Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers
Abstract
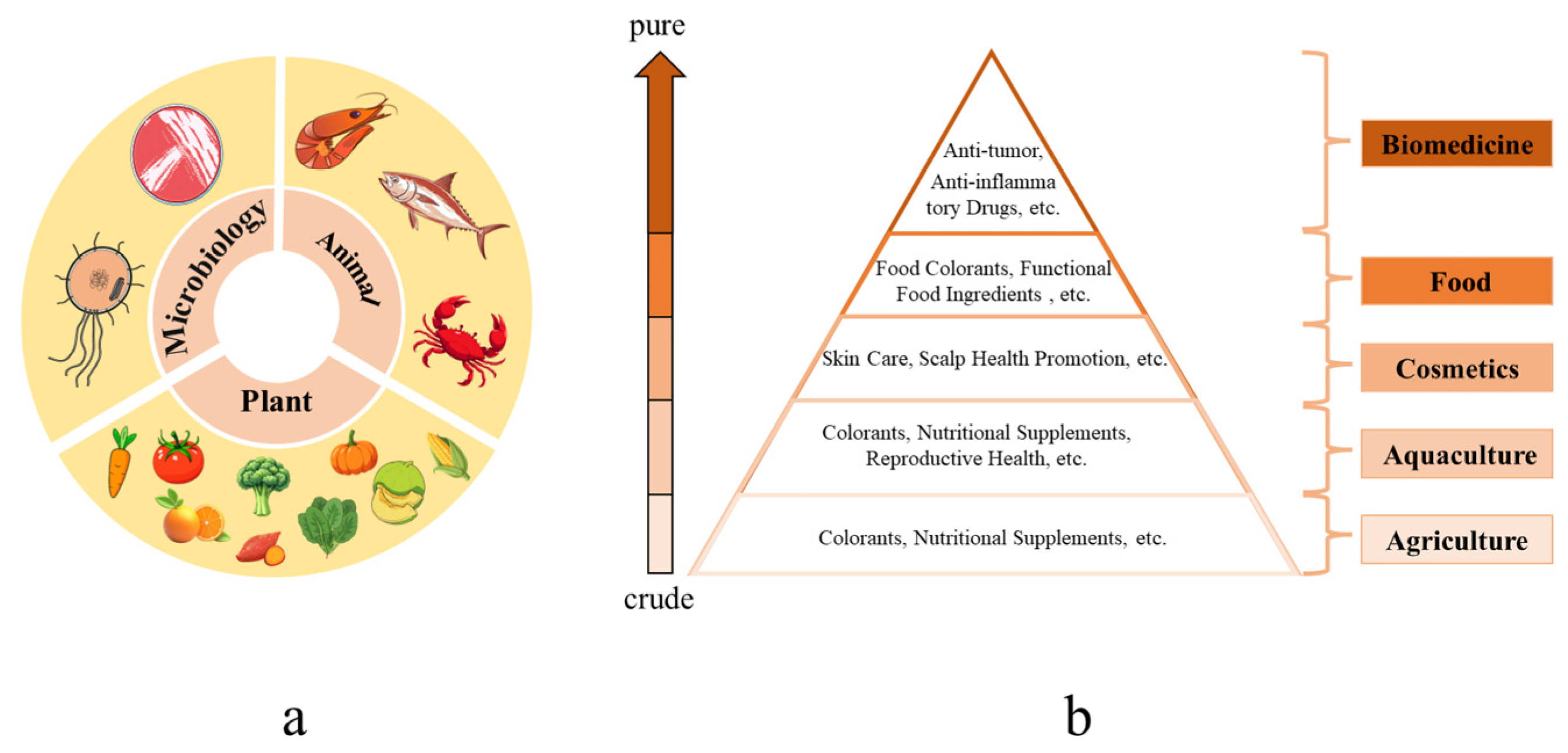
1. Introduction
2. Skin Photoaging
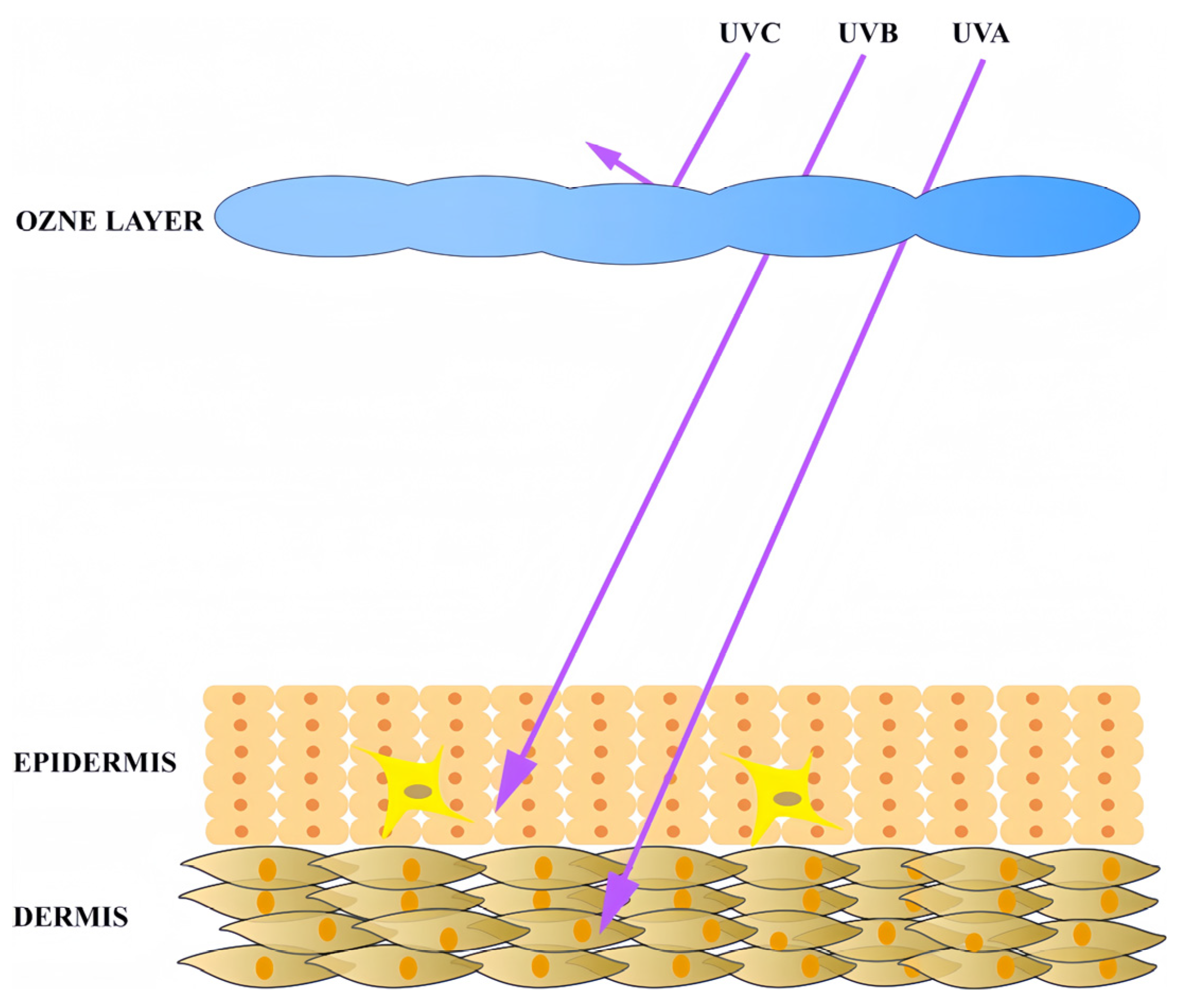
2.1. Ultraviolet Radiation as the Primary Trigger of Skin Photoaging
2.2. Skin Photoaging and Oxidative Stress
2.2.1. Bio-Macromolecule Damage by Oxidative Stress
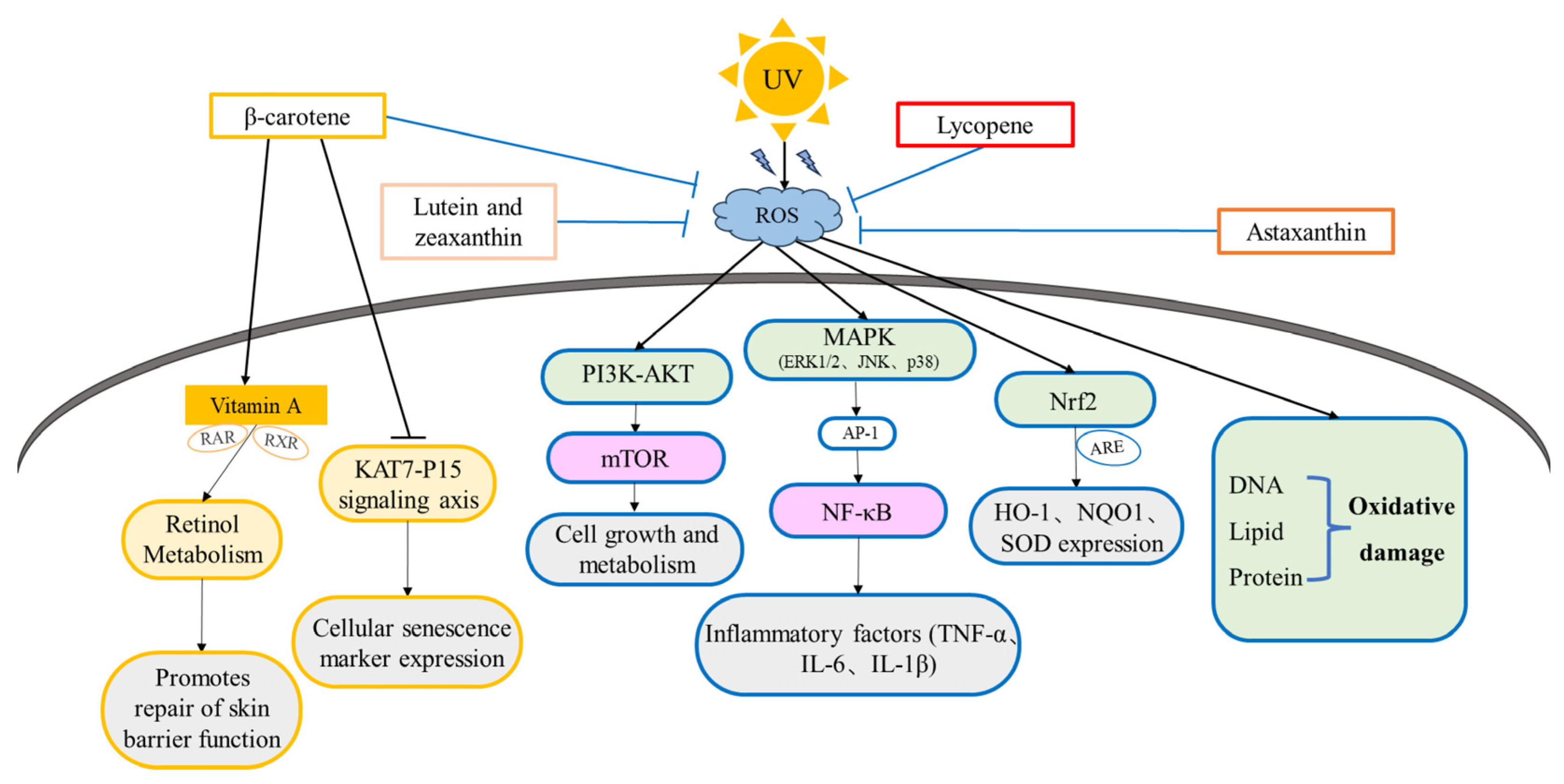
2.2.2. Signal Pathways Activated by Oxidative Stress
3. The Anti-Photoaging Effect and Mechanism of Carotenoids
3.1. The Anti-Photoaging Effect and Mechanism of C40 Carotenoids
3.1.1. The Anti-Photoaging Effects and Mechanism of β-Carotene
3.1.2. The Anti-Photoaging Effects and Mechanism of Lycopene
3.1.3. The Anti-Photoaging Effects and Mechanism of Astaxanthin
3.1.4. The Anti-Photoaging Effects and Mechanism of Lutein and Zeaxanthin
| Name | Antioxidant Capacity | In Vitro Experiment | In Vivo Experiment | Signal Pathways | References |
|---|---|---|---|---|---|
| β-carotene | Singlet oxygen (all-E-isomer-rich, IC50 0.38 μg/mL; Z-isomer-rich IC50 = 0.95 μg/mL) | Human skin fibroblasts (HSFs), human229 neonatal skin fibroblasts (NB1RGB), and B16274 mouse melanoma cells: enhancing hyaluronic acid production, promoting proliferation, anti-elastase activity, anti-melanogenic activity and anti-tyrosinase activity, inhibition of type I collagen production, and inhibition of melanin precursor darkening | - | - | [130] |
| - | Keratinocyte: inhibition of UVA-induced ECM degradation and enhancement of UVA-induced expression of tanning-related protease-activated receptor 2, promotes cell differentiation | - | - | [117,118] | |
| - | Mesenchymal stem cells (MSCs): reducing the expression of cellular senescence markers (e.g., SA-β-gal, p21, p53), enhancing cellular antioxidant capacity, and reducing oxidative stress-induced cell damage | C57 mice: improving the aging state of many tissues and organs, reducing expression of inflammatory factors | KAT7-P15 | [116] | |
| - | Human mammary cancer cells (MCF-7) and human hepatocellular carcinoma cells (HepG 2): activating ARE, inducing the expression of antioxidant enzymes and phase II detoxification enzymes, and enhancing cellular antioxidant and detoxification capacity | - | Nrf2/ARE | [113] | |
| - | Rat Small Intestine Crypt Epithelial Cells (IEC): Down-regulation of caspase-3, Bax levels and LC3II/I ratio, and up-regulation of Bcl-2 and p62 levels were used to reduce autophagy and inhibit apoptosis | - | PI3K/AKT/mTOR | [114] | |
| - | - | Mice: decreasing malondialdehyde, TNF-α and IL-6 levels, and increasing glutathione peroxidase and superoxide dismutase levels | NF-κB/MAPK/Nrf2 | [115] | |
| - | - | Healthy female subjects: improving facial wrinkles and elasticity, increases collagen type I mRNA levels, reduces UV-induced DNA damage | - | [105] | |
| - | - | Hairless mice: inhibiting MMP-9 expression and reducing skin wrinkles and sagging | - | [107] | |
| - | - | 11 male and 11 female subjects: protecting human skin from UVA and UVB-induced erythema, reducing serum lipid peroxidation | - | [106] | |
| Lycopene | Singlet oxygen (all-E-isomer-rich IC50 0.26 μg/mL, Z-isomer-rich IC50 1.06 μg/mL) | HSF: Enhancing hyaluronic acid production, promoting proliferation | Anti-elastase activity, anti-melanogenic activity, and anti-tyrosinase activity, inhibition of melanin precursor darkening | - | [130] |
| - | - | Human oral intake: reducing erythematous reaction | - | [131] | |
| - | Chinese hamster ovary cell (M146L cell): reduction in oxidative stress and apoptosis, upregulation of antioxidant and anti-apoptotic proteins, downregulation of pro-apoptotic proteins | - | PI3K/Akt/Nrf2 | [139] | |
| - | Macrophages: inhibiting LPS-induced IκB phosphorylation, IκB degradation, and NF-κB translocation, blocking phosphorylation of ERK1/2 and p38 MAP kinase | - | MAPK/NF-κB | [140] | |
| - | Primary mouse neurons cell: enhancing cell viability, restoring mitochondrial membrane potential, and reducing ROS production | - | PI3K/Akt | [138] | |
| - | HSF: decreasing the content of ROS, β-galactosidases, and AGEs and increases mitochondrial membrane potential | - | - | [128] | |
| - | Human neuroblastoma cells (SH-SY5Y): blocking neuro-inflammation and apoptosis | - | - | [129] | |
| - | HSF: Combined with vitamin E, enhancing the inhibition of MMP-1 expression after UVA radiation | - | - | [137] | |
| - | - | 60 female subjects: improving skin elasticity, firmness, brightness, tone, and fine lines and wrinkles | - | [132] | |
| - | - | 5 male and 5 female subjects: reduction in markers of inflammatory oxidative damage (e.g., malondialdehyde, protein carbonyls, etc.) and low-density lipoprotein peroxidase protein levels | - | [133] | |
| - | - | 33 healthy male volunteers aged 20 to 30 years old: enhancing skin hydration and elasticity, reducing erythema, melanin, and sebum levels | - | [134] | |
| - | - | 20 volunteers between 40 and 50 years of age: reducing the number of wrinkles and roughness | - | [135] | |
| Astaxanthin | ABTS(IC50 7.7 μg/mL); DPPH(IC50 17.5 μg/mL) | - | - | - | [161] |
| ABTS(IC50 17.56 μg/mL); DPPH(IC50 50.93 μg/mL) | - | - | - | [162] | |
| - | HSF: inhibiting cellular damage caused by free radicals and reducing UVA radiation-induced elevation of IL6 expression | - | - | [151] | |
| - | Mouse photoreceptor cells (661W): reducing ROS production and attenuating apoptosis | - | PI3K/Akt/Nrf2 | [153] | |
| - | - | Male hairless mice: reducing UV-induced collagen degradation and elastic fiber damage, reducing the expression of MMPs (e.g., MMP-1, MMP-3, MMP-9) | Nrf2/NF-κB/MAPK | [154] | |
| - | HaCaT keratinocytes: inhibition of cell apoptosis by reducing INOS and COX-2 | - | - | [152] | |
| - | - | Between 30 and 56 years of age, 21 women and 2 men: improving skin elasticity and hydration | - | [149] | |
| - | - | 30 healthy female subjects: improving wrinkles, elasticity, transepidermal water loss, moisture content, and sebum oil levels | - | [150] | |
| Lutein and zeaxanthin | - | Human RPE cell: directly quenching ROS and facilitating glutathione synthesis | - | - | [156] |
| - | Rat tracheal epithelial cells: reduction in UVA radiation-induced DNA damage | - | - | [157] | |
| - | - | Human subjects: improving skin hydration, elasticity, and photoprotective activity | - | [158] | |
| - | - | Hairless mice: decreasing UVB-induced epidermal hyper-proliferation and acute inflammation in hairless mice | - | [159] | |
| - | - | Mice: reducing wrinkles and dryness | - | [160] |
3.2. The Potential of Novel Carotenoids on Anti-Photoaging
3.2.1. C30 Carotenoids
3.2.2. C50 Carotenoids
4. Safety and Bioavailability of Carotenoids
4.1. Oral Administration: Safety Considerations
4.2. Topical Application: Efficacy and Safety Profile
5. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Millar, S.E. Revitalizing Aging Skin through Diet. Cell 2018, 175, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729. [Google Scholar] [CrossRef] [PubMed]
- Tsatsou, F.; Trakatelli, M.; Patsatsi, A.; Kalokasidis, K.; Sotiriadis, D. Extrinsic Aging. Dermato-Endocrinology 2012, 4, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Chien, A.L. Photoaging: A Review of Current Literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a Modulator of Skin Aging and Inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic Acid, a Promising Anti-Photoaging Agent: Evidences of Its Antioxidant and Anti-Wrinkle Potentials, and Its Ability to Prevent Photodamage and MMP-1 Expression in L929 Fibroblasts Exposed to UVB. Free. Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin Aging Properties of Protocatechuic Acid in Vitro and in Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef]
- Yutani, R.; Kikuchi, T.; Teraoka, R.; Kitagawa, S. Efficient Delivery and Distribution in Skin of Chlorogenic Acid and Resveratrol Induced by Microemulsion Using Sucrose Laurate. Chem. Pharm. Bull. 2014, 62, 274–280. [Google Scholar] [CrossRef]
- Galano, A.; Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Alarcón-Ángeles, G.; Rojas-Hernández, A. Role of the Reacting Free Radicals on the Antioxidant Mechanism of Curcumin. Chem. Phys. 2009, 363, 13–23. [Google Scholar] [CrossRef]
- Ciardullo, G.; Orlando, C.; Russo, N.; Marchese, E.; Galano, A.; Marino, T.; Prejanò, M. On the Dual Role of (+)-Catechin as Primary Antioxidant and Inhibitor of Viral Proteases. Comput. Biol. Med. 2024, 180, 108953. [Google Scholar] [CrossRef] [PubMed]
- Grivard, A.; Goubet, I.; Duarte Filho, L.M.D.S.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Mar. Drugs 2022, 20, 524. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.; Grosso, C.; Delerue-Matos, C. Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Mar. Drugs 2024, 22, 153. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Suárez-Montenegro, Z.J.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Anti-Inflammatory and Neuroprotective Evaluation of Diverse Microalgae Extracts Enriched in Carotenoids. Algal Res. 2022, 67, 102830. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and Natural Carotenoids: Biochemical Activities and Anti-Tumor Effects. Biochim. Et Biophys. Acta (Bba)-Rev. Cancer 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in Cancer Metastasis—Status Quo and Outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef]
- Satomi, Y. Antitumor and Cancer-Preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer. Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Hirakida, H.; Nakamura, S.; Inagaki, S.; Tsuji, S.; Hayashi, M.; Shimazawa, M.; Hara, H. Anti-Diabetic Effects of Astaxanthin-Rich Extract Derived from Paracoccus carotinifaciens on Pancreatic β Cells. J. Funct. Foods 2022, 97, 105252. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Zuluaga Tamayo, M.; Rodriguez-Ruiz, V.; Thibaudeau, O.; Aboulhassanzadeh, S.; Abdolalizadeh, J.; Meddahi-Pellé, A.; Gueguen, V.; Barzegari, A.; Pavon-Djavid, G. Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress. Pharmaceutics 2024, 16, 840. [Google Scholar] [CrossRef]
- Maoka, T. Recent Progress in Structural Studies of Carotenoids in Animals and Plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef]
- Naik, A.A.; Gadgoli, C.H.; Naik, A.B. Formulation Containing Phytosomes of Carotenoids from Nyctanthes Arbor-Tristis and Tagetes Patula Protect D-Galactose Induced Skin Aging in Mice. Clin. Complement. Med. Pharmacol. 2023, 3, 100070. [Google Scholar] [CrossRef]
- Imokawa, G. The Xanthophyll Carotenoid Astaxanthin Has Distinct Biological Effects to Prevent the Photoaging of the Skin Even by Its Postirradiation Treatment. Photochem. Photobiol. 2019, 95, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Intracellular Signaling Mechanisms Involved in the Biological Effects of the Xanthophyll Carotenoid Astaxanthin to Prevent the Photo-Aging of the Skin in a Reactive Oxygen Species Depletion-Independent Manner: The Key Role of Mitogen and Stress-Activated Protein Kinase 1. Photochem. Photobiol. 2019, 95, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Amer, R.I.; Ezzat, S.M.; Aborehab, N.M.; Ragab, M.F.; Mohamed, D.; Hashad, A.; Attia, D.; Salama, M.M.; El Bishbishy, M.H. Downregulation of MMP1 Expression Mediates the Anti-Aging Activity of Citrus sinensis Peel Extract Nanoformulation in UV Induced Photoaging in Mice. Biomed. Pharmacother. 2021, 138, 111537. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New Insights in Photoaging, UVA Induced Damage and Skin Types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, K.; Chen, Y.; Zhao, J.; Jing, R.; Wang, L.; Li, X.; Hu, Z.; Xu, N.; Li, X. Keratinocyte Growth Factor 2 Ameliorates UVB-Induced Skin Damage via Activating the AhR/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 655281. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha Pinene Modulates UVA-Induced Oxidative Stress, DNA Damage and Apoptosis in Human Skin Epidermal Keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Travers, J.B.; Kemp, M.G. Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environ. Mol. Mutagen. 2018, 59, 438–460. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and Function of Skin, Hair and Nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Mu, J.; Chen, H.; Ye, M.; Zhang, X.; Ma, H. Acacetin Resists UVA Photoaging by Mediating the SIRT3/ROS/MAPKs Pathway. J. Cell Mol. Med. 2022, 26, 4624–4628. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.F.D.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA Photoprotective Activity of Brown Macroalgae Sargassum Cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef]
- Lohakul, J.; Chaiprasongsuk, A.; Jeayeng, S.; Saelim, M.; Muanjumpon, P.; Thanachaiphiwat, S.; Tripatara, P.; Soontrapa, K.; Lumlerdkij, N.; Akarasereenont, P.; et al. The Protective Effect of Polyherbal Formulation, Harak Formula, on UVA-Induced Photoaging of Human Dermal Fibroblasts and Mouse Skin via Promoting Nrf2-Regulated Antioxidant Defense. Front. Pharmacol. 2021, 12, 649820. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef]
- Morales-García, B.C.; Pérez-González, A.; Galano, A. Spirochromene and Spiroindene Compounds as Antioxidants. J. Mol. Struct. 2024, 1318, 139355. [Google Scholar] [CrossRef]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Cutaneous Oxidative Stress. J. Cosmet. Dermatol. 2012, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Romanhole, R.C.; Ataide, J.A.; Moriel, P.; Mazzola, P.G. Update on Ultraviolet A and B Radiation Generated by the Sun and Artificial Lamps and Their Effects on Skin. Int. J. Cosmet. Sci. 2015, 37, 366–370. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C.; Golebiewski, C.; Bernerd, F. Diversity of Biological Effects Induced by Longwave UVA Rays (UVA1) in Reconstructed Skin. PLoS ONE 2014, 9, e105263. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Miyata, Y.; Okada, K.; Fujimoto, A.; Hata, K.-I.; Kagami, H.; Tomita, Y.; Ueda, M. The Effect of the Long-Term Cultivation on Telomere Length and Morphology of Cultured Epidermis. J. Dermatol. Sci. 2004, 34, 221–230. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular Dynamic Simulations of Oxidized Skin Lipid Bilayer and Permeability of Reactive Oxygen Species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Ling, H.; Lou, Y. Total Flavones from Elsholtzia Blanda Reduce Infarct Size during Acute Myocardial Ischemia by Inhibiting Myocardial Apoptosis in Rats. J. Ethnopharmacol. 2005, 101, 169–175. [Google Scholar] [CrossRef]
- Andrés Juan, C.; Pérez de Lastra, J.M.; Plou Gasca, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Vasilyeva, A.D.; Yurina, L.V.; Bychkova, A.V. Oxidation of Proteins: Is It a Programmed Process? Free Radic. Res. 2018, 52, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Grune, T. Redox Regulation in Aging: Role of Protein Aggregates. Free Radic. Biol. Med. 2018, 124, 564. [Google Scholar] [CrossRef]
- Höhn, A.; König, J.; Grune, T. Protein Oxidation in Aging and the Removal of Oxidized Proteins. J. Proteom. 2013, 92, 132–159. [Google Scholar] [CrossRef]
- Jang, H.-Y.; Kim, G.-B.; Kim, J.-M.; Kang, S.Y.; Youn, H.-J.; Park, J.; Ro, S.Y.; Chung, E.-Y.; Park, K.-H.; Kim, J.-S. Fisetin Inhibits UVA-Induced Expression of MMP-1 and MMP-3 through the NOX/ROS/MAPK Pathway in Human Dermal Fibroblasts and Human Epidermal Keratinocytes. Int. J. Mol. Sci. 2023, 24, 17358. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Zhang, J.; Bowden, G.T. Activation of P38 MAP Kinase and JNK Pathways by UVA Irradiation. Photochem. Photobiol. Sci. 2012, 11, 54–61. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, C. P38 MAPK in Regulating Cellular Responses to Ultraviolet Radiation. J. Biomed. Sci. 2007, 14, 303–312. [Google Scholar] [CrossRef]
- Lasa, M.; Mahtani, K.R.; Finch, A.; Brewer, G.; Saklatvala, J.; Clark, A.R. Regulation of Cyclooxygenase 2 mRNA Stability by the Mitogen-Activated Protein Kinase P38 Signaling Cascade. Mol. Cell Biol. 2000, 20, 4265–4274. [Google Scholar] [CrossRef]
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular Basis of Skin Photoaging and Therapeutic Interventions by Plant-Derived Natural Product Ingredients: A Comprehensive Review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef]
- Yoshioka, H.; Hino, Y.; Iwata, K.; Ogawa, T.; Yoshioka, M.; Ishihama, N.; Adachi, H. Dynamics of Plant Immune MAPK Activity and ROS Signaling in Response to Invaders. Physiol. Mol. Plant Pathol. 2023, 125, 102000. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Mol. Cell Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-Derived Exosomes Protect against Oxidative Stress-Induced Skin Injury via Adaptive Regulation of the NRF2 Defense System. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef]
- Tian, F.F.; Zhang, F.F.; Lai, X.D.; Wang, L.J.; Yang, L.; Wang, X.; Singh, G.; Zhong, J.L. Nrf2-Mediated Protection against UVA Radiation in Human Skin Keratinocytes. Biosci. Trends 2011, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer Chemoprevention Mechanisms Mediated through the Keap1-Nrf2 Pathway. Antioxid. Redox Signal 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Suzuki, T.; Hiramoto, K.; Asami, S.; Naganuma, E.; Suda, H.; Iso, T.; Yamamoto, H.; Morita, M.; Baird, L.; et al. Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response. Mol. Cell Biol. 2016, 36, 271–284. [Google Scholar] [CrossRef]
- Hu, N.; An, R.; Yu, K.; Chang, Y.; Gao, W. PF4 Induces Inflammatory Response through NF-kB Signal Pathway in Rats with Intracerebral Haemorrhage. Folia Neuropathol. 2023, 61, 379–386. [Google Scholar] [CrossRef]
- Barber, K.; Mendonca, P.; Evans, J.A.; Soliman, K.F.A. Antioxidant and Anti-Inflammatory Mechanisms of Cardamonin through Nrf2 Activation and NF-kB Suppression in LPS-Activated BV-2 Microglial Cells. Int. J. Mol. Sci. 2023, 24, 10872. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and Target Genes of Rel/NF-kappaB Transcription Factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB Signaling in Skin Aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Youssef, N.S.; Elzaitony, A.S.; Baky, N.A.A. Diacerein Attenuate LPS-Induced Acute Lung Injury via Inhibiting ER Stress and Apoptosis: Impact on the Crosstalk between SphK1/S1P, TLR4/NFκB/STAT3, and NLRP3/IL-1β Signaling Pathways. Life Sci. 2022, 308, 120915. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Chen, W.; Feng, F.; Deng, Y. Resveratrol Inhibits Lipopolysaccharide-Induced Extracellular Matrix Accumulation and Inflammation in Rat Glomerular Mesangial Cells by SphK1/S1P2/NF-κB Pathway. Diabetes Metab. Syndr. Obes. 2020, 13, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.A.; Grougnet, R.; Nicolau, E.; Picot, L.; de Oliveira Junior, R.G. Carotenoids from Marine Microalgae as Antimelanoma Agents. Mar. Drugs 2022, 20, 618. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Saglam-Metiner, P.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. Aligned with Sustainable Development Goals: Microwave Extraction of Astaxanthin from Wet Algae and Selective Cytotoxic Effect of the Extract on Lung Cancer Cells. Prep. Biochem. Biotechnol. 2023, 53, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective Effects of Astaxanthin Supplementation against Ultraviolet-Induced Photoaging in Hairless Mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive Effect of Dietary Astaxanthin on UVA-Induced Skin Photoaging in Hairless Mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Arslansoy, N.; Fidan, O.; Arslansoy, N.; Fidan, O. Carotenoids and Their Antioxidant Power. In The Power of Antioxidants-Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2024; ISBN 978-0-85466-205-0. [Google Scholar]
- Zeb, A.; Hussain, A. Chemo-Metric Analysis of Carotenoids, Chlorophylls, and Antioxidant Activity of Trifolium Hybridum. Heliyon 2020, 6, e03195. [Google Scholar] [CrossRef]
- Chu, X.; Liu, J.; Gu, W.; Tian, L.; Tang, S.; Zhang, Z.; Jiang, L.; Xu, X. Study of the Properties of Carotenoids and Key Carotenoid Biosynthesis Genes from Deinococcus Xibeiensis R13. Biotechnol. Appl. Biochem. 2022, 69, 1459–1473. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Bonete, M.-J.; León, R.; Kharroub, K. Characterization and Biological Activities of Carotenoids Produced by Three Haloarchaeal Strains Isolated from Algerian Salt Lakes. Arch. Microbiol. 2021, 204, 6. [Google Scholar] [CrossRef] [PubMed]
- Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Mandić, A.; Četojević-Simin, D. Tomato Waste: Carotenoids Content, Antioxidant and Cell Growth Activities. Food Chem. 2015, 172, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium Halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.-L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba Sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Zargar, M.; Zolfaghari, M.R.; Amoozegar, M.A. Carotenoid Pigment of Halophilic Archaeon Haloarcula Sp. A15 Induces Apoptosis of Breast Cancer Cells. Cell Biochem. Funct. 2023, 41, 344–354. [Google Scholar] [CrossRef]
- Gómez-Villegas, P.; Vigara, J.; Vila, M.; Varela, J.; Barreira, L.; Léon, R. Antioxidant, Antimicrobial, and Bioactive Potential of Two New Haloarchaeal Strains Isolated from Odiel Salterns (Southwest Spain). Biology 2020, 9, 298. [Google Scholar] [CrossRef]
- Sheokand, P.; Tiwari, S.K. Characterization of Carotenoids Extracted from Haloferax Larsenii NCIM 5678 Isolated from Pachpadra Salt Lake, Rajasthan. Extremophiles 2024, 28, 33. [Google Scholar] [CrossRef]
- Fariq, A.; Yasmin, A.; Jamil, M. Production, Characterization and Antimicrobial Activities of Bio-Pigments by Aquisalibacillus Elongatus MB592, Salinicoccus Sesuvii MB597, and Halomonas Aquamarina MB598 Isolated from Khewra Salt Range, Pakistan. Extremophiles 2019, 23, 435–449. [Google Scholar] [CrossRef]
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium Salinarium against DNA-Damaging Agents. J. Radiat. Res. 1998, 39, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, R.; Taslim, N.A.; Aman, A.M.; Syauki, A.Y.; Bukhari, A.; Bahar, B.; Mayulu, N.; Putra, D.E.; Syahputra, R.A.; Kim, B.; et al. Pharmacoinformatics and ex vivo Studies of Carotenoids from Green Algae Caulerpa Racemosa as Functional Biomolecules to Modulate Type-2 Diabetes Markers. S. Afr. J. Bot. 2025, 178, 348–359. [Google Scholar] [CrossRef]
- Mao, M.; Lei, H.; Liu, Q.; He, R.; Zuo, Z.; Zhang, N.; Zhou, C. Lycopene Inhibits Neointimal Hyperplasia through Regulating Lipid Metabolism and Suppressing Oxidative Stress. Mol. Med. Rep. 2014, 10, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gao, R.; Song, X.; Li, X.; Zhu, J. Cardio-Protective and Anti-Atherosclerosis Effect of Crocetin on Vitamin D3 and HFD-Induced Atherosclerosis in Rats. J. Oleo Sci. 2021, 70, 1447–1459. [Google Scholar] [CrossRef]
- Liu, S.; Mohri, S.; Tsukamoto, M.; Yanai, Y.; Manabe, Y.; Sugawara, T. Preventive Effects of Dietary Fucoxanthin on Ultraviolet A Induced Photoaging in Hairless Mice. J. Sci. Food Agric. 2025, 105, 453–464. [Google Scholar] [CrossRef]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef]
- Hata, T.R.; Scholz, T.A.; Ermakov, I.V.; McClane, R.W.; Khachik, F.; Gellermann, W.; Pershing, L.K. Non-Invasive Raman Spectroscopic Detection of Carotenoids in Human Skin. J. Investig. Dermatol. 2000, 115, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef]
- Novikov, V.S.; Kuzmin, V.V.; Darvin, M.E.; Lademann, J.; Sagitova, E.A.; Prokhorov, K.A.; Ustynyuk, L.Y.; Nikolaeva, G.Y. Relations between the Raman Spectra and Molecular Structure of Selected Carotenoids: DFT Study of α-Carotene, β-Carotene, γ-Carotene and Lycopene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 270, 120755. [Google Scholar] [CrossRef]
- Lin, H.-W.; Chang, T.-J.; Yang, D.-J.; Chen, Y.-C.; Wang, M.; Chang, Y.-Y. Regulation of Virus-Induced Inflammatory Response by β-Carotene in RAW264.7 Cells. Food Chem. 2012, 134, 2169–2175. [Google Scholar] [CrossRef]
- Dembińska-Kieć, A.; Malczewska-Malec, M.; Polus, A.; Kieć-Wilk, B.; Grzybowska, J.; Stachura, J.; Dyduch, G.; Pryjma, J.; Skrzeczyńska, J.; Langman, T.; et al. β-Carotene and Angiogenesis. Pure Appl. Chem. 2006, 78, 1519–1537. [Google Scholar] [CrossRef]
- Białek-Bylka, G.E.; Jazurek, B.; Dedic, R.; Hala, J.; Skrzypczak, A. Unique Spectroscopic Properties of Synthetic 15-Cis Beta-Carotene, an Important Compound in Photosynthesis, and a Medicine for Photoprotective Function. Cell Mol. Biol. Lett. 2003, 8, 689–697. [Google Scholar]
- Cui, B.; Liu, S.; Wang, Q.; Lin, X. Effect of β-Carotene on Immunity Function and Tumour Growth in Hepatocellular Carcinoma Rats. Molecules 2012, 17, 8595–8603. [Google Scholar] [CrossRef]
- Cho, S.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Lee, S.; Lee, M.-J.; Chung, J.H. Differential Effects of Low-Dose and High-Dose Beta-Carotene Supplementation on the Signs of Photoaging and Type I Procollagen Gene Expression in Human Skin in Vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef]
- Lee, J.; Jiang, S.; Levine, N.; Watson, R.R. Carotenoid Supplementation Reduces Erythema in Human Skin after Simulated Solar Radiation Exposure. Proc. Soc. Exp. Biol. Med. 2000, 223, 170–174. [Google Scholar] [CrossRef]
- Minami, Y.; Kawabata, K.; Kubo, Y.; Arase, S.; Hirasaka, K.; Nikawa, T.; Bando, N.; Kawai, Y.; Terao, J. Peroxidized Cholesterol-Induced Matrix Metalloproteinase-9 Activation and Its Suppression by Dietary Beta-Carotene in Photoaging of Hairless Mouse Skin. J. Nutr. Biochem. 2009, 20, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the Link between Nutrition and Skin Aging. Dermato-Endocrinology 2012, 4, 298–307. [Google Scholar] [CrossRef]
- White, A.L.; Jahnke, L.S. Contrasting Effects of UV-A and UV-B on Photosynthesis and Photoprotection of Beta-Carotene in Two Dunaliella spp. Plant Cell Physiol. 2002, 43, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, Canthaxanthin and Beta-Carotene Differently Affect UVA-Induced Oxidative Damage and Expression of Oxidative Stress-Responsive Enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef]
- Wertz, K.; Seifert, N.; Hunziker, P.B.; Riss, G.; Wyss, A.; Hunziker, W.; Goralczyk, R. β-Carotene Interference with UVA-Induced Gene Expression by Multiple Pathways. Pure Appl. Chem. 2006, 78, 1539–1550. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids Activate the Antioxidant Response Element Transcription System. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef]
- Xu, G.; Ma, T.; Zhou, C.; Zhao, F.; Peng, K.; Li, B. β-Carotene Attenuates Apoptosis and Autophagy via PI3K/AKT/mTOR Signaling Pathway in Necrotizing Enterocolitis Model Cells IEC-6. Evidence-Based Complement. Altern. Med. 2022, 2022, 2502263. [Google Scholar] [CrossRef]
- Wu, T.; Xie, Y.; Wu, Z.; Li, Y.; Jiang, M.; Yu, H.; Li, X.; Wang, J.; Zhou, E.; Yang, Z. β-Carotene Protects Mice against Lipopolysaccharide and D-Galactosamine Induced Acute Liver Injury via Regulation of NF-κB, MAPK, and Nrf2 Signaling. J. Oleo Sci. 2023, 72, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-Aging Effect of β-Carotene through Regulating the KAT7-P15 Signaling Axis, Inflammation and Oxidative Stress Process. Cell Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Wertz, K.; Hunziker, P.B.; Seifert, N.; Riss, G.; Neeb, M.; Steiner, G.; Hunziker, W.; Goralczyk, R. Beta-Carotene Interferes with Ultraviolet Light A-Induced Gene Expression by Multiple Pathways. J. Investig. Dermatol. 2005, 124, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Wertz, K.; Seifert, N.; Hunziker, P.B.; Riss, G.; Wyss, A.; Lankin, C.; Goralczyk, R. β-Carotene Inhibits UVA-Induced Matrix Metalloprotease 1 and 10 Expression in Keratinocytes by a Singlet Oxygen-Dependent Mechanism. Free Radic. Biol. Med. 2004, 37, 654–670. [Google Scholar] [CrossRef]
- Bayerl, C. Beta-Carotene in Dermatology: Does It Help? Acta Dermatoven APA 2008, 17, 161. [Google Scholar]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let Food Be Your Medicine: Nutraceutical Properties of Lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia Coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of Carotenoids: The Challenges and Prospects—A Review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, J.-H.; Kim, N.-H.; Yeom, S.-J.; Kim, S.-W.; Oh, D.-K. Increase of Lycopene Production by Supplementing Auxiliary Carbon Sources in Metabolically Engineered Escherichia Coli. Appl. Microbiol. Biotechnol. 2011, 90, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Jeong, S.-W.; Yang, J.E.; Choi, Y.J. High-Yield Production of Lycopene from Corn Steep Liquor and Glycerol Using the Metabolically Engineered Deinococcus Radiodurans R1 Strain. J. Agric. Food Chem. 2020, 68, 5147–5153. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Huang, C.-C.; Lin, W.-T.; Hsieh, C.-C.; Huang, S.-Y.; Lin, S.-J.; Yang, S.-C. Lycopene Supplementation Attenuated Xanthine Oxidase and Myeloperoxidase Activities in Skeletal Muscle Tissues of Rats after Exhaustive Exercise. Br. J. Nutr. 2005, 94, 595–601. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Yang, X.; Ding, H. Lycopene Ameliorates Skin Aging by Regulating the Insulin Resistance Pathway and Activating SIRT1. Food Funct. 2022, 13, 11307–11320. [Google Scholar] [CrossRef]
- Almeer, R.; Alyami, N.M. Effect of Lycopene on TiO2 Nanoforms Induced Oxidative Stress and Neuroinflammation in SH-SY5Y Cells: An in Vitro Study. Drug Chem. Toxicol. 2025, 48, 51–61. [Google Scholar] [CrossRef]
- Honda, M. Z-Isomers of Lycopene and β-Carotene Exhibit Greater Skin-Quality Improving Action than Their All-E-Isomers. Food Chem. 2023, 421, 135954. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-Rich Products and Dietary Photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Tarshish, E.; Hermoni, K.; Sharoni, Y.; Muizzuddin, N. Effect of Lumenato Oral Supplementation on Plasma Carotenoid Levels and Improvement of Visual and Experiential Skin Attributes. J. Cosmet. Dermatol. 2022, 21, 4042–4052. [Google Scholar] [CrossRef]
- Chernyshova, M.P.; Pristenskiy, D.V.; Lozbiakova, M.V.; Chalyk, N.E.; Bandaletova, T.Y.; Petyaev, I.M. Systemic and Skin-Targeting Beneficial Effects of Lycopene-Enriched Ice Cream: A Pilot Study. J. Dairy. Sci. 2019, 102, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Baig, M.M.F.A.; Akhtar, N.; Chen, Y.; Xie, B.; Li, B. Topical Lycopene Emulgel Significantly Improves Biophysical Parameters of Human Skin. Eur. J. Pharm. Biopharm. 2022, 180, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Patzelt, A.; Gehse, S.; Schanzer, S.; Benderoth, C.; Sterry, W.; Lademann, J. Cutaneous Concentration of Lycopene Correlates Significantly with the Roughness of the Skin. Eur. J. Pharm. Biopharm. 2008, 69, 943–947. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.B.; Rhodes, L.E. Tomato Paste Rich in Lycopene Protects against Cutaneous Photodamage in Humans in Vivo: A Randomized Controlled Trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Offord, E.A.; Gautier, J.-C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective Potential of Lycopene, β-Carotene, Vitamin E, Vitamin C and Carnosic Acid in UVA-Irradiated Human Skin Fibroblasts. Free Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene Protects against T-BHP-Induced Neuronal Oxidative Damage and Apoptosis via Activation of the PI3K/Akt Pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef]
- Fang, Y.; Ou, S.; Wu, T.; Zhou, L.; Tang, H.; Jiang, M.; Xu, J.; Guo, K. Lycopene Alleviates Oxidative Stress via the PI3K/Akt/Nrf2pathway in a Cell Model of Alzheimer’s Disease. PeerJ 2020, 8, e9308. [Google Scholar] [CrossRef]
- Feng, D.; Ling, W.-H.; Duan, R.-D. Lycopene Suppresses LPS-Induced NO and IL-6 Production by Inhibiting the Activation of ERK, p38MAPK, and NF-κB in Macrophages. Inflamm. Res. 2010, 59, 115–121. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Mutale-Joan, C.; El Arroussi, H. Biotechnological Strategies Overcoming Limitations to H. Pluvialis-Derived Astaxanthin Production and Morocco’s Potential. Crit. Rev. Food Sci. Nutr. 2025, 65, 1404–1419. [Google Scholar] [CrossRef]
- Cunha, F.F.M.D.; Tonon, A.P.; Machado, F.; Travassos, L.R.; Grazzia, N.; Possatto, J.F.; Sant’ana, A.K.C.D.; Lopes, R.D.M.; Rodrigues, T.; Miguel, D.C.; et al. Astaxanthin Induces Autophagy and Apoptosis in Murine Melanoma B16F10-Nex2 Cells and Exhibits Antitumor Activity in Vivo. J. Chemother. 2024, 36, 222–237. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. In Vitro Antitumor Potential of Astaxanthin Nanoemulsion against Cancer Cells via Mitochondrial Mediated Apoptosis. Int. J. Pharm. 2019, 560, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.-W. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, e2100252. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Suganuma, K.; Nakajima, H.; Ohtsuki, M.; Imokawa, G. Astaxanthin Attenuates the UVA-Induced up-Regulation of Matrix-Metalloproteinase-1 and Skin Fibroblast Elastase in Human Dermal Fibroblasts. J. Dermatol. Sci. 2010, 58, 136–142. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a Xanthophyll Carotenoid, Inhibits Ultraviolet-Induced Apoptosis in Keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-T.; Yang, C.-M.; Yang, C.-H. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Turner, S.D.; Brautigan, D.L. Xanthophylls Lutein and Zeaxanthin Modify Gene Expression and Induce Synthesis of Hyaluronan in Keratinocyte Model of Human Skin. Biochem. Biophys. Rep. 2015, 4, 52–58. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Influence of Astaxanthin, Zeaxanthin and Lutein on DNA Damage and Repair in UVA-Irradiated Cells. J. Photochem. Photobiol. B Biol. 2006, 85, 205–215. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Pathak, M.A.; Goukassian, D. Dietary Lutein/Zeaxanthin Decreases Ultraviolet B-Induced Epidermal Hyperproliferation and Acute Inflammation in Hairless Mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and Zeaxanthin in Eye and Skin Health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef]
- Abd El-Ghany, M.N.; Hamdi, S.A.; Elbaz, R.M.; Aloufi, A.S.; El Sayed, R.R.; Ghonaim, G.M.; Farahat, M.G. Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste. Fermentation 2023, 9, 505. [Google Scholar] [CrossRef]
- Siziya, I.N.; Hwang, C.Y.; Seo, M.-J. Antioxidant Potential and Capacity of Microorganism-Sourced C30 Carotenoids—A Review. Antioxidants 2022, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Seo, D.-H.; Park, Y.-S.; Cha, I.-T.; Seo, M.-J. Isolation of Lactobacillus Plantarum Subsp. Plantarum Producing C30 Carotenoid 4,4’-Diaponeurosporene and the Assessment of Its Antioxidant Activity. J. Microbiol. Biotechnol. 2019, 29, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Cho, E.-S.; Yoon, D.J.; Seo, M.-J. Probiotic and Antioxidant Properties of C30 Carotenoid-Producing Lactiplantibacillus Plantarum Isolated from Kimchi. Food Sci. Biotechnol. 2023, 32, 543–552. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, B.Y.; Lee, P.C. Generation of Structurally Novel Short Carotenoids and Study of Their Biological Activity. Sci. Rep. 2016, 6, 21987. [Google Scholar] [CrossRef]
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Zanjani, B.M.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of Carotenoid Production by Halorubrum Sp. TBZ126; an Extremely Halophilic Archeon from Urmia Lake. Adv. Pharm. Bull. 2014, 4, 61–67. [Google Scholar] [CrossRef]
- Alvares, J.J.; Furtado, I.J. Characterization of Multicomponent Antioxidants from Haloferax Alexandrinus GUSF-1 (KF796625). 3 Biotech 2021, 11, 58. [Google Scholar] [CrossRef]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the Extreme Halophilic Archaeon Haloterrigena Turkmenica: Identification and Antioxidant Activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef]
- Moopantakath, J.; Imchen, M.; Anju, V.T.; Busi, S.; Dyavaiah, M.; Martínez-Espinosa, R.M.; Kumavath, R. Bioactive Molecules from Haloarchaea: Scope and Prospects for Industrial and Therapeutic Applications. Front. Microbiol. 2023, 14, 1113540. [Google Scholar] [CrossRef]
- Flegler, A.; Lipski, A. The C50 Carotenoid Bacterioruberin Regulates Membrane Fluidity in Pink-Pigmented Arthrobacter Species. Arch. Microbiol. 2021, 204, 70. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Cho, E.-S.; Kim, S.; Kim, K.; Seo, M.-J. Optimization of Bacterioruberin Production from Halorubrum Ruber and Assessment of Its Antioxidant Potential. Microb. Cell Factories 2024, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Bouhamed, S.B.H.; Chaari, M.; Baati, H.; Zouari, S.; Ammar, E. Extreme Halophilic Archaea: Halobacterium Salinarum Carotenoids Characterization and Antioxidant Properties. Heliyon 2024, 10, e36832. [Google Scholar] [CrossRef]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetović, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena Sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated From Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Gómez-Villegas, P.; de Carvalho, C.C.C.R.; Vigara, J.; Motilva, V.; León, R.; Talero, E. Up-Regulation of the Nrf2/HO-1 Antioxidant Pathway in Macrophages by an Extract from a New Halophilic Archaea Isolated in Odiel Saltworks. Antioxidants 2023, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Silva, T.D.C.D.; Vidal, E.E.; de Souza, R.D.F.R.; da Cunha Schmidt, K.; Mendes, P.V.D.; da Silva Andrade, R.F.; da Silva Oliveira, F.G.; de Lucena, B.T.L.; de Oliveira, M.B.M.; dos Santos Correia, M.T.; et al. Production of Carotenoid Sarcinaxanthin by Kocuria Palustris Isolated from Northeastern Brazil Caatinga Soil and Their Antioxidant and Photoprotective Activities. Electron. J. Biotechnol. 2021, 53, 44–53. [Google Scholar] [CrossRef]
- Wang, J.; Kong, W.; Liu, M.; Wang, Y.; Zheng, Y.; Zhou, Y. Association between Dietary Carotenoids Intake and Chronic Constipation in American Men and Women Adults: A Cross-Sectional Study. BMC Public Health 2023, 23, 1597. [Google Scholar] [CrossRef]
- Bates, C.A.; Vincent, M.J.; Buerger, A.N.; Santamaria, A.B.; Maier, A.; Jack, M. Investigating the Relationship between β-Carotene Intake from Diet and Supplements, Smoking, and Lung Cancer Risk. Food Chem. Toxicol. 2024, 194, 115104. [Google Scholar] [CrossRef]
- Edwards, J.A.; Bellion, P.; Beilstein, P.; Rümbeli, R.; Schierle, J. Review of Genotoxicity and Rat Carcinogenicity Investigations with Astaxanthin. Regul. Toxicol. Pharmacol. 2016, 75, 5–19. [Google Scholar] [CrossRef]
- Ramos-Souza, C.; De Rosso, V.V. Recent Approaches for the Bioaccessibility and Bioavailability of Carotenoids. In Carotenoids: Trends and Advances; Campos Chisté, R., Helena de Aguiar Andrade, E., Santana de Oliveira, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 153–178. ISBN 978-3-031-75322-0. [Google Scholar]
- Bouwstra, J.A.; Ponec, M. The Skin Barrier in Healthy and Diseased State. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Hsieh, Y.-S.; Ko, H.-H.; Wu, Y.-T. Formulation Approaches to Crystalline Status Modification for Carotenoids: Impacts on Dissolution, Stability, Bioavailability, and Bioactivities. Pharmaceutics 2023, 15, 485. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as Delivery Systems for Carotenoids: Comparative Studies of Loading Ability, Storage Stability and in Vitro Release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Jivan, M.; Rostamabadi, H.; Assadpour, E.; Tomas, M.; Capanoglu, E.; Alizadeh-Sani, M.; Kharazmi, M.S.; Jafari, S.M. Recent Progresses in the Delivery of β-Carotene: From Nano/Microencapsulation to Bioaccessibility. Adv. Colloid. Interface Sci. 2022, 307, 102750. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-Carotene Nanoemulsion-Based Delivery Systems Using Dual-Channel Microfluidization: Physical and Chemical Stability. J. Colloid. Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef] [PubMed]


| Name | DPPH IC50 (μM) |
|---|---|
| 4,4′-Diapolycopene | 8.7 |
| 4,4′-Diaponeurosporene | 11.6 |
| 4,4′-Diaponeurosporen-4′-al | 10.2 |
| 4,4′-Diapolycopendial | 7.5 |
| 4,4′-Diaponeurosporen-4′-oic acid | 9.7 |
| 4,4′-Diapotorulene | 70.3 |
| 4,4′-Diapo-β-carotene | 77.8 |
| Name | Antioxidant Capacity | In Vitro Experiment | References |
|---|---|---|---|
| Bacterioruberin | ABTS (IC50 9.8 μg/mL), FRAP (IC50 2.1 μg/mL) | - | [172] |
| DPPH (IC50 86.67 μg/mL) | - | [173] | |
| - | Protection of cells against oxidizing DNA damaging agent | [92] | |
| - | Protection of erythrocytes from H2O2 | [86] | |
| ABTS (IC50 20.5 μg/mL), | Macrophages: reducing ROS levels and reducing levels of pro-inflammatory cytokines TNF-α and IL-6 | [175] | |
| Sarcinaxanthin | 1O2 | Displaying an SPF of 9.36 ± 0.52, exhibiting in vitro photoprotective activity | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, C.; Su, W.; Sun, Z.; Gao, S.; Xie, W.; Zhang, B.; Sui, L. Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers. Antioxidants 2025, 14, 577. https://doi.org/10.3390/antiox14050577
Ma Y, Li C, Su W, Sun Z, Gao S, Xie W, Zhang B, Sui L. Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers. Antioxidants. 2025; 14(5):577. https://doi.org/10.3390/antiox14050577
Chicago/Turabian StyleMa, Yingchao, Chengxiang Li, Wanping Su, Zhongshi Sun, Shuo Gao, Wei Xie, Bo Zhang, and Liying Sui. 2025. "Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers" Antioxidants 14, no. 5: 577. https://doi.org/10.3390/antiox14050577
APA StyleMa, Y., Li, C., Su, W., Sun, Z., Gao, S., Xie, W., Zhang, B., & Sui, L. (2025). Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers. Antioxidants, 14(5), 577. https://doi.org/10.3390/antiox14050577







