The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota
Abstract
1. Introduction
2. Methods
3. Oxidative Stress in Ischaemic Stroke
3.1. Pathophysiological Mechanisms Underlying Oxidative Stress
3.2. Oxidative Stress and Ischaemic Stroke: Advanced Mechanistic Insights
3.3. Cerebral Ischaemia-Reperfusion Injury and ROS Generation
4. Gut Microbiota as a New Player in Ischaemic Stroke Pathophysiology
4.1. Overview of the Gut–Brain Axis in Ischaemic Stroke
4.2. Gut Microbiota and Ischaemic Stroke
5. Mechanisms Linking the Gut Microbiota to Cerebral Ischaemia and Oxidative Stress
5.1. Gut Microbiota and Oxidative Stress
5.2. Metabolites Derived from Gut Microbiota and Oxidative Stress
6. Clinical Implications of Targeting Gut–Brain Axis in Acute Ischaemic Stroke and Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| Cl− | Chloride ion |
| CNS | Central nervous system |
| GPx | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| mRS | Modified Rankin Scale |
| Na+ | Sodium ion |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa B |
| NIHSS | National Institutes of Health Stroke Scale |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| O2•− | Superoxide anion radical |
| •OH | Hydroxyl radical |
| ONOO− | Peroxynitrite |
| RNS | Reactive nitrogen species |
| RONS | Reactive oxygen and nitrogen species |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| TMAO | Trimethylamine N-oxide |
| TNF-α | Tumor necrosis factor alpha |
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef] [PubMed]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the Pathophysiology of Ischemic Stroke: The Basis of Current Therapies and Opportunity for New Ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.M.; Thundyil, J.; Tang, S.-C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, Treatment, and Animal and Cellular Models of Human Ischemic Stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Pawluk, H.; Tafelska-Kaczmarek, A.; Sopońska, M.; Porzych, M.; Modrzejewska, M.; Pawluk, M.; Kurhaluk, N.; Tkaczenko, H.; Kołodziejska, R. The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke. Biomolecules 2024, 14, 1130. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in Acute Stroke: Targeting Excitotoxicity, Oxidative and Nitrosative Stress, and Inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Patil, S.; Rossi, R.; Jabrah, D.; Doyle, K. Detection, Diagnosis and Treatment of Acute Ischemic Stroke: Current and Future Perspectives. Front. Med. Technol. 2022, 4, 748949. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Golenia, A.; Leśkiewicz, M.; Regulska, M.; Budziszewska, B.; Szczęsny, E.; Jagiełła, J.; Wnuk, M.; Ostrowska, M.; Lasoń, W.; Basta-Kaim, A.; et al. Catalase Activity in Blood Fractions of Patients with Sporadic ALS. Pharmacol. Rep. 2014, 66, 704–707. [Google Scholar] [CrossRef]
- Benakis, C.; Liesz, A. The Gut-Brain Axis in Ischemic Stroke: Its Relevance in Pathology and as a Therapeutic Target. Neurol. Res. Pract. 2022, 4, 57. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; He, J.; Xiong, X. The Role of the Gut Microbiota in the Development of Ischemic Stroke. Front. Immunol. 2022, 13, 845243. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants That Penetrate the Blood Brain Barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Anaya-Fernández, R.; Anaya-Prado, R.; Anaya-Fernandez, M.M.; Guerrero-Palomera, M.A.; Garcia-Ramirez, I.F.; Gonzalez-Martinez, D.; Azcona-Ramirez, C.C.; Guerrero-Palomera, C.S.; Garcia-Perez, C.; Tenorio-Gonzalez, B.; et al. Oxidative Stress in Cerebral Ischemia/Reperfusion Injury. OBM Neurobiol. 2024, 8, 239. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2024, 14, 36. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial Respiratory Chain-Dependent Generation of Superoxide Anion and Its Release into the Intermembrane Space. Biochem. J. 2001, 353, 411. [Google Scholar] [CrossRef]
- Reis, P.A.; de Albuquerque, C.F.G.; Maron-Gutierrez, T.; Silva, A.R.; Neto, H.C.D.C.F. Role of Nitric Oxide Synthase in the Function of the Central Nervous System under Normal and Infectious Conditions. In Nitric Oxide Synthase—Simple Enzyme-Complex Roles; InTech: Vienna, Austria, 2017. [Google Scholar]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative Stress and Its Role in the Pathogenesis of Ischaemic Stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some New Prospects in the Understanding of the Molecular Basis of the Pathogenesis of Stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, L.; Simats, A.; García-Berrocoso, T.; Montaner, J. Inflammatory Molecules Might Become Both Biomarkers and Therapeutic Targets for Stroke Management. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789340. [Google Scholar] [CrossRef]
- Gloire, G.; Piette, J. Redox Regulation of Nuclear Post-Translational Modifications During NF-ΚB Activation. Antioxid. Redox Signal 2009, 11, 2209–2222. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The Immunology of Stroke: From Mechanisms to Translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory Mechanisms in Ischemic Stroke: Role of Inflammatory Cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-Reperfusion Injury: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Prado, R.; Toledo-Pereyra, L.H.; Lentsch, A.B.; Ward, P.A. Ischemia/Reperfusion Injury. J. Surg. Res. 2002, 105, 248–258. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Yu, Z. Ischemia-Reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies. Biochem. Pharmacol. 2016, 5, 213. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Yamato, M.; Egashira, T.; Utsumi, H. Application of in Vivo ESR Spectroscopy to Measurement of Cerebrovascular ROS Generation in Stroke. Free Radic. Biol. Med. 2003, 35, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, G.S.; Chen, H.; Maier, C.M.; Narasimhan, P.; Song, Y.S.; Niizuma, K.; Katsu, M.; Okami, N.; Yoshioka, H.; et al. Reperfusion and Neurovascular Dysfunction in Stroke: From Basic Mechanisms to Potential Strategies for Neuroprotection. Mol. Neurobiol. 2010, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef]
- Tabata Fukushima, C.; Dancil, I.-S.; Clary, H.; Shah, N.; Nadtochiy, S.M.; Brookes, P.S. Reactive Oxygen Species Generation by Reverse Electron Transfer at Mitochondrial Complex I Under Simulated Early Reperfusion Conditions. Redox Biol. 2024, 70, 103047. [Google Scholar] [CrossRef]
- Kleikers, P.W.M.; Wingler, K.; Hermans, J.J.R.; Diebold, I.; Altenhöfer, S.; Radermacher, K.A.; Janssen, B.; Görlach, A.; Schmidt, H.H.H.W. NADPH Oxidases as a Source of Oxidative Stress and Molecular Target in Ischemia/Reperfusion Injury. J. Mol. Med. 2012, 90, 1391–1406. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the Gut Microbiota in Stroke Pathogenesis and Potential Therapeutic Implications. Ann. Nutr. Metab. 2021, 77, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of Gut Microbiota and Brain via Immune and Neuroendocrine Signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S. Gut Microbiota Neurotransmitters: Influence on Risk and Outcome of Ischemic Stroke. Neural Regen. Res. 2022, 18, 1707. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, M.; Ren, J.; Sun, X.; Zhang, Z.; Luo, Y.; Sun, X. New Insight into Gut Microbiota and Their Metabolites in Ischemic Stroke: A Promising Therapeutic Target. Biomed. Pharmacother. 2023, 162, 114559. [Google Scholar] [CrossRef]
- Peh, A.; O’Donnell, J.A.; Broughton, B.R.S.; Marques, F.Z. Gut Microbiota and Their Metabolites in Stroke: A Double-Edged Sword. Stroke 2022, 53, 1788–1801. [Google Scholar] [CrossRef]
- Chen, G.; Du, X.; Cui, J.; Song, J.; Xiong, M.; Zeng, X.; Yang, H.; Xu, K. Role of Gut Microbiota in Ischemic Stroke: A Narrative Review of Human and Animal Studies. Neuroprotection 2024, 2, 120–136. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Vajdi, M.; Asghari-Jafarabadi, M. Gut Microbiota-Associated Metabolite Trimethylamine N-Oxide and the Risk of Stroke: A Systematic Review and Dose–Response Meta-Analysis. Nutr. J. 2020, 19, 76. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, J.; Xu, J.; Gu, A.; Deng, D.; Jia, X.; Wang, B. Trimethylamine-N-Oxide: A Potential Biomarker and Therapeutic Target in Ischemic Stroke. Front. Neurol. 2023, 14, 1156879. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, M.; Qian, J.; Wang, C.; Zhang, J. The Bridge Between Ischemic Stroke and Gut Microbes: Short-Chain Fatty Acids. Cell Mol. Neurobiol. 2023, 43, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-S.; Yang, I.-H.; Kuo, C.-M.; Wu, M.-N.; Lin, T.-C.; Fong, Y.-O.; Juan, C.-H.; Lai, C.-L. The Prognostic Biomarkers of Plasma Trimethylamine N-Oxide and Short-Chain Fatty Acids for Recanalization Therapy in Acute Ischemic Stroke. Int. J. Mol. Sci. 2023, 24, 10796. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.; Frank, J.; McLouth, C.; Trout, A.L.; Morris, A.; Chen, J.; Stowe, A.M.; Fraser, J.F.; Pennypacker, K. Short Chain Fatty Acids Taken at Time of Thrombectomy in Acute Ischemic Stroke Patients Are Independent of Stroke Severity But Associated With Inflammatory Markers and Worse Symptoms at Discharge. Front. Immunol. 2022, 12, 797302. [Google Scholar] [CrossRef]
- Chang, Y.; Woo, H.G.; Jeong, J.H.; Kim, G.H.; Park, K.D.; Song, T.-J. Microbiota Dysbiosis and Functional Outcome in Acute Ischemic Stroke Patients. Sci. Rep. 2021, 11, 10977. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Sun, H.; Gu, M.; Li, Z.; Chen, X.; Zhou, J. Gut Microbiota Dysbiosis in Acute Ischemic Stroke Associated With 3-Month Unfavorable Outcome. Front. Neurol. 2022, 12, 799222. [Google Scholar] [CrossRef]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut Dysbiosis Is Associated with Metabolism and Systemic Inflammation in Patients with Ischemic Stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shan, W.; Lai, Z.; Zuo, Z. Gut Microbiota of Old Mice Worsens Neurological Outcome after Brain Ischemia via Increased Valeric Acid and IL-17 in the Blood. Microbiome 2023, 11, 204. [Google Scholar] [CrossRef]
- He, P.; Jiang, C.; Ni, J.; Zhang, X.; Wu, Z.; Chen, G.; Huang, J.; Dai, Z.; Ji, W.; Li, L.; et al. Identifying Gut Microbiota with High Specificity for Ischemic Stroke with Large Vessel Occlusion. Sci. Rep. 2024, 14, 14086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ling, L.; Xiang, L.; Li, W.; Bao, P.; Yue, W. Role of the Gut Microbiota in Complications after Ischemic Stroke. Front. Cell Infect. Microbiol. 2024, 14, 1334581. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Wang, S.; Liu, W.; Song, Z.; Liao, H. The Impact of Gut Microbiota on the Occurrence, Treatment, and Prognosis of Ischemic Stroke. Neurobiol. Dis. 2025, 207, 106836. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative Stress Tolerance and Antioxidant Capacity of Lactic Acid Bacteria as Probiotic: A Systematic Review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Wanchao, S.; Chen, M.; Zhiguo, S.; Futang, X.; Mengmeng, S. Protective Effect and Mechanism of Lactobacillus on Cerebral Ischemia Reperfusion Injury in Rats. Braz. J. Med. Biol. Res. 2018, 51, e7172. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium Breve and Bifidobacterium Longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef]
- Long, J.; Wang, J.; Li, Y.; Chen, S. Gut Microbiota in Ischemic Stroke: Where We Stand and Challenges Ahead. Front. Nutr. 2022, 9, 1008514. [Google Scholar] [CrossRef]
- Deng, J.; Li, J.; Li, S.; Zhang, D.; Bai, X. Progress of Research on Short-Chain Fatty Acids, Metabolites of Gut Microbiota, and Acute Ischemic Stroke. Clin. Neurol. Neurosurg. 2025, 249, 108725. [Google Scholar] [CrossRef] [PubMed]
- Mathias, K.; Machado, R.S.; Stork, S.; Martins, C.D.; dos Santos, D.; Lippert, F.W.; Prophiro, J.S.; Petronilho, F. Short-Chain Fatty Acid on Blood-Brain Barrier and Glial Function in Ischemic Stroke. Life Sci. 2024, 354, 122979. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xin, L.; Yang, L.; Xu, M.; Li, F.; Zhou, M.; Yan, T. Butyrate Promotes Post-Stroke Outcomes in Aged Mice via Interleukin-22. Exp. Neurol. 2023, 363, 114351. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.A.E.; Bast, A.; Vanhoutvin, S.A.L.W.; Fischer, M.A.J.G.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.-J.M. Butyrate Modulates Oxidative Stress in the Colonic Mucosa of Healthy Humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef]
- Wang, R.-X.; Li, S.; Sui, X. Sodium Butyrate Relieves Cerebral Ischemia-Reperfusion Injury in Mice by Inhibiting JNK/STAT Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1762–1769. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Guo, Z.-N.; Yang, Y.; Qu, Y.; Jin, H. Gut-Brain Axis: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke through Immune Functions. Front. Neurosci. 2023, 17, 1081347. [Google Scholar] [CrossRef]
- Qu, D.; Jiang, D.; Xin, Y.; Yang, G.; Liang, H.; Wang, L. Gut Microbiota and Functional Outcome after Ischemic Stroke: A Mendelian Randomization Study. Front. Immunol. 2024, 15, 1414653. [Google Scholar] [CrossRef]
- Murthy, P.M.; CA, J.; Kandi, V.; Reddy, M.K.; Harikrishna, G.V.; Reddy, K.; JP, R.; Reddy, A.N.; Narang, J. Connecting the Dots: The Interplay Between Stroke and the Gut-Brain Axis. Cureus 2023, 15, e37324. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Yuan, X.; Yang, J.; Li, K. Effect of Early Enteral Nutrition Combined with Probiotics in Patients with Stroke: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2022, 76, 592–603. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, X.; Li, P.; Li, H.; Feng, G.; Wang, G. Bifidobacterium Bifidum Supplementation Improves Ischemic Stroke Outcomes in Elderly Patients: A Retrospective Study. Medicine 2024, 103, e37682. [Google Scholar] [CrossRef]
- Savigamin, C.; Samuthpongtorn, C.; Mahakit, N.; Nopsopon, T.; Heath, J.; Pongpirul, K. Probiotic as a Potential Gut Microbiome Modifier for Stroke Treatment: A Systematic Scoping Review of In Vitro and In Vivo Studies. Nutrients 2022, 14, 3661. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The Anxiolytic Effect of Bifidobacterium Longum NCC3001 Involves Vagal Pathways for Gut-Brain Communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Pasokh, A.; Farzipour, M.; Mahmoudi, J.; Sadigh-Eteghad, S. The Effect of Fecal Microbiota Transplantation on Stroke Outcomes: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2022, 31, 106727. [Google Scholar] [CrossRef]
- Hediyal, T.A.; Vichitra, C.; Anand, N.; Bhaskaran, M.; Essa, S.M.; Kumar, P.; Qoronfleh, M.W.; Akbar, M.; Kaul-Ghanekar, R.; Mahalakshmi, A.M.; et al. Protective Effects of Fecal Microbiota Transplantation against Ischemic Stroke and Other Neurological Disorders: An Update. Front. Immunol. 2024, 15, 1324018. [Google Scholar] [CrossRef]
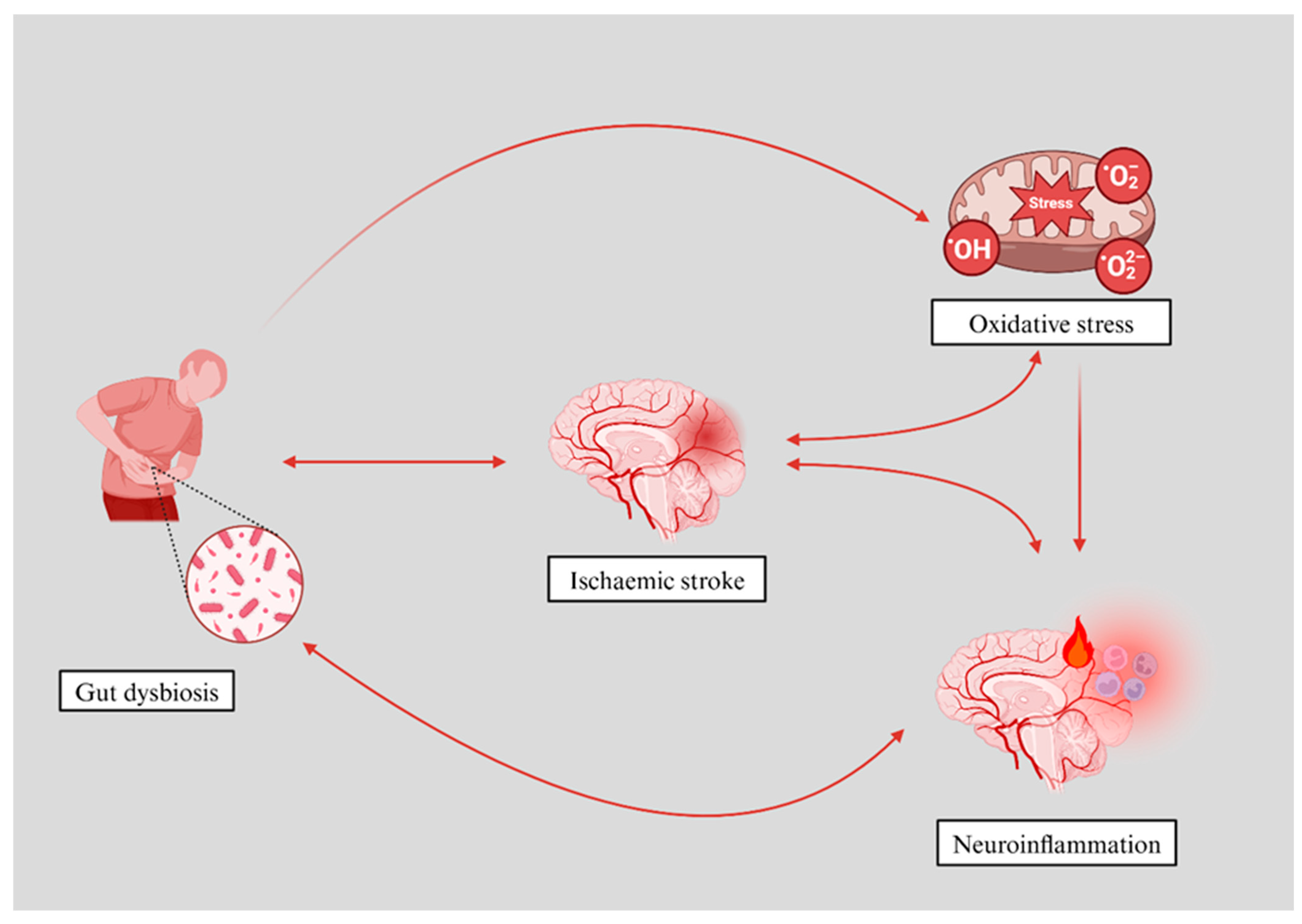
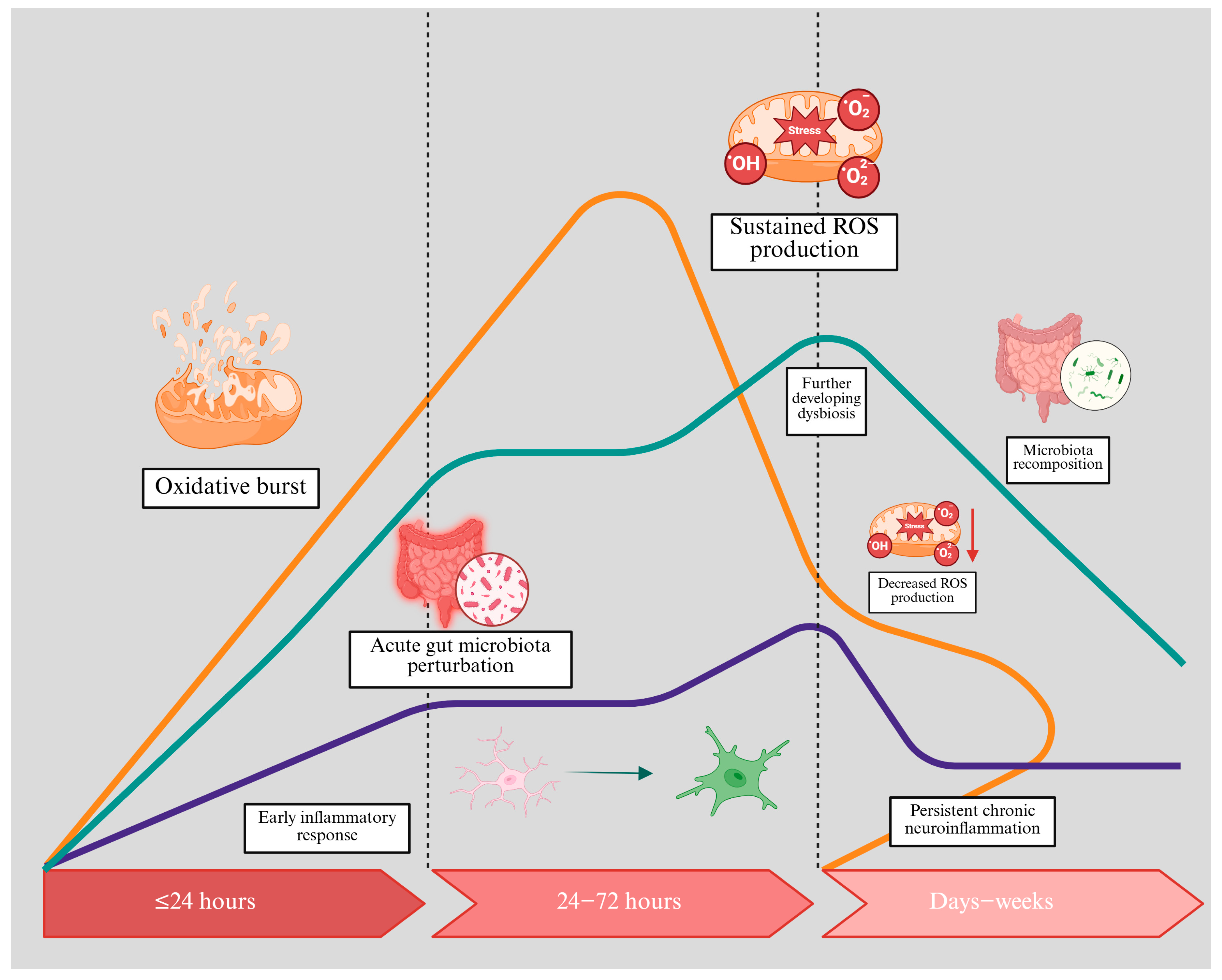
| Topic | Key Points | Implications |
|---|---|---|
| Oxidative stress | Excess RONS produced by mitochondrial dysfunction, NADPH oxidase, and xanthine oxidase directly damage lipids, proteins, and nucleic acids, and activate inflammatory pathways. | Reducing oxidative stress may be a target for neuroprotective therapies. |
| Reperfusion injury | The sudden influx of oxygen intensifies RONS production and inflammation. | Interventions to modulate reperfusion-induced oxidative damage may improve functional outcomes. |
| Gut–brain axis | Dysbiosis can exacerbate oxidative stress and systemic inflammation. | Addressing gut dysbiosis may improve stroke outcomes and reduce post-stroke complications. |
| Gut microbiota metabolites | SCFAs (e.g., butyrate, acetate, propionate): generally neuroprotective, enhance BBB integrity, reduce inflammation. TMAO: implicated in increased thrombosis risk and unfavourable stroke outcomes. | Targeting these metabolites can modulate oxidative stress and inflammation. Potential for personalised nutrition/probiotics. |
| Therapeutic strategies | Antioxidants: counteract RONS (SOD mimetics, vitamin E). Probiotics/faecal microbiota transplantation: rebalance gut flora and potentially reduce oxidative stress and inflammation. Dietary interventions: high-fiber intake and specific prebiotic ingredients to foster SCFA production. | Adjunctive therapies can be combined with standard acute stroke care (e.g., thrombolysis, thrombectomy). Larger clinical trials are needed to confirm their efficacy. |
| Future directions | Larger-scale, randomised trials are needed to test microbiota-targeting and antioxidant therapies. Further research is required to elucidate the precise molecular mechanisms linking dysbiosis and oxidative stress. Exploring personalised medicine approaches could enhance treatment effectiveness. | A better understanding of the complex gut–brain oxidative pathways may lead to novel interventions that optimise stroke recovery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golenia, A.; Olejnik, P. The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants 2025, 14, 542. https://doi.org/10.3390/antiox14050542
Golenia A, Olejnik P. The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants. 2025; 14(5):542. https://doi.org/10.3390/antiox14050542
Chicago/Turabian StyleGolenia, Aleksandra, and Piotr Olejnik. 2025. "The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota" Antioxidants 14, no. 5: 542. https://doi.org/10.3390/antiox14050542
APA StyleGolenia, A., & Olejnik, P. (2025). The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants, 14(5), 542. https://doi.org/10.3390/antiox14050542






