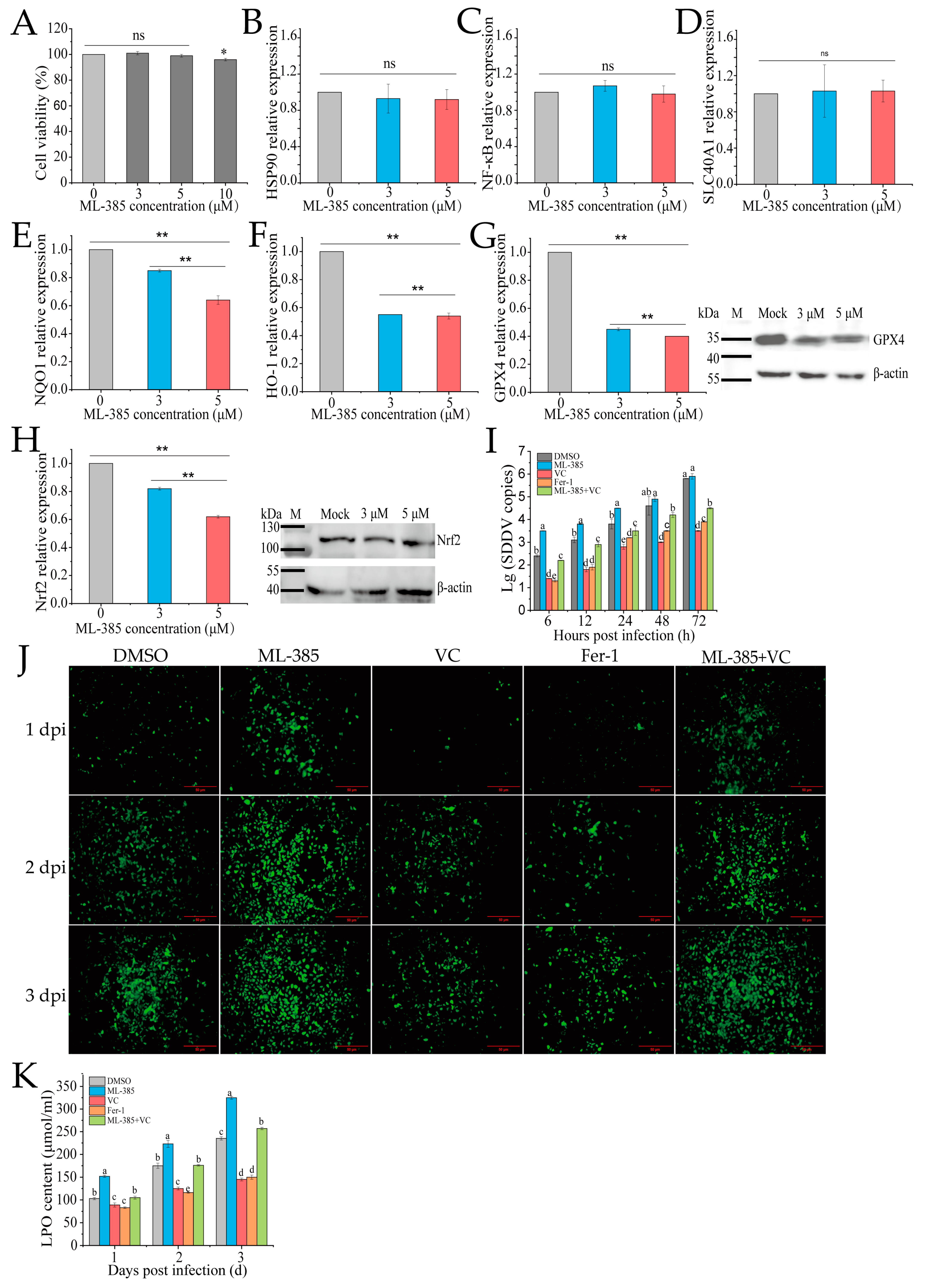
3.5. Inhibition of Nrf2 Promotes Ferroptosis and SDDV Infection in MFF-1 Cells
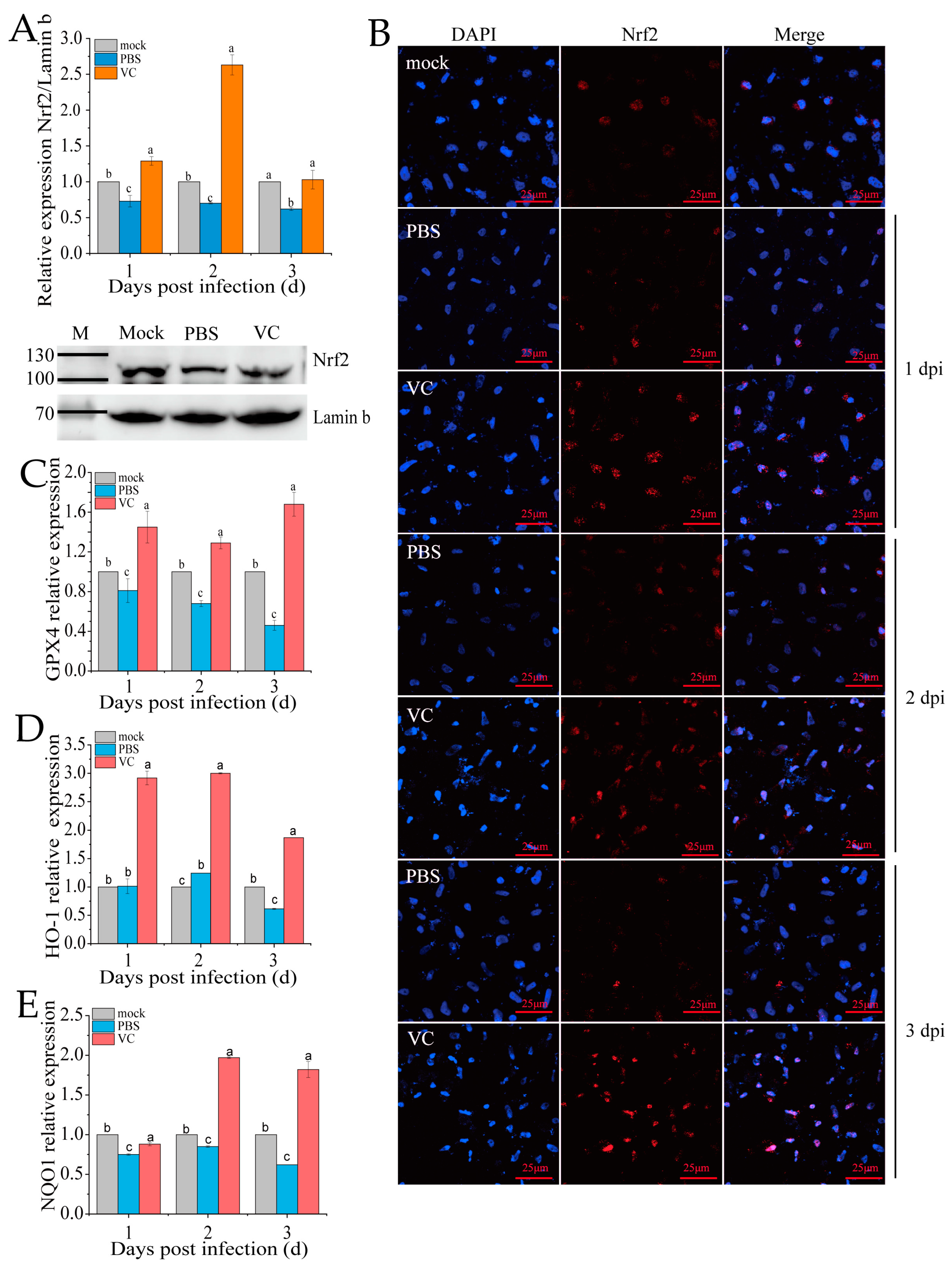
3.6. VC Inhibits SDDV-Induced Ferroptosis by Activating Nrf2 Nuclear Translocation
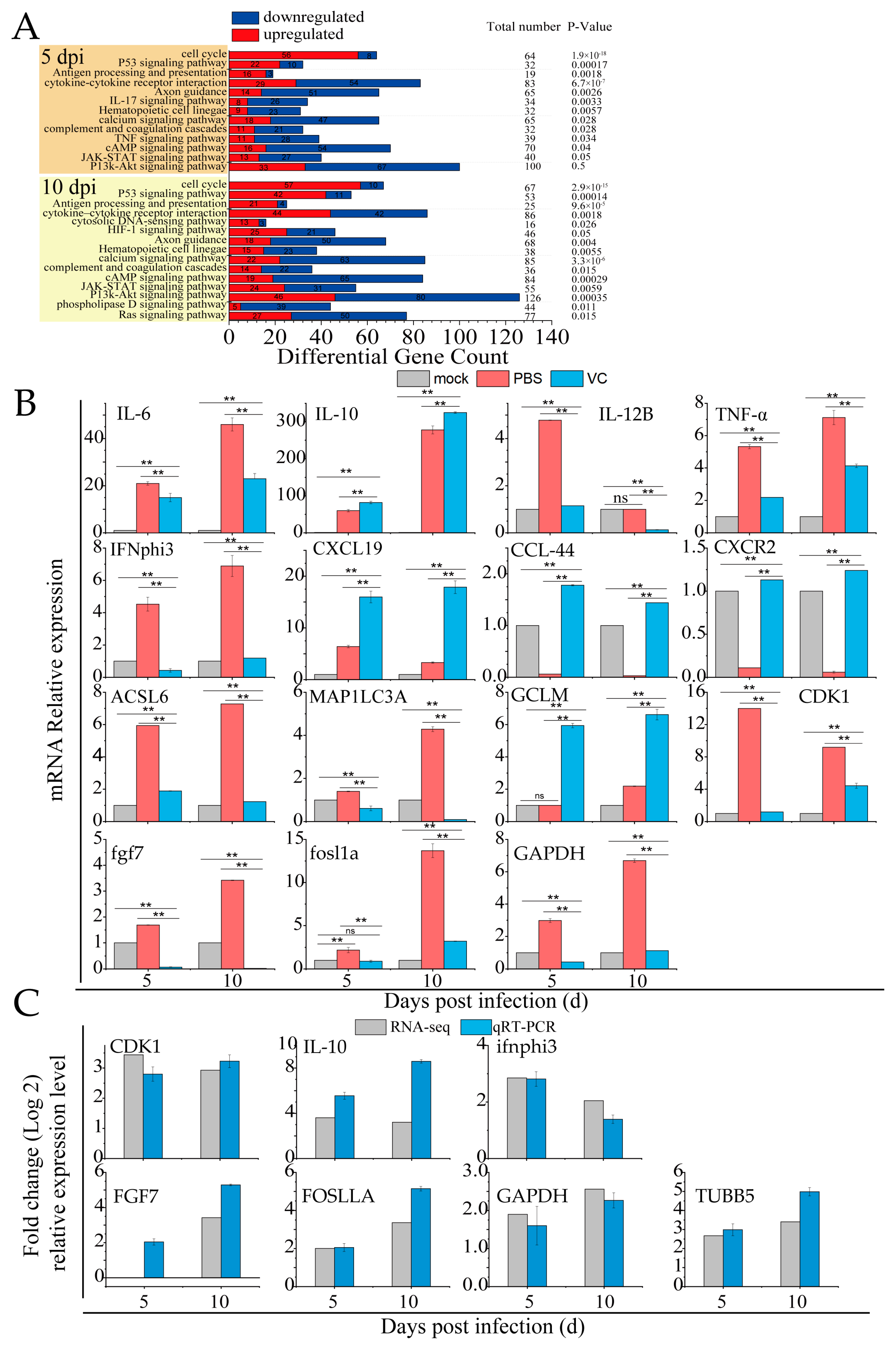
3.7. VC Suppresses Inflammation Induced by SDDV in Mandarin Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDDV | Scale drop disease virus |
| VC | Vitamin C |
| DEGs | Differentially expressed genes |
| ROS | Reactive oxygen species |
| LPO | Lipid peroxidation |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| MCP | Major capsid protein |
| SD | Standard deviation |
| ANOVA | Analysis of Variance |
References
- de Groof, A.; Guelen, L.; Deijs, M.; van der Wal, Y.; Miyata, M.; Ng, K.S.; van Grinsven, L.; Simmelink, B.; Biermann, Y.; Grisez, L.; et al. A Novel Virus Causes Scale Drop Disease in Lates calcarifer. PLoS Pathog. 2015, 11, e1005074. [Google Scholar] [CrossRef] [PubMed]
- Nurliyana, M.; Lukman, B.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Dong, H.T.; Rodkhum, C.; Amal, M.N.A. First Evidence of Scale Drop Disease Virus in Farmed Asian Seabass (Lates calcarifer) in Malaysia. Aquaculture 2020, 528, 735600. [Google Scholar] [CrossRef]
- Gibson, K.S.; Chee, D.; Chen, J.; Wang, Y.H.; Tay, S.; Leong, L.N.; Ng, M.L.; Jones, J.B.; Nicholls, P.K.; Ferguson, H.W. The Pathology of “scale Drop Syndrome” in Asian Seabass, Lates calcarifer Bloch, a First Description. J. Fish Dis. 2012, 35, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Senapin, S.; Dong, H.T.; Meemetta, W.; Gangnonngiw, W.; Sangsuriya, P.; Vanichviriyakit, R.; Sonthi, M.; Nuangsaeng, B. Mortality from Scale Drop Disease in Farmed Lates calcarifer in Southeast Asia. J. Fish Dis. 2019, 42, 119–127. [Google Scholar] [CrossRef]
- Halaly, M.A.; Subramaniam, K.; Koda, S.A.; Popov, V.L.; Stone, D.; Way, K.; Waltzek, T.B. Characterization of a Novel Megalocytivirus Isolated from European Chub (Squalius cephalus). Viruses 2019, 11, 440. [Google Scholar] [CrossRef]
- Fu, Y.T.; Li, Y.; Fu, W.X.; Su, H.B.; Zhang, L.; Huang, C.L.; Weng, S.P.; Yu, F.Z.; He, J.G.; Dong, C.F. Scale Drop Disease Virus Associated Yellowfin Seabream (Acanthopagrus latus) Ascites Diseases, Zhuhai, Guangdong, Southern China: The First Description. Viruses 2021, 13, 1617. [Google Scholar] [CrossRef]
- Shahin, K.; Subramaniam, K.; Camus, A.C.; Yazdi, Z.; Yun, S.; Koda, S.A.; Waltzek, T.B.; Pierezan, F.; Hu, R.; Soto, E. Isolation, Identification and Characterization of a Novel Megalocytivirus from Cultured Tilapia (Oreochromis Spp.) from Southern California, USA. Animals 2021, 11, 3524. [Google Scholar] [CrossRef]
- Fu, Y.T.; Li, Y.; Chen, J.M.; Yu, F.Z.; Liu, X.R.; Fu, W.X.; Pan, H.; Li, W.; Weng, S.P.; He, J.G. A Mandarinfish Siniperca chuatsi Infection and Vaccination Model for SDDV and Efficacy Evaluation of the Formalin-Killed Cell Vaccine in Yellowfin Seabream Acanthopagrus latus. Aquaculture 2023, 570, 739428. [Google Scholar] [CrossRef]
- Chen, J.M.; Fu, Y.T.; Chen, S.Y.; Weng, S.P.; He, J.G.; Dong, C.F. Scale Drop Disease Virus (SDDV) Triggering Ferroptosis Both In Vivo and In Vitro Facilitates Virus Infection via Targeting Transferrin Receptor 1 (TfR1). bioRxiv 2025. bioRxiv:2025.03.18.643978. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.H.; Luo, Z.S.; Zhao, H.Y.; Wang, X.M.; Guan, X.R. Effect of Autophagy on Ferroptosis in Foam Cells via Nrf2. Mol. Cell Biochem. 2022, 477, 1597–1606. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M. Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 2017, 6, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Guo, X.J.; Sun, y. Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration. Aging Dis. 2021, 12, 529–551. [Google Scholar] [CrossRef]
- Han, S.X.; Chen, J.J.; Hua, J.J.; Hu, X.J.; Jian, S.H.; Zheng, G.X.; Wang, J.; Li, H.R.; Yang, J.L.; Hejtmancik, J.F. MITF Protects against Oxidative Damage-Induced Retinal Degeneration by Regulating the NRF2 Pathway in the Retinal Pigment Epithelium. Redox Biol. 2020, 34, 101537. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, H.; Su, J.J.; Wang, X.C.; Wang, Q.X.; Chu, J.; Li, Q.L. Gastrodin Protects against Glutamate-Induced Ferroptosis in HT-22 Cells through Nrf2/HO-1 Signaling Pathway. Toxicol. Vitr. 2020, 62, 104715. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Lőrincz, T.; Szarka, A. Concentration Does Matter: The Beneficial and Potentially Harmful Effects of Ascorbate in Humans and Plants. Antioxid. Redox Signal 2018, 29, 1516–1533. [Google Scholar] [CrossRef]
- Panday, S.; Kar, S.; Kavdia, M. How Does Ascorbate Improve Endothelial Dysfunction?-A Computational Analysis. Free Radic. Biol. Med. 2021, 165, 111–126. [Google Scholar] [CrossRef]
- Szarka, A.; Kapuy, O.; Lőrincz, T.; Bánhegyi, G. Vitamin C and Cell Death. Antioxid. Redox Signal 2021, 34, 831–844. [Google Scholar] [CrossRef]
- Lőrincz, T.; Holczer, M.; Kapuy, O.; Szarka, A. The Interrelationship of Pharmacologic Ascorbate Induced Cell Death and Ferroptosis. Pathol. Oncol. Res. 2019, 25, 669–679. [Google Scholar] [CrossRef]
- Sun, G.Q.; Liu, C.; Lu, Z.Q.; Zhang, J.Y.; Cao, H.S.; Huang, T.; Dai, M.G.; Liu, H.Y.; Feng, T.T.; Tang, W.W. Metabolomics Reveals Ascorbic Acid Inhibits Ferroptosis in Hepatocytes and Boosts the Effectiveness of Anti-PD1 Immunotherapy in Hepatocellular Carcinoma. Cancer Cell Int. 2024, 24, 192. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-ΚB Activation by TNF via the Activation of P38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef]
- Schaefer, L. Complexity of Danger: The Diverse Nature of Damage-Associated Molecular Patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo, J.M.; Bórquez, O.O.; Golde, D.W. Vitamin C Inhibits Granulocyte Macrophage–Colony-Stimulating Fac tor–Induced Signaling Pathways. Blood 2002, 99, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’ai, H.; Abed, Y. Effect of Vitamin C on Inflammation and Metabolic Markers in Hypertensive and/or Diabetic Obese Adults: A Randomized Controlled Trial. Drug Des. Devel Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of High-Dose Intravenous Vitamin C on Inflammation in Cancer Patients. J. Transl. Med. 2012, 10, 1–10. [Google Scholar] [CrossRef]
- Block, G.; Jensen, C.; Dietrich, M.; Norkus, E.P.; Hudes, M.; Packer, L. Plasma C-Reactive Protein Concentrations in Active and Passive Smokers: Influence of Antioxidant Supplementation. J. Am. Coll. Nutr. 2004, 23, 141–147. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Huynh, Q.K.; Wijesinghe, D.S.; Chalfant, C.E.; Brophy, D.F.; Fowler III, A.A.; Natarajan, R. Resolution of Sterile Inflammation: Role for Vitamin C. Mediat. Inflamm. 2014, 2014, 173403. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.B.; Kang, R.; Klionsky, D.J.; Tang, D.L. Ferroptosis: Machinery and Regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Dong, C.F.; Weng, S.P.; Shi, X.J.; Xu, X.P.; Shi, N.; He, J.G. Development of a Mandarin Fish Siniperca chuatsi Fry Cell Line Suitable for the Study of Infectious Spleen and Kidney Necrosis Virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.X.; Xie, F.; Zhang, L.; Yan, H.Y.; Huang, J.; Zhang, C.; Zhou, F.F.; Chen, J.; Zhang, L. Ferroptosis in Cancer and Cancer Immunotherapy. Cancer Commun. 2022, 42, 88–116. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X. The Chemistry and Biology of Ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef] [PubMed]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)-Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.J.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.J.; Stockwell, B.R. Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Gu, W. P53 in Ferroptosis Regulation: The New Weapon for the Old Guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wang, J.M.; Hu, W.W.; Feng, Z.H. The Regulation of Ferroptosis by Tumor Suppressor P53 and Its Pathway. Int. J. Mol. Sci. 2020, 21, 8387. [Google Scholar] [CrossRef]
- Bao, J.Q.; Wang, Z.X.; Yang, Y.T.; Yu, X.F.; Yuan, W.L.; Sun, W.L.; Chen, L.L. Interleukin-17 Alleviates Erastin-induced Alveolar Bone Loss by Suppressing Ferroptosis via Interaction between NRF2 and P-STAT3. J. Clin. Periodontol. 2024, 51, 233–250. [Google Scholar] [CrossRef]
- Weigelt, B.; Downward, J. Genomic Determinants of PI3K Pathway Inhibitor Response in Cancer. Front. Oncol. 2012, 2, 109. [Google Scholar] [CrossRef]
- Yi, J.M.; Zhu, J.J.; Wu, J.; Thompson, C.B.; Jiang, X.J. Oncogenic Activation of PI3K-AKT-MTOR Signaling Suppresses Ferroptosis via SREBP-Mediated Lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/MTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The Antioxidant Role of Vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Chambers, I.; Yun, S.J.; Phillips, J.; Krause, M.; Hamza, I. Hrg1 Promotes Heme-Iron Recycling during Hemolysis in the Zebrafish Kidney. PLoS Genet. 2018, 14, e1007665. [Google Scholar] [CrossRef]
- Furukawa, M.; Xiong, Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef]
- Shin, C.S.; Mishra, P.; Watrous, J.D.; Carelli, V.; D’Aurelio, M.; Jain, M.; Chan, D.C. The Glutamate/Cystine XCT Antiporter Antagonizes Glutamine Metabolism and Reduces Nutrient Flexibility. Nat. Commun. 2017, 8, 15074. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear Factor Erythroid-derived 2-like 2 (NFE2L2, Nrf2) Mediates Exercise-induced Mitochondrial Biogenesis and the Anti-oxidant Response in Mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.M.; Liddell, J.R. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef]
- Ma, H.D.; Wang, X.D.; Zhang, W.L.; Li, H.T.; Zhao, W.; Sun, J.; Yang, M.W. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid. Med. Cell Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef]
- Ouyang, X.J.; Li, X.C.; Liu, J.; Liu, Y.; Xie, Y.P.; Du, Z.C.; Xie, H.L.; Chen, B.; Lu, W.B.; Chen, D.F. Structure-Activity Relationship and Mechanism of Four Monostilbenes with Respect to Ferroptosis Inhibition. RSC Adv. 2020, 10, 31171–31179. [Google Scholar] [CrossRef]
- Kong, L.; Xu, C.; Sun, N.; Liang, F.; Wei, M.; Su, X. Melatonin Alleviates Myocardial Ischemia-Reperfusion Injury in Mice by Inhibiting Inflammatory Response via Activating Nrf2 Signaling. J. South Med. Univ. 2021, 41, 1165–1170. [Google Scholar]
- Juszczak, M.; Tokarz, P.; Woźniak, K. Potential of NRF2 Inhibitors—Retinoic Acid, K67, and ML-385—In Overcoming Doxorubicin Resistance in Promyelocytic Leukemia Cells. Int. J. Mol. Sci. 2024, 25, 10257. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Wang, L.; Jiang, Z.J.; Xu, G.; Li, L.H.; Zhang, Y.; Tan, Y.N. Peiminine Triggers Ferroptosis to Inhibit Breast Cancer Growth through Triggering Nrf2 Signaling. Tissue Cell 2024, 87, 102323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Q.F. Tranilast Reduces Cardiomyocyte Injury Induced by Ischemia-reperfusion via Nrf2/HO-1/NF-κB Signaling. Exp. Ther. Med. 2023, 25, 160. [Google Scholar] [CrossRef]

| Gene | GenBank Accession No. | Primer | Primer Sequence (5′-3′) |
|---|---|---|---|
| β-actin | AY885683 | β-actin-F | AGAGGGAAATCGTGCGTG |
| β-actin-R | GAAGGAAGGCTGGAAGAGG | ||
| Nrf2 | MT270449 | Nrf2-F | AGACGAAAGCGAAAGCTCCT |
| Nrf2-R | GCTCTCTTCCAGAATGGCGT | ||
| GPX4 | XM_044200772 | GPX4-F | ACGCATCCTTGCTTTCCCTT |
| GPX4-R | TGCTCTTTCAGCCACTTCCA | ||
| HO-1 | KJ765334 | HO-1-F | CCAGAAGGGACAGATCACCC |
| HO-1-R | CCTCCTCTAGCGCCTTGTAG | ||
| NQO1 | XM_044189953 | NQO1-F | CCAGCATGGGTGACCTGAA |
| NQO1-R | CTCTGTCCATCCAGCCCTTC | ||
| IL-6 | MT126997 | IL-6-F | AGCCCATCGCAGAGAAAAGG |
| IL-6-R | GCACTTGTTGAGTTTCCGCT | ||
| IL-10 | MH644036 | IL-10-F | TTATCTGGGCACGGTTCTGC |
| IL-10-R | GGACTCCATGTGCGGCTTTA | ||
| IL-12B | XM_044195110 | IL-12B-F | AGGATGCCAACTGCCCATAC |
| IL-12B-R | TGAACACCGTCCCATCACTG | ||
| TNFα | XM_044171435 | TNFα-F | TCGTATGGGAACGATGACGC |
| TNFα-R | ATCGCTGCTGGCTGTCTTTT | ||
| IFNphi3 | XM_044181379 | IFNphi3-F | TCACCTGGGACAATGCGAAA |
| IFNphi3-R | TGGACAAACAGCTTCCCTCC | ||
| CXCL19 | XM_044184584 | CXCL19-F | TGCTTGAACCCTCGGTCTTC |
| CXCL19-R | CTTGGGAAGTGCTTGTCCCT | ||
| CCL44 | XM_044200386 | CCL44-F | TGCTGCATGTTGTACTCCCA |
| CCL44-R | GTCTCTGGGGTCTGCACATT | ||
| CXCR2 | XM_044217766 | CXCR2-F | AATGTGCAGACCCCAGAGAC |
| CXCR2-R | AGGAAGGTGCCACATGGTTC | ||
| ACSL6 | XM_044221798 | ACSL6-F | CTATCAGGGCGACATCCGTC |
| ACSL6-R | CAGCCAACGCTTCAATGGAC | ||
| MAP1LC3A | XM_044183152 | MAP1LC3A-F | AGGAGGACATTTGCTGACCG |
| MAP1LC3A-R | ATCACGGGGATCTTGTTGGG | ||
| GCLM | XM_044199155 | GCLM-F | TGAAGCCCAGCAGTAACCAG |
| GCLM-R | CCAGTCAGCGATGTTCAGGT | ||
| CDK1 | XM_044213061 | CDK1-F | GAGTTCTTCACCGGGACCTG |
| CDK1-R | CGACAGGGGTTGAGTATCGG | ||
| fgf7 | XM_044189235 | fgf7-F | CCTCCACAACTACCCTGTCG |
| fgf7-R | ACCCTTGTGTTGTGACCTCC | ||
| fosl1a | XM_044204836 | fosl1a-F | GGCTCACCGTCCCATTTGTA |
| fosl1a-R | GCTTGCAGTTTGGTTGAGGG | ||
| GAPDH | XM_044209071 | GAPDH-F | CCAGAACATCATCCCCGCTT |
| GAPDH-R | CGTCGTATTTGGCGGGTTTC | ||
| tubb5 | XM_044209030 | tubb5-F | ACAGGGACTTACCACGGAGA |
| tubb5-R | AAGGGTCCAGACCTCACAGA | ||
| FGF7 | XM_044189235 | FGF7-F | CCTCCACAACTACCCTGTCG |
| FGF7-R | ACCCTTGTGTTGTGACCTCC | ||
| MAP1LC3C | XM_044189420 | MAP1LC3C-F | TACGAGGAAACACGAGGTGG |
| MAP1LC3C-R | ACTTGGTTTTGTCCAGCAGC | ||
| NF-κB | EF492047 | NF-κB-F | ACAACAGGACAGACTGGCTC |
| NF-κB-R | CTGGATTGCCAGAAGGACCC | ||
| HSP90 | XM_044180633 | HSP90-F | TAGCCCACGCTAGGTTCTTG |
| HSP90-R | TTCGGGTGGGTGTCATCTTC | ||
| SLC40A1 | XM_044217457 | SLC40A1-F | TGATCCGCACAGGCTTCATT |
| SLC40A1-R | CAAAGACAGAGGCAACGCAC |
| KEGG Pathway | Pathway ID | Differentially Expressed Genes |
|---|---|---|
| Cell cycle | hsa04110 | LOC122879039, BUB1, BUB1BB, CCNA2, CCNB1, CCNB3, CDC20, CDK1, CDKN2C, DBF4B, ESPL1, MAD2L1, PLK1, CCNDX, CDK18 |
| p53 signaling pathway | hsa04115 | LCCNB1, CDC20, CDK1, CTSL.1, IGFBP3, SERPINE1, TUBB5, TUBB6, CCNDX, CDK18 |
| Antigen processing and presentation | hsa04612 | CTSL.1, CTSLA, HSPA1B, PSME2 |
| Cytokine–cytokine receptor interaction | hsa04060 | BMP7B, CXCL19, IFNphi3, IL10, IL12BB, NRADD, VEGFAA, LOC122871787, CCL44, CXCL12B, CXCR2, PRLRA |
| Cytosolic DNA-sensing pathway | hsa04623 | LOC122864465, CGASA, CXCL19, IFNphi3 |
| HIF-1 signaling pathway | hsa04066 | ANGPT2A, ANGPTL4, CPB2, EGLN2, EGLN3, ENO1A, GAPDHS, SERPINE1, SLC2A3B, VEGFAA, LOC122885592, CPB1, EPAS1B, GAPDH, PFKFB1 |
| Axon guidance | hsa04360 | CPB2, LINGO2B, ABLIM1A, ABLIM3, CPB1, CXCL12B, EPHB6, LRRC4BA, NTNG1A, SEMA3AB |
| IL-17 signaling pathway | hsa04657 | FOSL1A, CCL44, ELAVL4, FOSAB, FOSB, MMP9 |
| Hematopoietic cell lineage | hsa04640 | LOC122864664, LOC122879019, ITGA8 |
| Calcium signaling pathway | hsa04020 | CHRNA11, CPB2, AGTR1B, ATP2B2, AVPR1AA, CACNA1EB, CACNA1IA, CCKAR, CPB1, DRD1B, GRIN2DA, GRM6B, HTR2CL1, NOS1, OXTRA P2RX2 P2RX4B PLCD1B STIM2A TACR2 TRHRA TRHRB |
| Complement and coagulation cascades | hsa04610 | C8GF2, FGF13B, SERPINF2B, SSTR1A |
| TNF signaling pathway | hsa04668 | LOC122864664, MMP19, LOC122876711, LOC122888378, FOSAB, FOSB, MMP9 |
| cAMP signaling pathway | hsa04024 | CNGA1B, CPB2, JUNE, AGTR1B, ATP2B2, DRD1B, DRD2L, FOSAB, FOSB, GRIN2DA, SSTR1A, VIPR2 |
| JAK-STAT signaling pathway | hsa04630 | IFNPHI3, IL10, IL13RA2 |
| PI3K-Akt signaling pathway | hsa04151 | FGF7, IFNPHI3, MYBL1, PDGFC, CCNDX, PRLRA |
| Phospholipase D signaling pathway | hsa04072 | AGTR1B, CPB1, CXCR2, DNM3B, GRM3, GRM6B, OXTRA, SHC2 |
| Ras signaling pathway | hsa04014 | FGF7, PDGFC, FGFR1B, FGFR4, GNB3B, GRIN2DA, LRRC74B, NTRK2A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Fu, Y.; Weng, S.; He, J.; Dong, C. Vitamin C Inhibits Scale Drop Disease Virus Infectivity by Targeting Nrf2 to Reduce Ferroptosis. Antioxidants 2025, 14, 576. https://doi.org/10.3390/antiox14050576
Chen J, Fu Y, Weng S, He J, Dong C. Vitamin C Inhibits Scale Drop Disease Virus Infectivity by Targeting Nrf2 to Reduce Ferroptosis. Antioxidants. 2025; 14(5):576. https://doi.org/10.3390/antiox14050576
Chicago/Turabian StyleChen, Jiaming, Yuting Fu, Shaoping Weng, Jianguo He, and Chuanfu Dong. 2025. "Vitamin C Inhibits Scale Drop Disease Virus Infectivity by Targeting Nrf2 to Reduce Ferroptosis" Antioxidants 14, no. 5: 576. https://doi.org/10.3390/antiox14050576
APA StyleChen, J., Fu, Y., Weng, S., He, J., & Dong, C. (2025). Vitamin C Inhibits Scale Drop Disease Virus Infectivity by Targeting Nrf2 to Reduce Ferroptosis. Antioxidants, 14(5), 576. https://doi.org/10.3390/antiox14050576






