Red Wine Grape Pomace Restores Gut Barrier Function and Improves Survival in Diet-Induced Ischemic Heart Disease
Abstract
1. Introduction
2. Materials and Methods
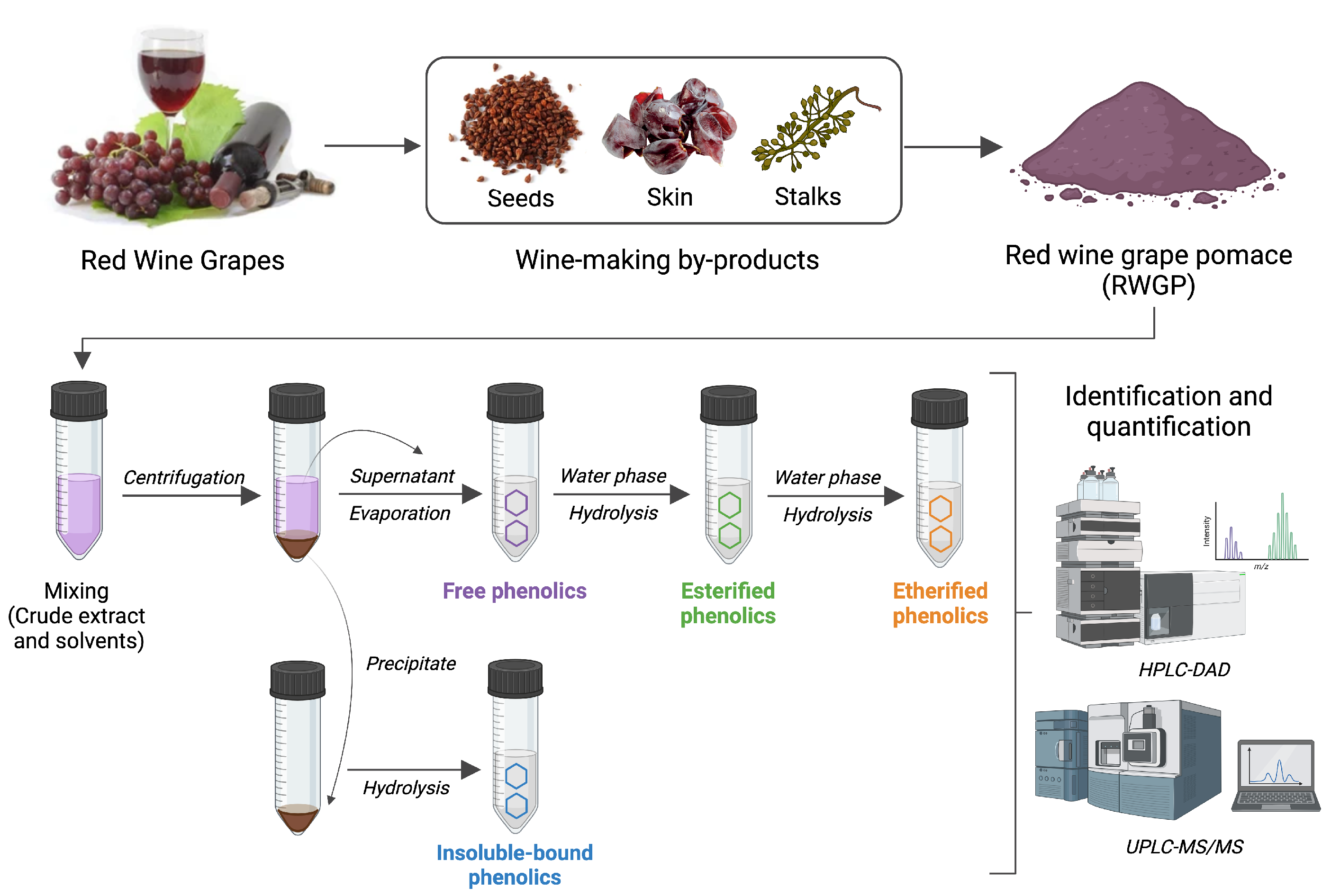
2.1. RWGP Material
2.2. Phenolic Compound Fractionation, Identification, and Quantification
2.2.1. Extraction of Soluble Free, Esterified, and Etherified Phenolics
2.2.2. Extraction of Insoluble-Bound Phenolics
2.2.3. Determination of Total Phenolic and Flavonoid Content
2.2.4. Characterization of Phenolic Compounds by HPLC-DAD
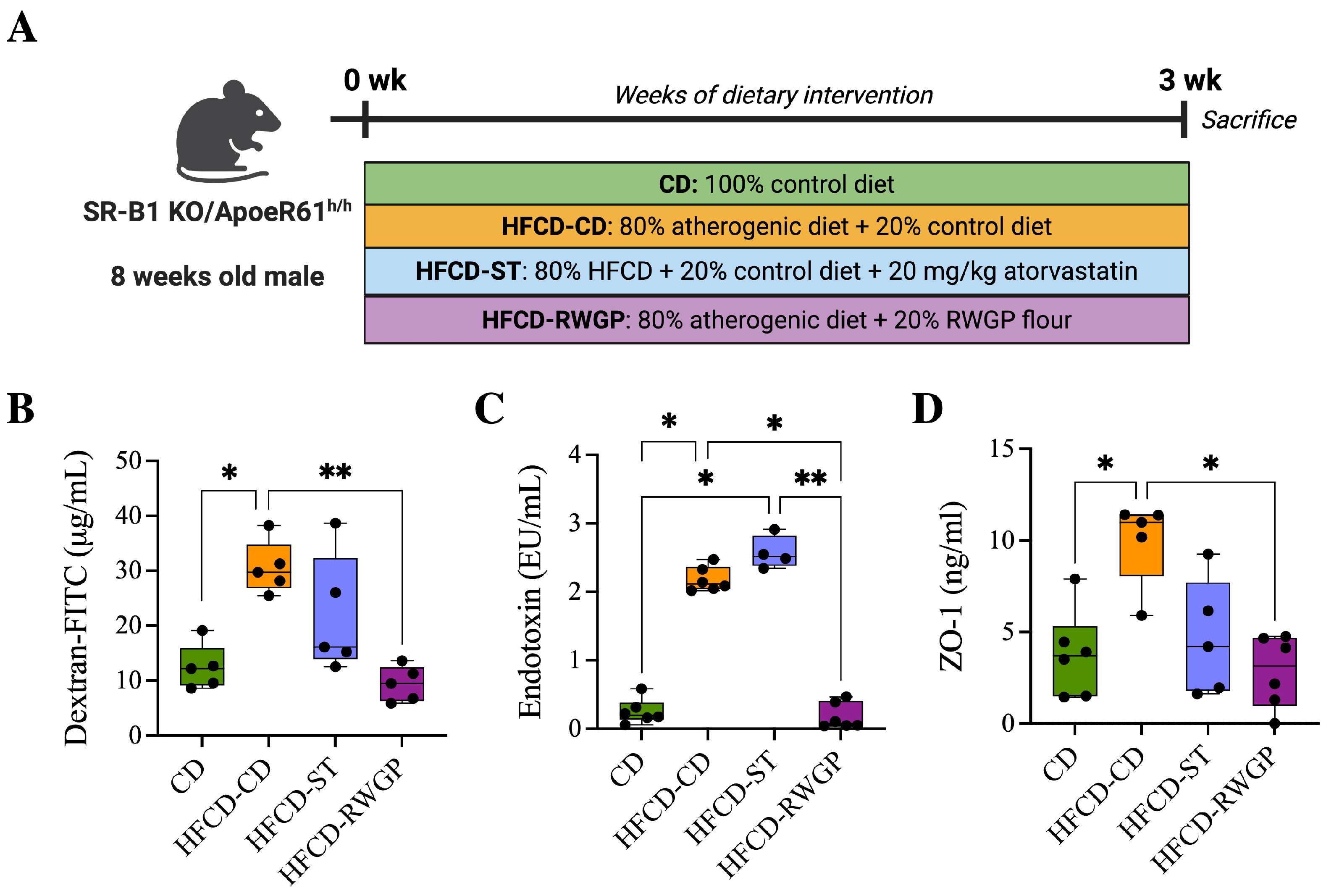
2.3. Animal Model and Diets
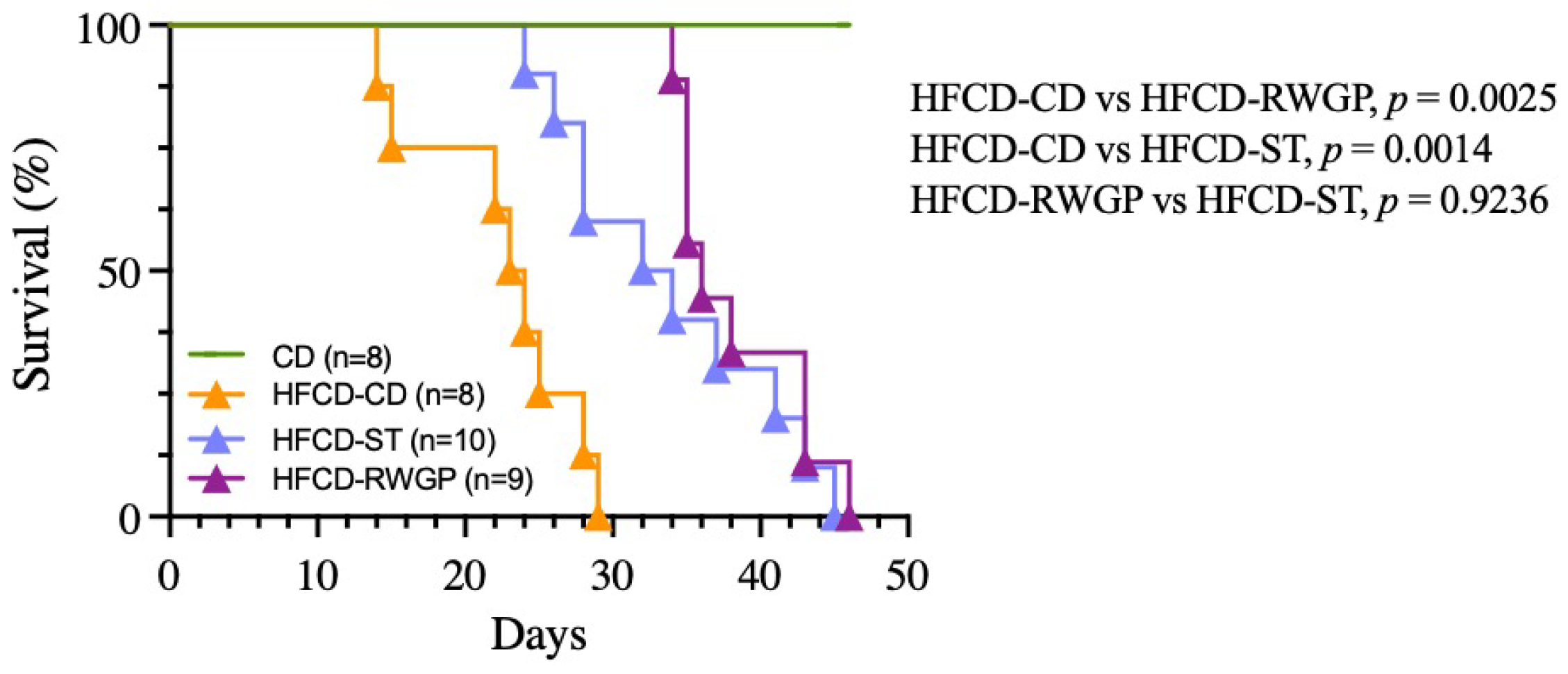
2.3.1. Survival Analysis
2.3.2. Intestinal Permeability Assay
2.3.3. Sample Collection
2.3.4. Biomarkers of Intestinal Permeability
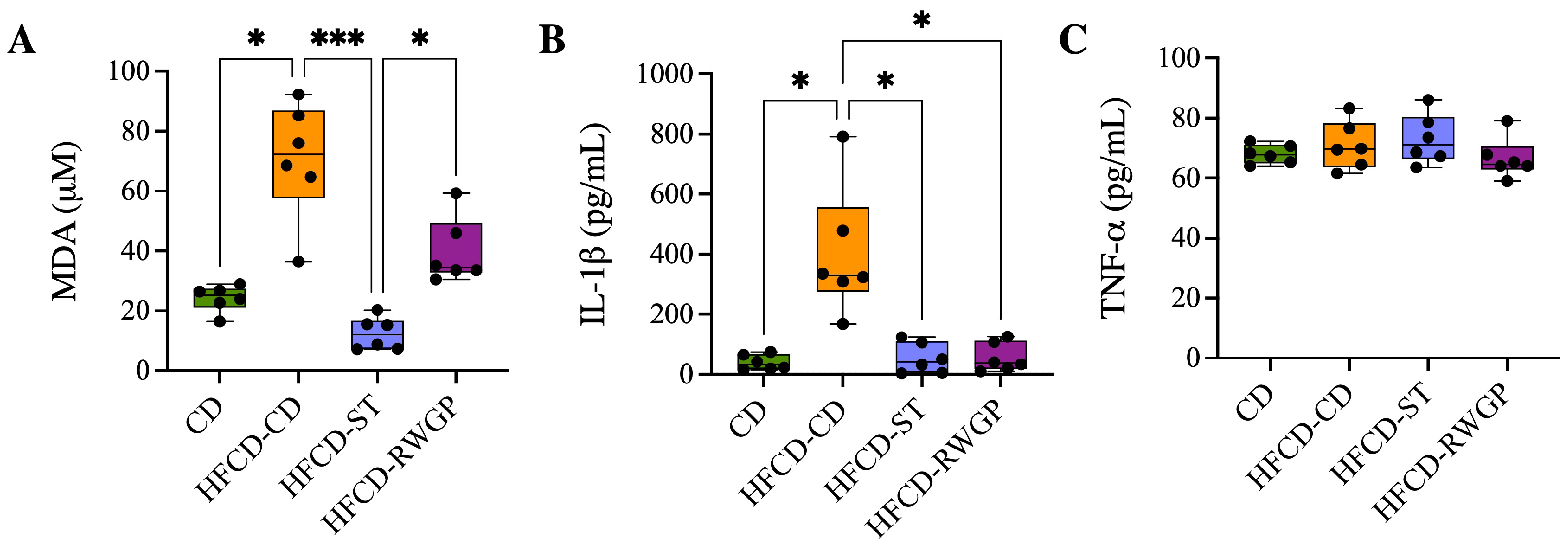
2.3.5. Oxidative Stress and Inflammatory Markers
2.3.6. Colon Histopathology Analysis
2.3.7. Statistical Analysis
3. Results
3.1. Total Phenolic and Flavonoid Content
3.2. Identification and Quantification of Phenolic Compounds
3.3. RWGP Supplementation Improves Survival and Gut Integrity
3.3.1. Survival Analysis and Body Weight
3.3.2. RWGP Reduces Gut Permeability and Endotoxemia
3.3.3. Effect of RWGP Supplementation on Oxidative Stress and Inflammatory Markers
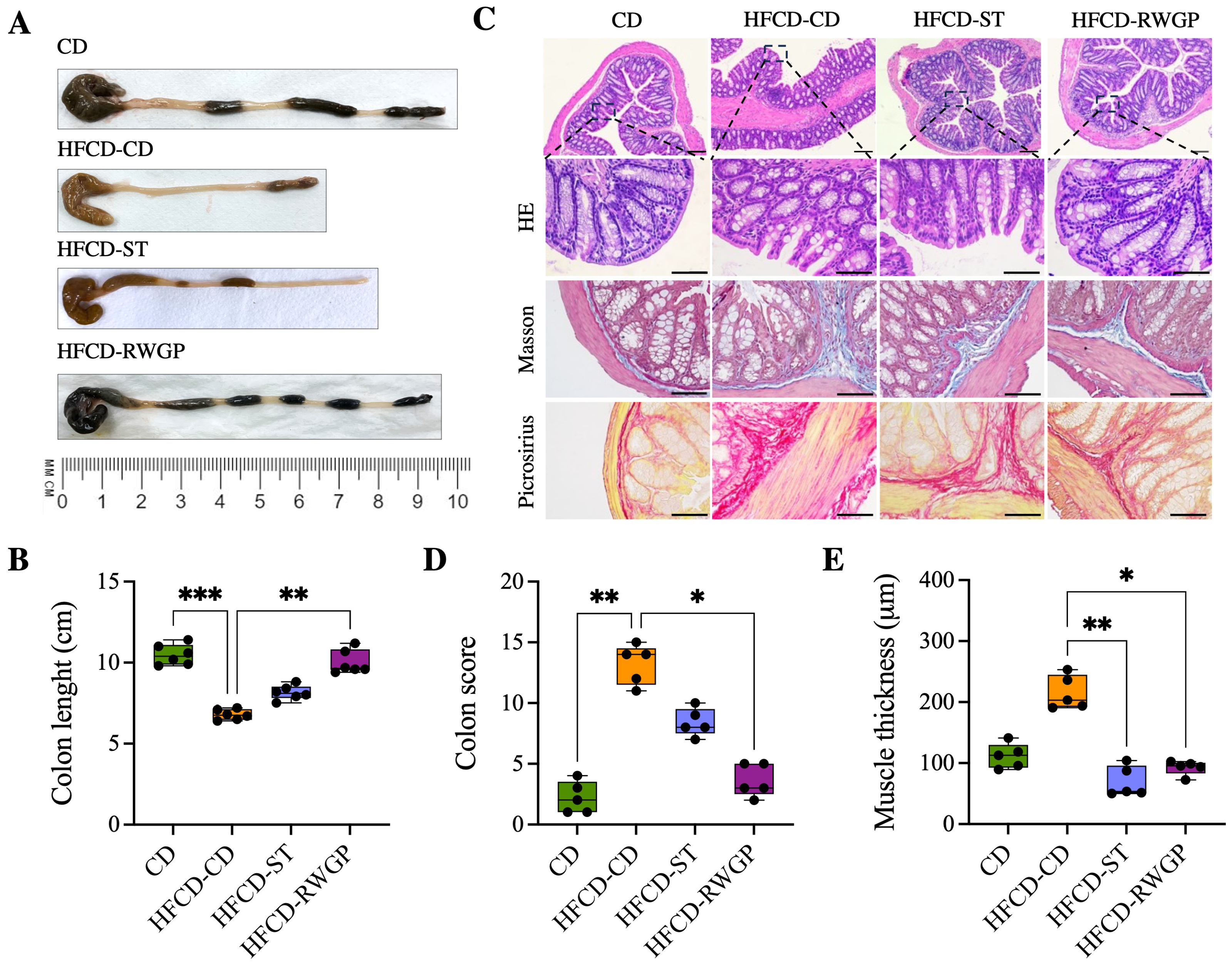
3.3.4. RWGP Mitigates Atherogenic Diet-Induced Colon Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, E347–E913. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, E472–E487. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2022, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.; Gonzalez, L.; Bravo, L.; Manjarres, L.; Andia, M.E. The Gut-Heart Axis: Molecular Perspectives and Implications for Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 12465. [Google Scholar] [CrossRef]
- Erlanson-Albertsson, C.; Stenkula, K.G. The Importance of Food for Endotoxemia and an Inflammatory Response. Int. J. Mol. Sci. 2021, 22, 9562. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Saito, Y.; Yamashita, T.; Yoshida, N.; Emoto, T.; Takeda, S.; Tabata, T.; Shinohara, M.; Kishino, S.; Sugiyama, Y.; Kitamura, N.; et al. Structural differences in bacterial lipopolysaccharides determine atherosclerotic plaque progression by regulating the accumulation of neutrophils. Atherosclerosis 2022, 358, 1–11. [Google Scholar] [CrossRef]
- Chagnon, F.; Metz, C.N.; Bucala, R.; Lesur, O. Endotoxin-induced myocardial dysfunction: Effects of macrophage migration inhibitory factor neutralization. Circ. Res. 2005, 96, 1095–1102. [Google Scholar] [CrossRef]
- Nguyen, M.; Putot, A.; Masson, D.; Cottin, Y.; Gautier, T.; Tribouillard, L.; Rérole, A.L.; Guinot, P.G.; Maza, M.; Pais de Barros, J.P.; et al. Risk factors and prognostic value of endotoxemia in patients with acute myocardial infarction. Front. Cardiovasc. Med. 2024, 11, 1419001. [Google Scholar] [CrossRef]
- Violi, F.; Castellani, V.; Menichelli, D.; Pignatelli, P.; Pastori, D. Gut barrier dysfunction and endotoxemia in heart failure: A dangerous connubium? Am. Heart J. 2023, 264, 40–48. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4865–4881. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape Pomace as a Natural Source of Phenolic Compounds: Solvent Screening and Extraction Optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-Sanjosé, M.L.; Muñiz, P. The protective effects of wine pomace products on the vascular endothelial barrier function. Food Funct. 2020, 11, 7878–7891. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Rivera, K.; Salas-Pérz, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; De La Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, Esterified, and Insoluble-Bound Phenolic Acids. 1. Extraction and Purification Procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Alvarez, A.C.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Pizarro, P.N.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals. Antioxidants 2022, 11, 1139. [Google Scholar] [CrossRef]
- Mudenuti, N.V.d.R.; de Camargo, A.C.; de Alencar, S.M.; Danielski, R.; Shahidi, F.; Madeira, T.B.; Hirooka, E.Y.; Spinosa, W.A.; Grossmann, M.V.E. Phenolics and alkaloids of raw cocoa nibs and husk: The role of soluble and insoluble-bound antioxidants. Food Biosci. 2021, 42, 101085. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mudenuti, N.V.d.R.; de Camargo, A.C.; Shahidi, F.; Madeira, T.B.; Hirooka, E.Y.; Grossmann, M.V.E. Soluble and insoluble-bound fractions of phenolics and alkaloids and their antioxidant activities in raw and traditional chocolate: A comparative study. J. Funct. Foods 2018, 50, 164–171. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Woting, A.; Blaut, M. Small Intestinal Permeability and Gut-Transit Time Determined with Low and High Molecular Weight Fluorescein Isothiocyanate-Dextrans in C3H Mice. Nutrients 2018, 10, 685. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Wu, J.C.; Huang, Q.; Shahidi, F.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Oikonomou, I.; Papageorgiou, A.; de Lastic, A.L.; Moulias, A.; Georgopoulou, G.A.; Mouzaki, A.; Koufou, E.E.; Tsigkas, G.; Gogos, C.; Davlouros, P.; et al. Gut barrier dysfunction, endotoxemia and inflammatory response in STEMI patients and effect of primary PCI. Am. J. Med. Sci. 2024, 368, 485–493. [Google Scholar] [CrossRef]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d’Amati, G.; Pignataro, M.G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: Implication for myocardial infarction. Eur. Heart J. 2020, 41, 3156–3165. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Picazo, Ó.; Fontes-Villalba, M.; Pareja-Galeano, H.; Lindeberg, S.; Martínez-Selles, M.; Lucia, A.; Emanuele, E. Serum Zonulin and Endotoxin Levels in Exceptional Longevity versus Precocious Myocardial Infarction. Aging Dis. 2018, 9, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Cheng, W.; Dai, Q.; Wei, Z.; Guo, M.; Chen, F.; Qiao, S.; Hu, J.; Wang, J.; et al. Heart–gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc. Res. 2023, 119, 1390. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Nery-Flores, S.D.; Castro-López, C.M.; Martínez-Hernández, L.; García-Chávez, C.V.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Flores-Gallegos, A.C.; Campos-Múzquiz, L.G.; Rodríguez-Herrera, R. Grape Pomace Polyphenols Reduce Acute Inflammatory Response Induced by Carrageenan in a Murine Model. Chem. Biodivers. 2024, 21, e202302065. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef]
- Fariña, E.; Daghero, H.; Bollati-Fogolín, M.; Boido, E.; Cantero, J.; Moncada-Basualto, M.; Olea-Azar, C.; Polticelli, F.; Paulino, M. Antioxidant Capacity and NF-kB-Mediated Anti-Inflammatory Activity of Six Red Uruguayan Grape Pomaces. Molecules 2023, 28, 3909. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, L.; Gentile, G.; Llorent-Martínez, E.J.; Zengin, G.; Masci, D.; Flamminii, F.; Stefanucci, A.; Mollica, A. Valorization of grape pomace extracts against cranberry, elderberry, rose hip berry, goji berry and raisin extracts: Phytochemical profile and in vitro biological activity. Food Chem. 2025, 463, 141323. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J.; Yang, J.; Hao, Y.; Fan, Y.; Wang, C.; Li, N. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT 2021, 135, 109995. [Google Scholar] [CrossRef]
- Jin, L.; Sun, S.; Ryu, Y.; Piao, Z.H.; Liu, B.; Choi, S.Y.; Kim, G.R.; Kim, H.S.; Kee, H.J.; Jeong, M.H. Gallic acid improves cardiac dysfunction and fibrosis in pressure overload-induced heart failure. Sci. Rep. 2018, 8, 9302. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Milajerdi, A.; Reiner, Ž.; Kolahdooz, F.; Amirani, E.; Mirzaei, H.; Barekat, M.; Asemi, Z. The effects of catechin on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 2369–2378. [Google Scholar] [CrossRef]
- Liu, J.; Du, X.; Yao, Q.; Jiang, T.; Cui, Q.; Xie, X.; Zhao, Z.; Lai, B.; Wang, N.; Xiao, L. Procyanidin B2 ameliorates endothelial dysfunction induced by nicotine via the induction of tetrahydrobiopterin synthesis. J. Funct. Foods 2022, 99, 105306. [Google Scholar] [CrossRef]
- Li, C.X.; Wu, R.M.; Xie, Q.L.; Wang, F.; Le Xu, X. Procyanidin B2 attenuates pathological cardiac fibrosis and inflammation: Role of PPARγ. J. Cardiovasc. Pharmacol. 2025, 85, 338–349. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Tokumaru, H.; Sadamoto, H.; Kobayashi, S.; Nochi, H. Effects of Phenolic Acids Produced from Food-Derived Flavonoids and Amino Acids by the Gut Microbiota on Health and Disease. Molecules 2024, 29, 5102. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease. Front. Cardiovasc. Med. 2022, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Rebollo-Hernanz, M.; Martin-Cabrejas, M.A.; Moreno-Arribas, M.V.; Bartolomé, B. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef]
- Taladrid, D.; de Llano, D.G.; Zorraquín-Peña, I.; Tamargo, A.; Silva, M.; Molinero, N.; Moreno-Arribas, M.V.; Bartolomé, B. Gastrointestinal digestion of a grape pomace extract: Impact on intestinal barrier permeability and interaction with gut microbiome. Nutrients 2021, 13, 2467. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.K.; Kalyan, M.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Pendyala, G.; Yelamanchili, S.V.; Yang, J.; Chidambaram, S.B.; Sakharkar, M.K.; et al. Role of the Gut Bacteria-Derived Metabolite Phenylacetylglutamine in Health and Diseases. ACS Omega 2023, 9, 3164–3172. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses Food Waste; FAO: Roma, Italy, 2011. [Google Scholar]
- Garrido, R.A.; Manrique, R.; Fredes, J.; Rodriguez, P.; Rodríguez, A.; Serafini, D.; Mena, M.; Masip, Y.; Díaz, I. Evaluating hydrogen production from grape pomace gasification: Unveiling the potential for Chile’s wine industry and its solid waste recovery as energy source. Renew. Energy 2024, 223, 119953. [Google Scholar] [CrossRef]
| Time (min) | Acetonitrile (%) | Acidified Water (%) |
|---|---|---|
| 0–30 | 15 | 85 |
| 30.1–45 | 25 | 75 |
| 45.1–55 | 40 | 60 |
| 55.1–60 | 50 | 50 |
| 60.1–65 | 80 | 20 |
| 65.1–75 | 15 | 85 |
| Fraction | Total Phenolics | Total Flavonoids |
|---|---|---|
| (mg GAE/100 g) | (mg QE/100 g) | |
| Free | 509 ± | 535 ± |
| Esterified | 270 ± | 116 ± |
| Etherified | 75 ± | 49 ± |
| Insoluble-bound | 830 ± | 656 ± |
| Total | 1684 | 1356 |
| Compound | Free | Esterified | Etherified | Insoluble |
|---|---|---|---|---|
| (mg/100 g) | (mg/100 g) | (mg/100 g) | (mg/100 g) | |
| Gallic acid | 9.95 ± | 17.05 ± | 5.73 ± | 72.38 ± |
| p-Coumaric acid | nd | 21.20 ± | nd | 34.91 ± |
| Catechin | 11.82 ± | 5.17 ± | 0.25 ± | 26.65 ± |
| Epicatechin | 40.75 ± | tr | 0.33 ± | 8.00 ± |
| Epigallocatechin gallate | 0.21 ± 0.01 | nd | nd | nd |
| Procyanidin B2 | nd | 1.16 ± | nd | 7.19 ± |
| Kaempferol | 2.16 ± 0.07 | nd | nd | nd |
| CD | HFCD-CD | HFCD-ST | HFCD-RWGP | |
|---|---|---|---|---|
| Dietary intake (g/day) | 4.30 (3.60–4.70 | 3.70 (3.30–4.30 | 3.85 (3.60–5.20 | 4.60 (3.60–5.30 |
| Fat intake (g/day) | 0.24 (0.20–0.26 | 0.54 (0.48–0.62 | 0.56 (0.52–0.75 | 0.69 (0.54–0.80 |
| Fiber intake (g/day) | 0.66 (0.55–0.72 | 0.56 (0.50–0.65 | 0.58 (0.54–0.78 | 1.00 (0.80–1.20 |
| Water intake (mL/day) | 5.70 (5.40–6.20 | 6.4 (5.10–9.90 | 5.50 (4.80–6.20 | 4.20 (3.90–8.70 |
| Starting body weight (g) | 28.2 (25.3–29.9 | 27.1 (26.4–30.1 | 27.1 (25.3–27.7 | 26.60 (25.6–27.7 |
| Final body weight (g) | 30.1 (28.7–31.3 | 23.80 (22.8–25.0 | 27.2 (26.5–28.1 | 27.2 (25.6–29.2 |
| Body weight gain (g) | 1.9 (0.2–3.4 | −3.9 (−5.1– 0.0 | 0.1 (−1.2–2.8 | 1.3 (−2.1–2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, K.; González, L.; Parra, L.; Oyarzún, J.E.; Concepción-Alvarez, A.; de Camargo, A.C.; Bridi, R.; Rigotti, A.; Andia, M.E. Red Wine Grape Pomace Restores Gut Barrier Function and Improves Survival in Diet-Induced Ischemic Heart Disease. Antioxidants 2025, 14, 574. https://doi.org/10.3390/antiox14050574
Rivera K, González L, Parra L, Oyarzún JE, Concepción-Alvarez A, de Camargo AC, Bridi R, Rigotti A, Andia ME. Red Wine Grape Pomace Restores Gut Barrier Function and Improves Survival in Diet-Induced Ischemic Heart Disease. Antioxidants. 2025; 14(5):574. https://doi.org/10.3390/antiox14050574
Chicago/Turabian StyleRivera, Katherine, Leticia González, Laura Parra, Juan E. Oyarzún, Alina Concepción-Alvarez, Adriano Costa de Camargo, Raquel Bridi, Attilio Rigotti, and Marcelo E. Andia. 2025. "Red Wine Grape Pomace Restores Gut Barrier Function and Improves Survival in Diet-Induced Ischemic Heart Disease" Antioxidants 14, no. 5: 574. https://doi.org/10.3390/antiox14050574
APA StyleRivera, K., González, L., Parra, L., Oyarzún, J. E., Concepción-Alvarez, A., de Camargo, A. C., Bridi, R., Rigotti, A., & Andia, M. E. (2025). Red Wine Grape Pomace Restores Gut Barrier Function and Improves Survival in Diet-Induced Ischemic Heart Disease. Antioxidants, 14(5), 574. https://doi.org/10.3390/antiox14050574









