Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical and Reagents
2.3. Enzyme Preparations
2.4. Chemical Analysis of Raw Material
2.5. Enzyme-Assisted Extraction
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Antioxidant Activity
2.9. Antibacterial Activity
2.10. Anti-Aging Activity (Tyrosinase Inhibition)
2.11. Anti-Diabetic Activity
2.11.1. α-Glucosidase Inhibition
2.11.2. α-Amylase Inhibition
2.12. Kinetic Modeling
2.12.1. First-Order Model
2.12.2. Second-Order Model
2.12.3. Peleg’s Model
2.12.4. Power Law Model
2.13. Statistical Analysis
3. Results
3.1. Composition of the Hypanthium of Pseudo-Fruit of Rosa canina L.
3.2. Total Phenolic Content (TPC)
| Extraction Method | Solvent System | Extraction Time (h) | SLR (%, w/v) | Temperature (°C) | TPC (mg GAE/g DM) | TFC (mg/g DM) | References |
|---|---|---|---|---|---|---|---|
| Maceration | EtOH:H2O (1:1, v/v) | 1 | 5 | 20 | 69.40 | - | [46] |
| EtOH:H2O (1:1, v/v) | 20 | 10 | 25 | 0.22 | - | [10] | |
| Boiling water | 1 | 10 | 25 | 61.00 | 1.14 ** | [12] | |
| EtOH:H2O (80:20, v/v) | 72 | 10 | 25 | 50.30 | 0.63 ** | [12] | |
| Methanol | 0.03 | 12 | 4 | 16.61 | - | [11] | |
| Methanol | 48 | 50 | - | 290 | 118 * | [48] | |
| MeOH:H2O (60:40, v/v) | - | - | 5 | 2.15 | 0.02 * | [51] | |
| MeOH:H2O (80:20, v/v) | 0.33 | 25 | 25 | 177 | 4.85 ** | [52] | |
| MeOH:H2O (1:1, v/v) | 2 | 10 | 25 | 48.7 | 6.7 | [53] | |
| MeOH:H2O:HCOOH (50:48.5:1.5, v/v/v) | - | 2 | 4 | 31.08 | - | [54] | |
| n-hexane:acetone:EtOH (2:1:1, v/v/v) plus 50 mg/L butylated hydroxytoluene | 0.25 | - | 5 | 2.98 | 1.45 * | [55] | |
| EtOH:H2O (40:60, v/v) | 0.83 | 50 | 25 | 21.61 | 1.8 *** | [47] | |
| UAE | EtOH:H2O (40:60, v/v) | 1.35 | - | 25 | 47.23 | - | [13] |
| EtOH:H2O (70:30, v/v) | 0.50 | - | 50 | 24.00 | 1.4 ** | [50] | |
| UAEE | 0.6 U/mL pectinase, 0.3 U/mL cellulase, 0.3 U/mL hemicellulase (2:1:1, v/v/v) in phosphate buffer pH 5.6 | 0.83 | 5 | 50 | 32.64 | - | [16] |
| PEF | Distilled water | 0.33 | 5 | 24 | 70 | - | [15] |
| MHG | Distilled water | 15 min pretreatment before MHG | - | 8 | 200 | 190 * | [14] |
| SFE | Supercritical CO2 | 0.5 | - | 25 | 5.12 | 1.95 * | [17] |
| EAE | 1.0% v/v Cellic Ctec3 | 6 | 6 | 50 | 168.3 | 72.3 * | Present study |
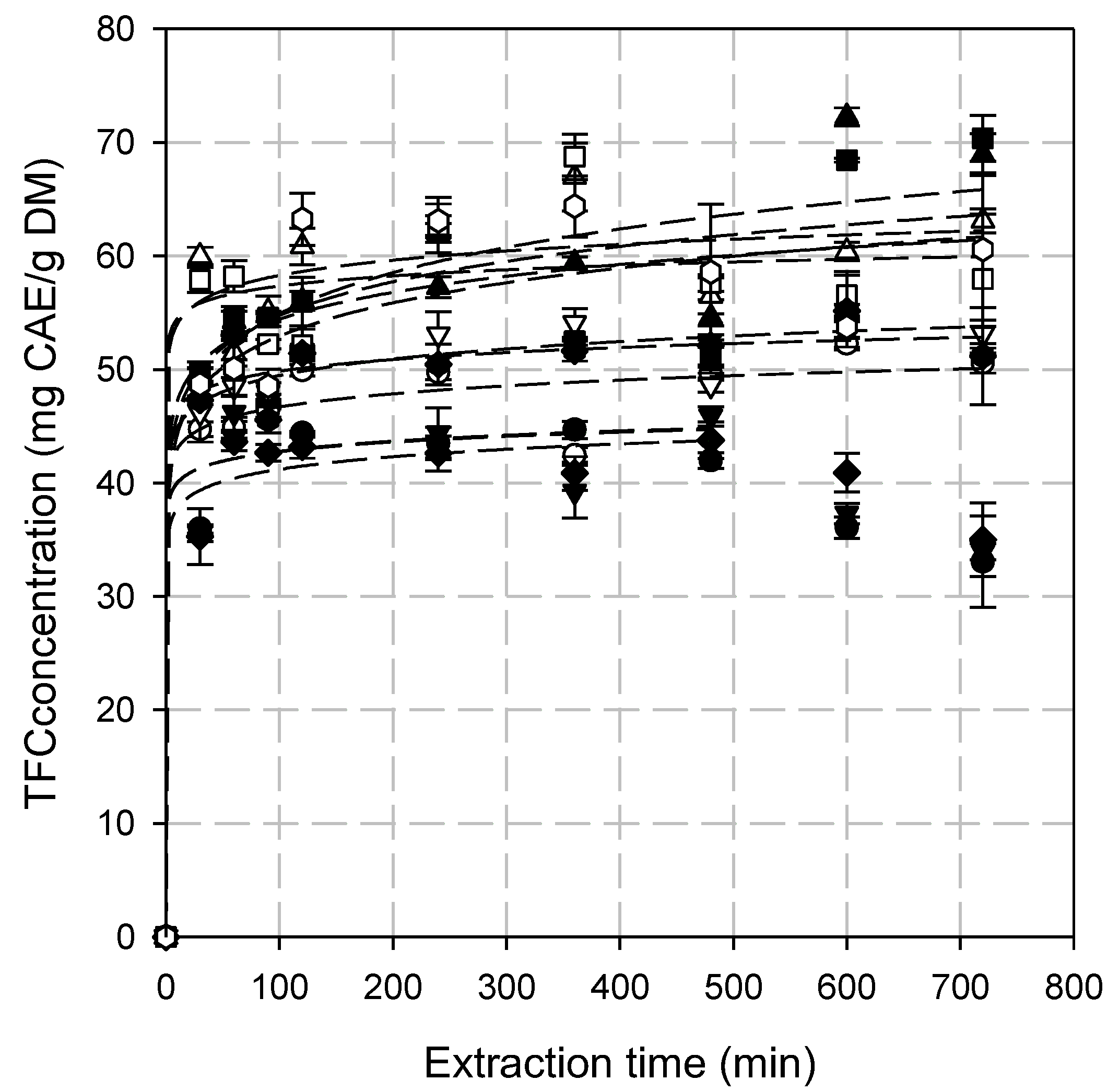
3.3. Total Flavonoid Content (TFC)
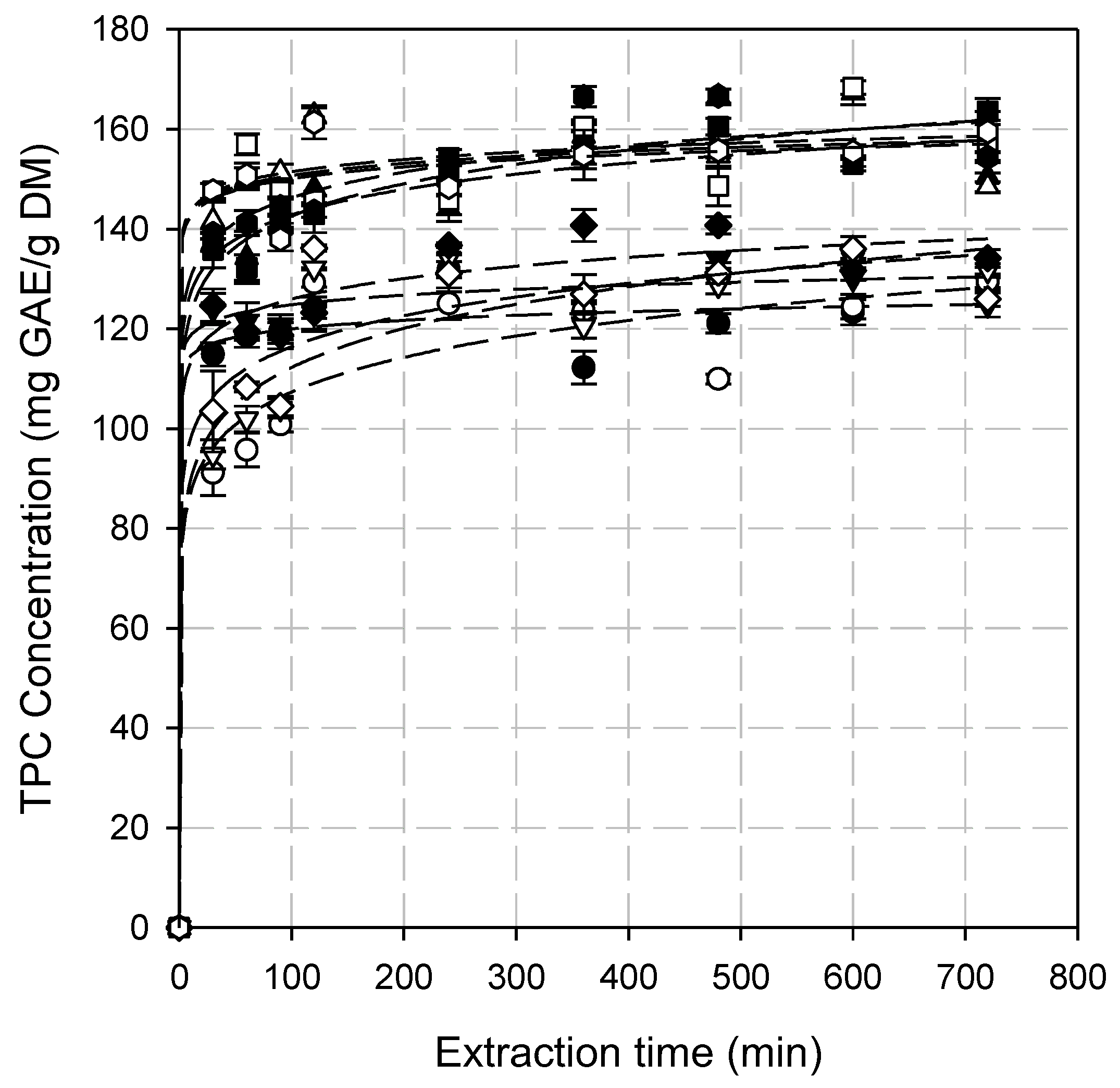
3.4. Kinetic Modeling of the Extraction Process
3.5. Biological Activities
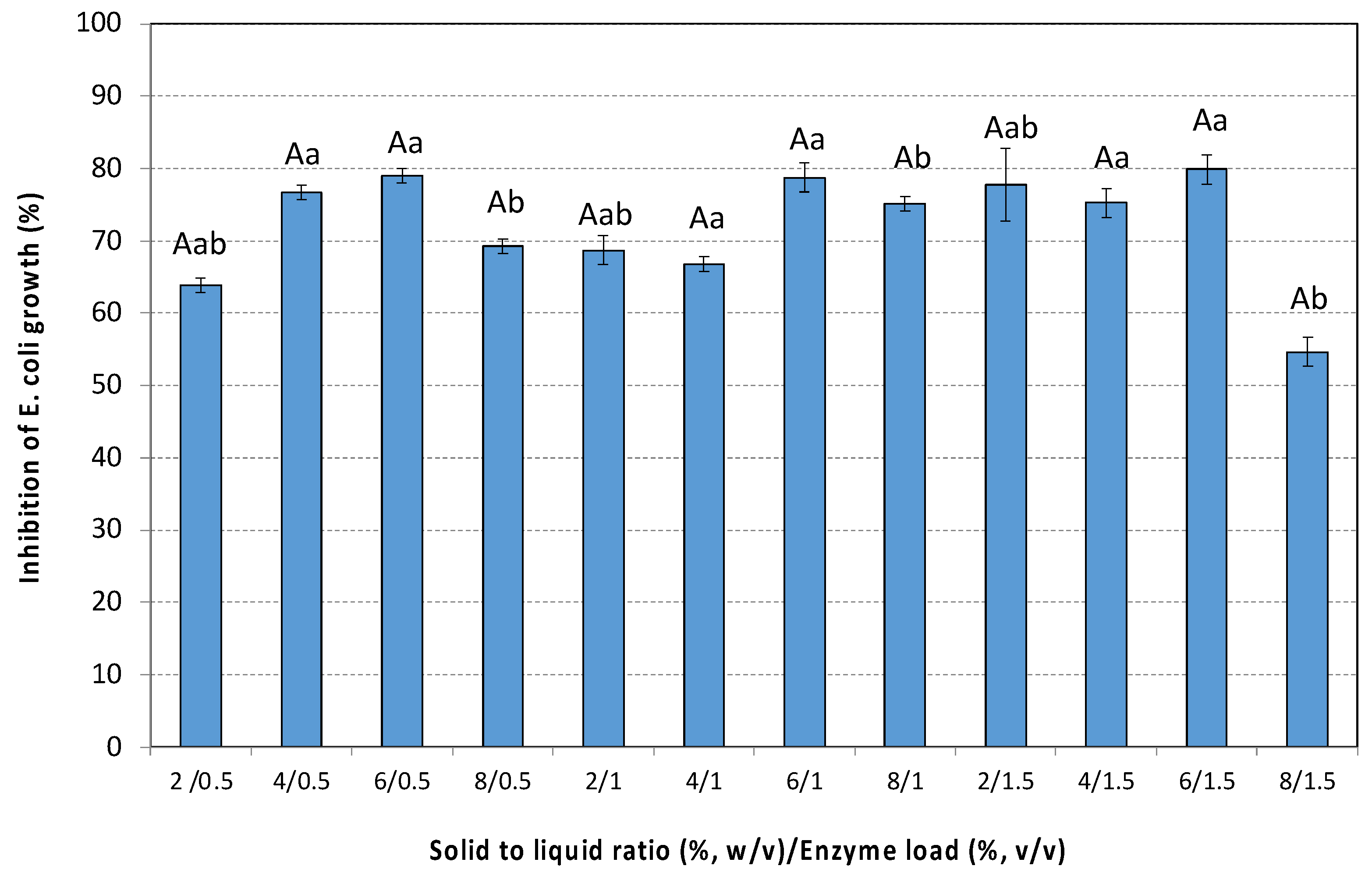
3.5.1. Antibacterial Activity
3.5.2. Antioxidant Activity
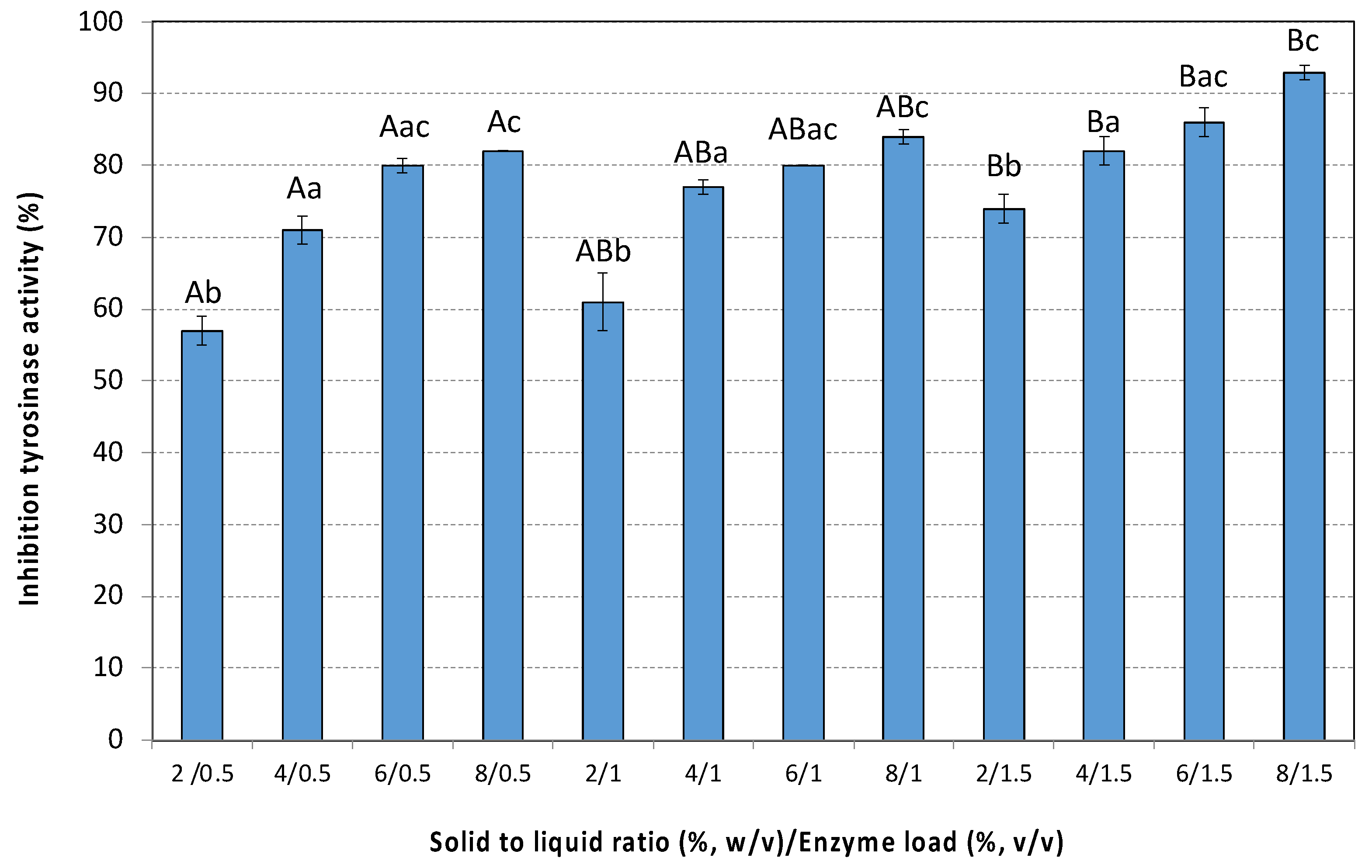
3.5.3. Anti-Aging Activity (Tyrosinase Inhibition)
3.5.4. Anti-Diabetic Activity (α-Amylase and α-Glucosidase Inhibition)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EAE | Enzyme-assisted extraction |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| GAE | Gallic Acid Equivalents |
| DM | Dry Matter |
| CAE | Catechin Equivalents |
| UAE | Ultrasound-Assisted Extraction |
| MAE | Microwave-Assisted Extraction |
| NADES | Natural Deep Eutectic Solvent |
| PEF | Pulsed Electric Field Extraction |
| MHG | Microwave Hydrodiffusion and Gravity |
| UAEE | Ultrasound-Assisted Enzymatic Extraction |
| SFE | Supercritical carbon dioxide extraction |
| SLR | Solid to liquid ratio |
| MIC | Minimum Inhibitory Concentration |
| ANOVA | Analysis of Variance |
| SD | Standard Deviation |
| NRMSD | Normalized Root Mean Square Deviation |
References
- Winther, K.; Campbell-Tofte, J.; Vinther Hansen, A.S. Bioactive Ingredients of Rose Hips (Rosa canina L.) with Special Reference to Antioxidative and Anti-Inflammatory Properties: In Vitro Studies. Botanics 2016, 6, 11. [Google Scholar] [CrossRef]
- Negrean, O.R.; Farcas, A.C.; Nemes, S.A.; Cic, D.E.; Socaci, S.A. Recent Advances and Insights into the Bioactive Properties and Applications of Rosa canina L. and Its by-products. Heliyon 2024, 10, e30816. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural Bioactive Products as Promising Therapeutics: A Review of Natural Product-Based Drug Development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Suastika, I.B.K.; Mahayu, W.M.; Wani, A.K. Enhancing Secondary Metabolite Production in Plants: Exploring Traditional and Modern Strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial Use of Cell Wall Degrading Enzymes: The Fine Line Between Production Strategy and Economic Feasibility. Front. Bioeng. Biotechnol. 2020, 8, 356. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Biologically Active Macromolecules: Extraction Strategies, Therapeutic Potential and Biomedical Perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.D.C.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.D.L.Á.; Silva, M.F.; Dias, F.D.S. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Zamaraeva, M.; Skłodowska, M.; Bryszewska, M.; et al. Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts. Agronomy 2020, 10, 1897. [Google Scholar] [CrossRef]
- Kayahan, S.; Ozdemir, Y.; Gulbag, F. Functional Compounds and Antioxidant Activity of Rosa Species Grown in Turkey. Erwerbs-Obstbau 2023, 65, 1079–1086. [Google Scholar] [CrossRef]
- Nadpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative Study of Biological Activities and Phytochemical Composition of Two Rose Hips and Their Preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Ilbay, Z.; Şahin, S.; Kirbaşlar, Ş.I. Optimisation of Ultrasound-Assisted Extraction of Rosehip (Rosa canina L.) with Response Surface Methodology. J. Sci. Food Agric. 2013, 93, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, E.; Caprioli, G.; Simonelli, G.; Mustafa, A.M.; Maggi, F.; Cespi, M. Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.). Foods 2023, 12, 3051. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Stavropoulos, G.; Samanidis, I.; Athanasiadis, V.; Dourtoglou, V.G.; Makris, D.P.; Lalas, S.I. Enhancement of Polyphenols Recovery from Rosa canina, Calendula Officinalis and Castanea Sativa Using Pulsed Electric Field. Beverages 2021, 7, 63. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized Ultrasound-Assisted Enzymatic Extraction of Phenolic Compounds from Rosa canina L. Pseudo-Fruits (Rosehip) and Their Biological Activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef]
- Mihalcea, L.; Păcularu-Burada, B.; Milea, S.A.; Aprodu, I.; Condurache Lazăr, N.N.; Cucolea, E.I.; Dănilă, G.M.; Cîrciumaru, A.; Nicoleta, S. CO2 Supercritical Extraction and Microencapsulation of Oleoresins from Rosehip Fruits for Getting Powders with Multiple Applications. Curr. Res. Food Sci. 2023, 6, 100449. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Int. Food Res. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Kumari Dubey, K.; Singhal, R.S. Improvements in the Extraction of Bioactive Compounds by Enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC Inc.: Rockville, MD, USA, 1990. [Google Scholar]
- Phatak, L.; Chang, K.C.; Brown, G. Isolation and Characterization of Pectin in Sugar-Beet Pulp. J. Food Sci. 1988, 53, 830–833. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent6s, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Con-centration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among Extracts of Seashore Plants and Identification of Potent Inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Swapna, M.; Ayesha, S.B.; Zehra, A.; Agawane, S.B.; Madhusudana, K. Identification of Proglycemic and Antihyperglycemic Activity in Antioxidant Rich Fraction of Some Common Food Grains. Int. Food Res. J. 2011, 18, 915–923. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum myrtifolium Boiss. Endemic to Turkey and Its Inhibitory Potential against Key Enzymes Involved in Alzheimer, Skin Diseases and Type II Diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.; Ho, Y.S. Comparison of Kinetic Models for the Aqueous Solid-Liquid Extraction of Tilia Sapwood a Continuous Stirred Tank Reactor. Chem. Eng. Commun. 2007, 194, 537–552. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Kinetics Studies on Effects of Extraction Techniques on Bioactive Compounds from Vernonia Cinerea Leaf. J. Food Sci. Technol. 2019, 56, 580–588. [Google Scholar] [CrossRef]
- Da Silva, L.A.; da Scapim, M.R.S.; da Silva, J.F.; Stafussa, A.P.; Aranha, A.C.R.; Jorge, L.M. de M.; Defendi, R.O.; Júnior, O. de O.S.; Madrona, G.S. Modeling the Extraction of Bioactive Compounds of Green and Red Camu-Camu Peel and Identification Using UPLC-MS/MS. Chem. Eng. Res. Des. 2023, 196, 1–12. [Google Scholar] [CrossRef]
- Ognyanov, M.; Denev, P.; Teneva, D.; Georgiev, Y.; Taneva, S.; Totseva, I.; Kamenova-Nacheva, M.; Nikolova, Y.; Mom-chilova, S. Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits. Molecules 2022, 27, 1765. [Google Scholar] [CrossRef]
- Ognyanov, M.H.; Hodzhova, M.M.; Petkova, N.T.; Denev, P.N.; Georgiev, Y.N.; Kratchanova, M.G. Isolation and Characterization of Plant Cell Wall Material from Rose Hip Fruits. Bulg. Chem. Commun. 2018, 50, 530. [Google Scholar]
- Fan, C.; Pacier, C.; Martirosyan, D.M.; Martirosyan, D. Rose Hip (Rosa canina L.): A Functional Food Perspective. Funct. Food Health Dis. 2014, 4, 493–509. [Google Scholar] [CrossRef]
- Kizil, S.; Toncer, O.; Sogut, T. Mineral Contents and Fatty Acid Compositions of Wild and Cultivated Rose Hip (Rosa canina L.). Fresenius Environ. Bull. 2017, 27, 744–748. [Google Scholar]
- Demir, F.; Özcan, M. Chemical and Technological Properties of Rose (Rosa canina L.) Fruits Grown Wild in Turkey. J. Food Eng. 2001, 47, 333–336. [Google Scholar] [CrossRef]
- Ilić, N.; Milić, M.; Beluhan, S.; Dimitrijević-Branković, S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies 2023, 16, 3598. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C. Kinetic Models for Conventional and Ultrasound Assistant Extraction of Polyphenols from Defatted Fresh and Distilled Grape Marc and Its Main Components Skins and Seeds. Chem. Eng. Res. Des. 2020, 156, 1–12. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á.D.; García-Martinez, J.B.; L’Abbate, P. Optimization of Enzyme-Assisted Extraction of Flavonoids from Corn Husks. Processes 2019, 7, 804. [Google Scholar] [CrossRef]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of Rose Hip (Rosa canina L.) Fruits Extracts and Evaluation of Their In Vitro Antioxidant Activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Liaudanskas, M.; Noreikienė, I.; Zymonė, K.; Juodytė, R.; Žvikas, V.; Janulis, V. Composition and Antioxidant Activity of Phenolic Compounds in Fruit of the Genus Rosa L. Antioxidants 2021, 10, 545. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in Human Endothelial Cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food. Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Moldovan, C.; Babota, M.; Mocan, A.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.; Vodnar, D.C.; Dias, M.I.; Pereira, C.; et al. Optimization of the Drying Process of Autumn Fruits Rich in Antioxidants: A Study Focusing on Rosehip (Rosa canina L.) and Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) and Their Bioactive Properties. Food Funct. 2021, 12, 3939–3953. [Google Scholar] [CrossRef]
- Maloupa, E.; Karapatzak, E.; Ganopoulos, I.; Karydas, A.; Papanastasi, K.; Kyrkas, D.; Yfanti, P.; Nikisianis, N.; Zahari-adis, A.; Kosma, I.S.; et al. Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation. Plants 2021, 10, 2634. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Kaszuba, M.; Konopacka, A.; Marzec-Wróblewska, U.; Wesolowski, M.; Waleron, K.; Buciński, A.; Viapiana, A. Phenolic Composition and Biological Properties of Wild and Commercial Dog Rose Fruits and Leaves. Molecules 2020, 25, 5272. [Google Scholar] [CrossRef]
- Goztepe, B.; Kayacan, S.; Bozkurt, F.; Tomas, M.; Sagdic, O.; Karasu, S. Drying Kinetics, Total Bioactive Compounds, Antioxidant Activity, Phenolic Profile, Lycopene and β-Carotene Content and Color Quality of Rosehip Dehydrated by Different Methods. LWT 2022, 153, 112476. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of Volatiles, Phenolic Compounds and Antioxidant Activities of Rose Hip (Rosa L.) Fruits in Turkey. LWT 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R.; Ercisli, S.; Karatas, N.; Ekiert, H.; Elansary, H.O.; Szopa, A. Morphological and Biochemical Diversity in Fruits of Unsprayed Rosa canina and Rosa dumalis Ecotypes Found in Different Agroecological Conditions. Sustainability 2021, 13, 8060. [Google Scholar] [CrossRef]
- Diaconeasa, Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their in Vitro Antiproliferative Effects against Melanoma. Molecules 2018, 23, 2534. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of Extraction Methods on Stability of Flavonoids. J. Chromatogr. A. 2011, 1218, 2505–2512. [Google Scholar] [CrossRef]
- Liu, R.; Chu, X.; Su, J.; Fu, X.; Kan, Q.; Wang, X.; Zhang, X. Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax Senticosus and Their Enrichment and Antioxidant Properties. Processes 2021, 9, 1708. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Papakosta-Tsigkri, L.; Lymperopoulou, T.; Mamma, D.; Kekos, D.; Detsi, A. Conventional and En-zyme-Assisted Extraction of Rosemary Leaves (Rosmarinus officinalis L.): Toward a Greener Approach to High Add-ed-Value Extracts. Appl. Sci. 2021, 11, 3724. [Google Scholar] [CrossRef]
- Milićević, N.; Kojić, P.; Sakač, M.; Mišan, A.; Kojić, J.; Perussello, C.; Banjac, V.; Pojić, M.; Tiwari, B. Kinetic Modelling of Ultrasound-Assisted Extraction of Phenolics from Cereal Brans. Ultrason. Sonochem. 2021, 79, 105761. [Google Scholar] [CrossRef]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction Kinetics of Total Poly-phenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Taweekayujan, S.; Somngam, S.; Pinnarat, T. Optimization and Kinetics Modeling of Phenolics Extraction from Coffee Silverskin in Deep Eutectic Solvent Using Ultrasound-Assisted Extraction. Heliyon 2023, 9, e17942. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Guo, Z.; Yu, G. Process Intensification and Kinetic Studies of Ultrasound-Assisted Extraction of Flavonoids from Peanut Shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Krasniewska, K.; Przybył, J.L.; Zielinska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [PubMed]
- Profillidis, V.A.; Botzoris, G.N. Statistical Methods for Transport Demand Modeling. In Modeling of Transport Demand; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–224. [Google Scholar]
- Ispiryan, A.; Atkociuniene, V.; Makstutiene, N.; Sarkinas, A.; Salaseviciene, A.; Urbonaviciene, D.; Viskelis, J.; Pakeltiene, R.; Raudone, L. Correlation between Antimicrobial Activity Values and Total Phenolic Content/Antioxidant Activity in Rubus idaeus L. Plants 2024, 13, 504. [Google Scholar] [CrossRef]
- Montazeri, N.; Baher, E.; Mirzajani, F.; Barami, Z.; Yousefian, S. Phytochemical Contents and Biological Activities of Rosa canina Fruit from Iran. J. Med. Plant Res. 2011, 5, 4584–4589. [Google Scholar]
- Oyedemi, S.O.; Oyedemi, B.O.; Prieto, J.M.; Coopoosamy, R.M.; Stapleton, P.; Gibbons, S. In Vitro Assessment of Antibi-otic-Resistance Reversal of a Methanol Extract from Rosa canina L. S. Afr. J. 2016, 105, 337–342. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, IPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Cock, I.E. Rosa canina L. Fruit Extracts Inhibit the Growth of Bacterial Triggers of Some Autoimmune Inflammatory Diseases and Potentiate the Activity of Conventional Antibiotics. Phcog Commn. 2019, 10, 7–17. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Syn-thetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Kim, H.D.; Choi, H.; Abekura, F.; Park, J.Y.; Yang, W.S.; Yang, S.H.; Kim, C.H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, M. Inhibitory Effect of Quercetin Isolated from Rose Hip (Rosa canina L.) against Melanogenesis by Mouse Melanoma Cells. Biosci. Biotechnol. Biochem. 2009, 73, 1989–1993. [Google Scholar] [CrossRef]
- Phetcharat, L.; Wongsuphasawat, K.; Winther, K. The Effectiveness of a Standardized Rose Hip Powder, Containing Seeds and Shells of Rosa canina, on Cell Longevity, Skin Wrinkles, Moisture, and Elasticity. Clin. Interv. Aging 2015, 10, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.I.; Savic, V.L.J.; Zivkovic, J.V.; Stanojevic, L.P.; Tadic, V.M.; Arsic, I.A. Tyrosinase Inhibitory and Antioxidant Activity of Wild Rosa canina L. and Sorbus aucuparia L. Fruit Extracts. Acta Pol. Pharm. 2019, 76, 523–533. [Google Scholar] [CrossRef]
- Azwanida, Z.N.; Jonathan, O.E.; Melanie-Jaynes, H. Antioxidant, Anti-Collagenase, Anti-Elastase and Anti-Tyrosinase Activities of an Aqueous Cosmos Caudatus kunth (Asteraceae) Leaf Extract. Trop. J. Nat. Prod. Res. 2020, 4, 1124–1130. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Zhong, Y.; Ni, D.; Yang, Y.; Li, Y.; Wang, L.; Tian, J.; Yang, F.; Ye, X. Inhibitory Effect and Mechanism of Tannic Acid against Two Starch Digestive Enzymes. Food Qual. Saf. 2024, 8, fyad057. [Google Scholar] [CrossRef]
- Fattahi, A.; Niyazi, F.; Shahbazi, B.; Farzaei, M.H.; Bahrami, G. Antidiabetic Mechanisms of Rosa canina Fruits: An In Vitro Evaluation. J. Evid. Based Complement. Altern. Med. 2017, 22, 127–133. [Google Scholar] [CrossRef]
- Jemaa, H.B.; Jemia, A.B.; Khlifi, S.; Ahmed, H.B.; Slama, F.B.; Benzarti, A.; Elati, J.; Aouidet, A. Antioxidant Activity and a-Amylase Inhibitory Potential of Rosa canina L. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Vasić, D.; Katanić Stanković, J.S.; Urošević, T.; Kozarski, M.; Naumovski, N.; Khan, H.; Popović-Djordjević, J. Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.). Beverages 2024, 10, 1. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Hoşbaş, S.; Deliorman Orhan, D. Antidiabetic Effect and Antioxidant Potential of Rosa canina Fruits. Pharmacogn. Mag. 2009, 5, 309–315. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Rashidi, A.A.; Taherian, A.A.; Vakili, Z.; Mehran, M. The Protective Effect of Hydroalcoholic Extract of Rosa canina (Dog Rose) Fruit on Liver Function and Structure in Streptozotocin-Induced Diabetes in Rats. J. Diet. Suppl. 2018, 15, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Salehi, P.; Moridi Farimani, M.; Nejad Ebrahimi, S. α-Glucosidase Inhibitors from Fruits of Rosa canina L. Rec. Nat. Prod. 2015, 9, 276. [Google Scholar]
- Haguet, Q.; Le Joubioux, F.; Chavanelle, V.; Groult, H.; Schoonjans, N.; Langhi, C.; Michaux, A.; Otero, Y.F.; Boisseau, N.; Peltier, S.L.; et al. Inhibitory Potential of α-Amylase, α-Glucosidase, and Pancreatic Lipase by a Formulation of Five Plant Extracts: TOTUM-63. Int. J. Mol. Sci. 2023, 24, 3652. [Google Scholar] [CrossRef]




| Component | Concentration (% w/w, Dry Basis) |
|---|---|
| Water-soluble compounds * | 40.3 ± 1.2 |
| Crude fat | 5.4 ± 0.1 |
| Pectin | 11.8 ± 1.9 |
| Cellulose | 9.9 ± 0.7 |
| Hemicellulose | 10.9 ± 0.3 |
| Crude protein | 7.5 ± 0.2 |
| Lignin | 9.5 ± 4.4 |
| Ash | 2.1 ± 0.2 |
| Solid to Liquid Ratio (%, w/v)/Enzyme Loading (%, v/v) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | 2/0.5 | 4/0.5 | 6/0.5 | 8/0.5 | 2/1.0 | 4/1.0 | 6/1.0 | 8/1.0 | 2/1.5 | 4/1.5 | 6/1.5 | 8/1.5 | ||
| First order | Constants | Ceq | 122.6 | 121.1 | 149.8 | 155.7 | 127.1 | 127.6 | 151.7 | 153.8 | 130.9 | 127.4 | 153.5 | 153.1 |
| k | 9.0 × 10−2 | 3.6 × 10−2 | 7.5 × 10−2 | 7.8 × 10−2 | 12 × 10−2 | 3.5 × 10−2 | 6.6 × 10−2 | 11 × 10−2 | 9.5 × 10−2 | 4.5 × 10−2 | 7.3 × 10−2 | 11 × 10−2 | ||
| Statistical Parameters | R2 | 0.981 | 0.940 | 0.968 | 0.986 | 0.985 | 0.949 | 0.965 | 0.978 | 0.964 | 0.946 | 0.970 | 0.982 | |
| R2-adj | 0.978 | 0.933 | 0.964 | 0.984 | 0.983 | 0.943 | 0.961 | 0.976 | 0.960 | 0.939 | 0.967 | 0.980 | ||
| NRMSD (%) | 4.45 | 8.28 | 5.79 | 3.86 | 3.95 | 7.59 | 6.06 | 4.71 | 6.11 | 7.71 | 5.57 | 4.27 | ||
| Power law | Constants | B | 109.4 | 70.8 | 113.2 | 136.2 | 112.8 | 71.8 | 106.2 | 135,8 | 101.8 | 82.6 | 116.2 | 135.2 |
| n | 2.0 × 10−2 | 9.0 × 10−2 | 5.1 × 10−2 | 2.3 × 10−2 | 2.2 × 10−2 | 9.7 × 10−2 | 6.4 × 10−2 | 2.3 × 10−2 | 4.6 × 10−2 | 7.5 × 10−2 | 5.0 × 10−2 | 2.3 × 10−2 | ||
| Statistical Parameters | R2 | 0.981 | 0.942 | 0.986 | 0.983 | 0.989 | 0.958 | 0.994 | 0.982 | 0.984 | 0.959 | 0.987 | 0.986 | |
| R2-adj | 0.979 | 0.935 | 0.985 | 0.981 | 0.988 | 0.953 | 0.993 | 0.980 | 0.982 | 0.954 | 0.985 | 0.985 | ||
| NRMSD (%) | 4.41 | 8.21 | 3.76 | 4.17 | 3.30 | 6.95 | 2.55 | 4.25 | 4.09 | 6.72 | 4.72 | 3.71 | ||
| Peleg’s | Constants | K1 | 2.0 × 10−2 | 1.1 × 10−2 | 3.6 × 10−2 | 2.1 × 10−2 | 3.6 × 10−2 | 1.1 × 10−2 | 4.6 × 10−2 | 1.2 × 10−2 | 3.5 × 10−2 | 7.9 × 10−2 | 3.6 × 10−2 | 1.4 × 10−2 |
| K2 | 8.0 × 10−3 | 7.9 × 10−3 | 6.5 × 10−3 | 6.3 × 10−3 | 6.5 × 10−3 | 7.5 × 10−3 | 6.3 × 10−3 | 6.4 × 10−3 | 7.4 × 10−3 | 7.5 × 10−3 | 6.3 × 10−3 | 6.4 × 10−3 | ||
| Ceq | 125.0 | 126.6 | 153.8 | 158.7 | 153.8 | 133.3 | 158.7 | 156.3 | 135.1 | 133.3 | 158.7 | 156.3 | ||
| Statistical Parameters | R2 | 0.982 | 0.954 | 0.981 | 0.986 | 0.981 | 0.968 | 0.986 | 0.980 | 0.977 | 0.965 | 0.983 | 0.985 | |
| R2-adj | 0.980 | 0.948 | 0.979 | 0.984 | 0.979 | 0.964 | 0.984 | 0.977 | 0.974 | 0.960 | 0.981 | 0.983 | ||
| NRMSD (%) | 4.31 | 7.30 | 4.40 | 5.61 | 17.48 | 6.07 | 3.86 | 4.56 | 4.86 | 6.22 | 4.18 | 3.95 | ||
| Second order | Constants | Ce | 124.3 | 126.9 | 154.9 | 158.2 | 129.2 | 134.2 | 158.3 | 158.2 | 135.2 | 133.2 | 158.7 | 155.2 |
| k | 3.2 × 10−3 | 5.8 × 10−4 | 1.2 × 10−3 | 2.0 × 10−3 | 3.5 × 10−3 | 5.1 × 10−4 | 8.6 × 10−4 | 2.0 × 10−3 | 1.6 × 10−3 | 7.2 × 10−4 | 1.1 × 10−3 | 3.1 × 10−3 | ||
| Statistical Parameters | R2 | 0.982 | 0.954 | 0.981 | 0.986 | 0.982 | 0.968 | 0.968 | 0.986 | 0.977 | 0.965 | 0.983 | 0.985 | |
| R2-adj | 0.980 | 0.948 | 0.979 | 0.984 | 0.980 | 0.964 | 0.964 | 0.984 | 0.974 | 0.960 | 0.981 | 0.983 | ||
| NRMSD (%) | 4.30 | 7.22 | 4.35 | 3.81 | 3.47 | 6.00 | 3.77 | 4.76 | 4.82 | 6.17 | 4.15 | 3.95 | ||
| Solid to Liquid Ratio (%, w/v)/Enzyme Loading (%, v/v) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | 2/0.5 | 4/0.5 | 6/0.5 | 8/0.5 | 2/1.0 | 4/1.0 | 6/1.0 | 8/1.0 | 2/1.5 | 4/1.5 | 6/1.5 | 8/1.5 | ||
| First order | Constants | Ceq | 44.6 | 48.4 | 60.4 | 60.9 | 44.5 | 51.7 | 59.2 | 58.3 | 42.9 | 51.3 | 57.6 | 58.7 |
| k | 5.8 × 10−2 | 8.2 × 10−2 | 5.1 × 10−2 | 6.2 × 10−2 | 5.9 × 10−2 | 7.0 × 10−2 | 5.6 × 10−2 | 1.7 × 10−1 | 6.0 × 10−2 | 8.6 × 10−2 | 5.2 × 10−2 | 4.8 × 10−2 | ||
| Statistical Parameters | R2 | 0.989 | 0.965 | 0.911 | 0.972 | 0.971 | 0.980 | 0.891 | 0.937 | 0.994 | 0.978 | 0.952 | 0.928 | |
| R2-adj | 0.987 | 0.961 | 0.900 | 0.969 | 0.967 | 0.977 | 0.878 | 0.929 | 0.993 | 0.975 | 0.946 | 0.919 | ||
| NRMSD (%) | 3.80 | 6.04 | 10.08 | 8.38 | 6.17 | 4.61 | 11.19 | 8.22 | 2.73 | 4.77 | 7.36 | 8.94 | ||
| Power | Constants | B | 37.4 | 39.7 | 35.9 | 50.1 | 37.5 | 40.7 | 38.6 | 51.8 | 34.6 | 43.6 | 37.1 | 41.1 |
| n | 2.9 × 10−2 | 3.6 × 10−2 | 9.2 × 10−2 | 3.3 × 10−2 | 2.8 × 10−2 | 4.2 × 10−2 | 7.6 × 10−2 | 2.2 × 10−1 | 3.8 × 10−2 | 2.9 × 10−2 | 7.7 × 10−2 | 6.1 × 10−2 | ||
| Statistical Parameters | R2 | 0.962 | 0.972 | 0.955 | 0.961 | 0.943 | 0.986 | 0.919 | 0.942 | 0.976 | 0.981 | 0.975 | 0.932 | |
| R2-adj | 0.955 | 0.969 | 0.949 | 0.956 | 0.933 | 0.985 | 0.909 | 0.935 | 0.972 | 0.979 | 0.971 | 0.923 | ||
| NRMSD (%) | 7.08 | 5.39 | 7.16 | 6.44 | 8.72 | 3.77 | 9.64 | 7.88 | 5.61 | 4.36 | 5.94 | 8.77 | ||
| Peleg’s | Constants | K1 | 1.1 × 10−1 | 7.8 × 10−2 | 1.7 × 10−1 | 4.8 × 10−2 | 1.1 × 10−1 | 9.7 × 10−2 | 1.4 × 10−1 | 3.1 × 10−2 | 1.2 × 10−1 | 5.9 × 10−2 | 1.5 × 10−1 | 1.5 × 10−1 |
| K2 | 2.2 × 10−2 | 2.0 × 10−2 | 1.6 × 10−2 | 1.6 × 10−2 | 2.2 × 10−2 | 1.9 × 10−2 | 1.6 × 10−2 | 1.7 × 10−2 | 2.3 × 10−2 | 1.9 × 10−2 | 1.7 × 10−2 | 1.6 × 10−2 | ||
| Ceq | 45.7 | 49.5 | 63.7 | 61.0 | 45.5 | 53.2 | 61.7 | 59.2 | 44.1 | 52.4 | 60.2 | 61.0 | ||
| Statistical Parameters | R2 | 0.974 | 0.970 | 0.935 | 0.956 | 0.955 | 0.987 | 0.907 | 0.939 | 0.985 | 0.980 | 0.966 | 0.943 | |
| R2-adj | 0.970 | 0.967 | 0.927 | 0.950 | 0.947 | 0.985 | 0.896 | 0.932 | 0.983 | 0.978 | 0.962 | 0.936 | ||
| NRMSD (%) | 5.81 | 5.56 | 8.56 | 8.82 | 7.78 | 3.66 | 10.34 | 8.04 | 4.40 | 4.51 | 6.43 | 8.00 | ||
| Second order | Constants | Ce | 45.6 | 49.5 | 63.7 | 61.1 | 45.4 | 61.1 | 61.8 | 59.2 | 44.1 | 52.2 | 60.1 | 61.1 |
| k | 4.3 × 10−3 | 5.3 × 10−3 | 1.5 × 10−3 | 5.6 × 10−3 | 4.5 × 10−3 | 5.6 × 10−3 | 1.9 × 10−3 | 9.1 × 10−3 | 4.3 × 10−3 | 6.2 × 10−3 | 1.8 × 10−3 | 1.8 × 10−3 | ||
| Statistical Parameters | R2 | 0.974 | 0.970 | 0.935 | 0.956 | 0.955 | 0.956 | 0.907 | 0.939 | 0.985 | 0.980 | 0.983 | 0.943 | |
| R2-adj | 0.970 | 0.967 | 0.927 | 0.950 | 0.947 | 0.950 | 0.896 | 0.932 | 0.983 | 0.978 | 0.981 | 0.936 | ||
| NRMSD (%) | 5.81 | 5.56 | 8.55 | 6.82 | 7.78 | 17.57 | 10.34 | 8.04 | 4.40 | 4.51 | 6.42 | 8.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemoni, Z.; Kalantzi, S.; Lymperopoulou, T.; Tzani, A.; Stavropoulos, G.; Detsi, A.; Mamma, D. Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction. Antioxidants 2025, 14, 558. https://doi.org/10.3390/antiox14050558
Lemoni Z, Kalantzi S, Lymperopoulou T, Tzani A, Stavropoulos G, Detsi A, Mamma D. Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction. Antioxidants. 2025; 14(5):558. https://doi.org/10.3390/antiox14050558
Chicago/Turabian StyleLemoni, Zafeiria, Styliani Kalantzi, Theopisti Lymperopoulou, Andromachi Tzani, Georgios Stavropoulos, Anastasia Detsi, and Diomi Mamma. 2025. "Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction" Antioxidants 14, no. 5: 558. https://doi.org/10.3390/antiox14050558
APA StyleLemoni, Z., Kalantzi, S., Lymperopoulou, T., Tzani, A., Stavropoulos, G., Detsi, A., & Mamma, D. (2025). Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction. Antioxidants, 14(5), 558. https://doi.org/10.3390/antiox14050558








