Bergamot Leaf Extract as an Agent Against Chronic Liver Diseases? In Vitro and In Vivo Findings on Oxidative Stress Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. BLE
2.3. In Vivo Study
2.3.1. Nutritional and Metabolic Parameters
2.3.2. Hepatic Triglycerides Levels
2.3.3. Oxidative Stress Markers and Antioxidant Enzymes Activity in Liver
2.4. In Vitro Study
2.4.1. Mitochondrial Assays
Mitochondrial Isolation and Treatment
Monitoring ROS Formation
Redox State of Pyridine Nucleotides (NAD(P)H) Evaluation
Mitochondrial Membrane Lipoperoxidation
Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Level Determination
2.5. Cell Culture Conditions, Treatments, and Viability
2.6. Statistical Analysis
3. Results
3.1. In Vivo
3.1.1. Nutritional and Metabolic Parameters: BLE Attenuated After 10 Weeks of Treatment the Metabolic Parameters
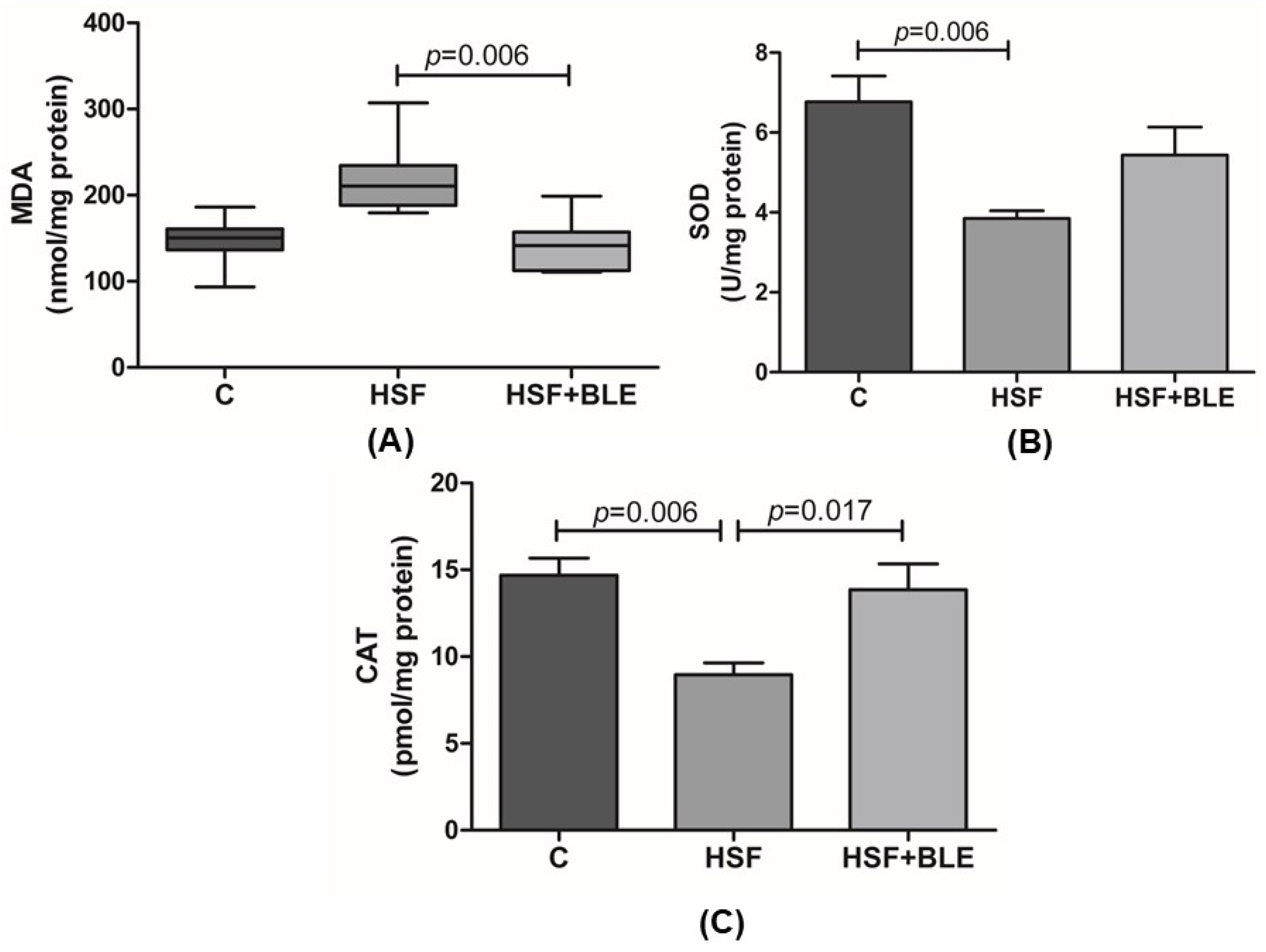
3.1.2. Hepatic Oxidative Stress Was Modulated by BLE
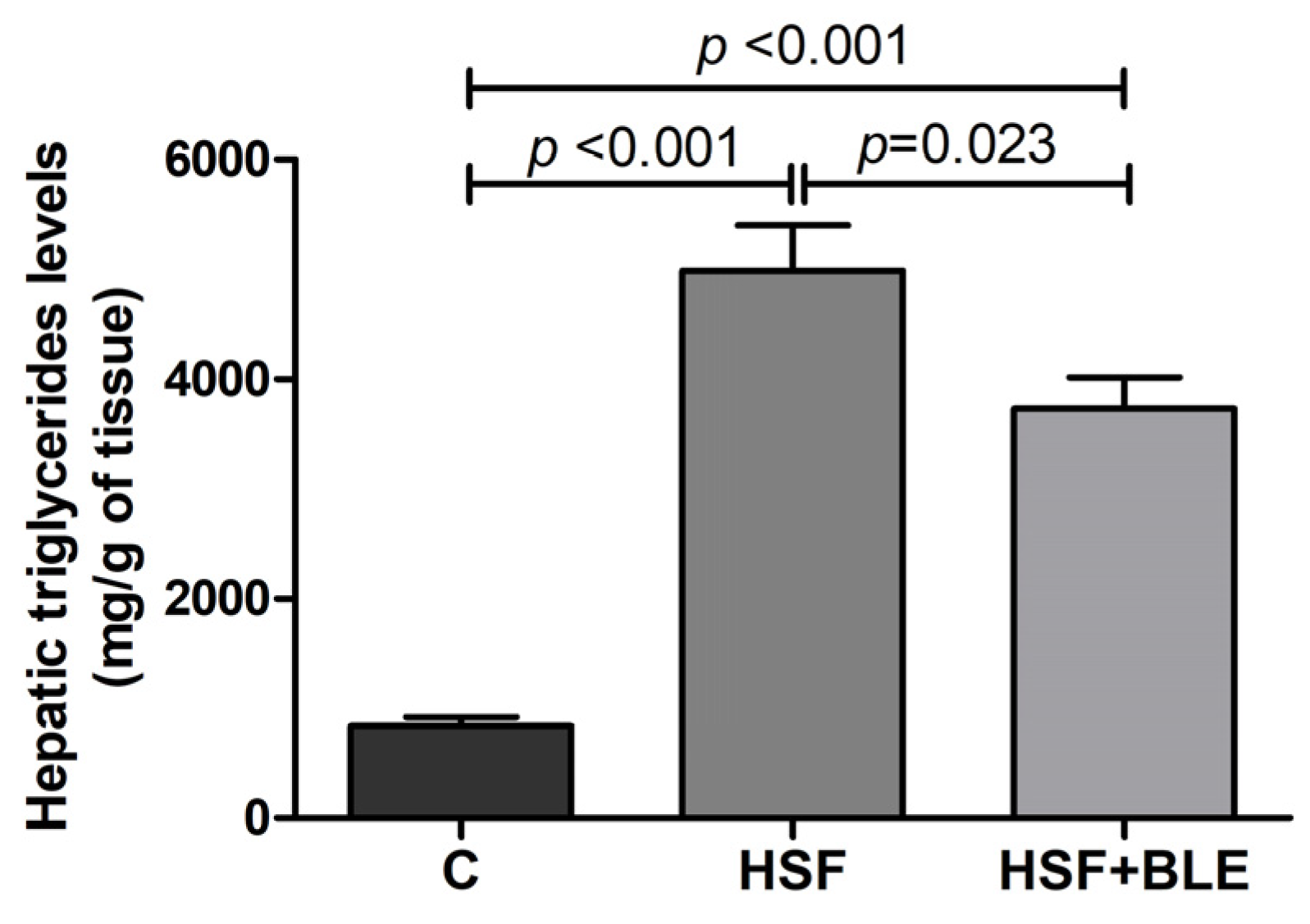
3.1.3. BLE-Modulated Hepatic Triglycerides Levels
3.2. In Vitro
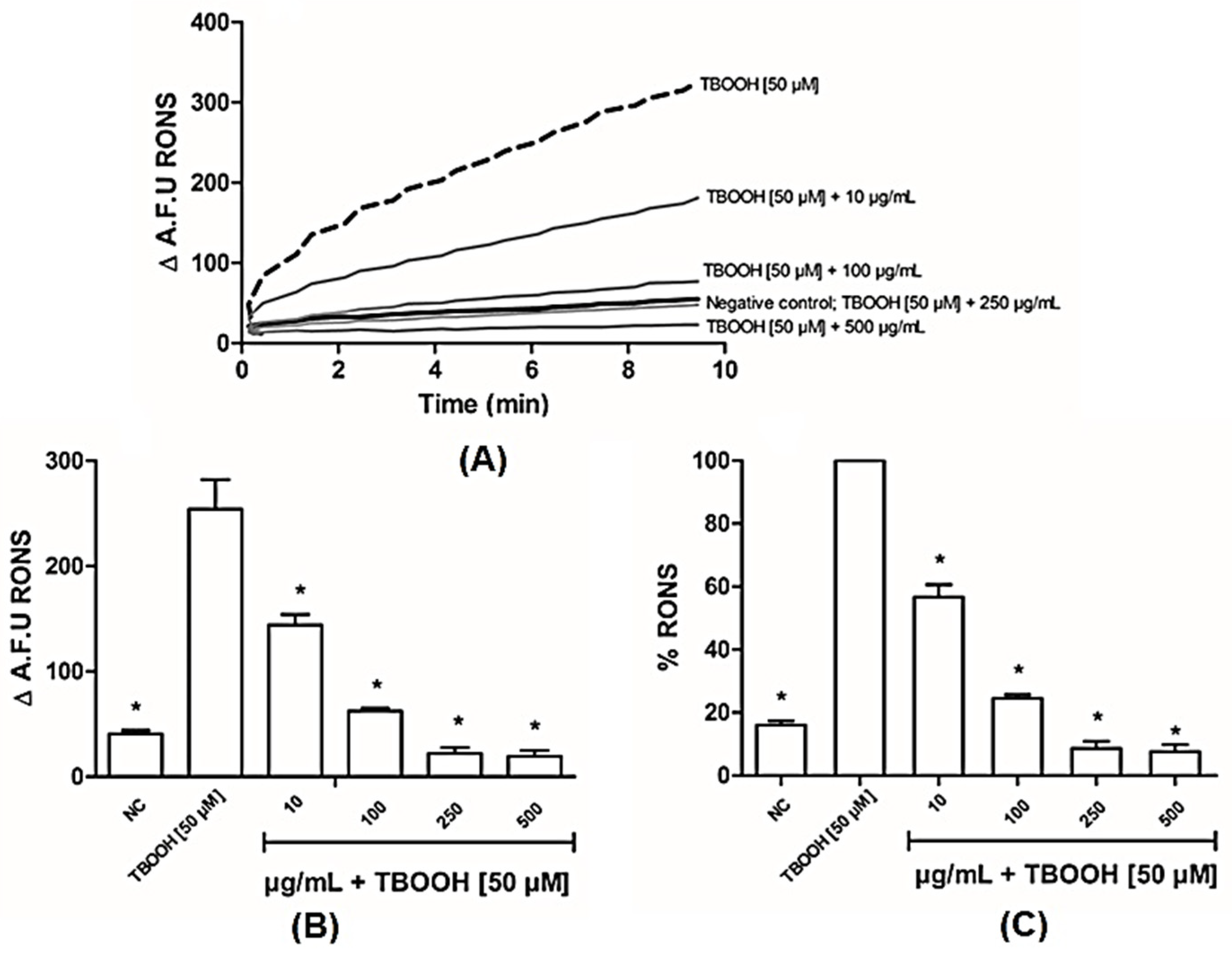
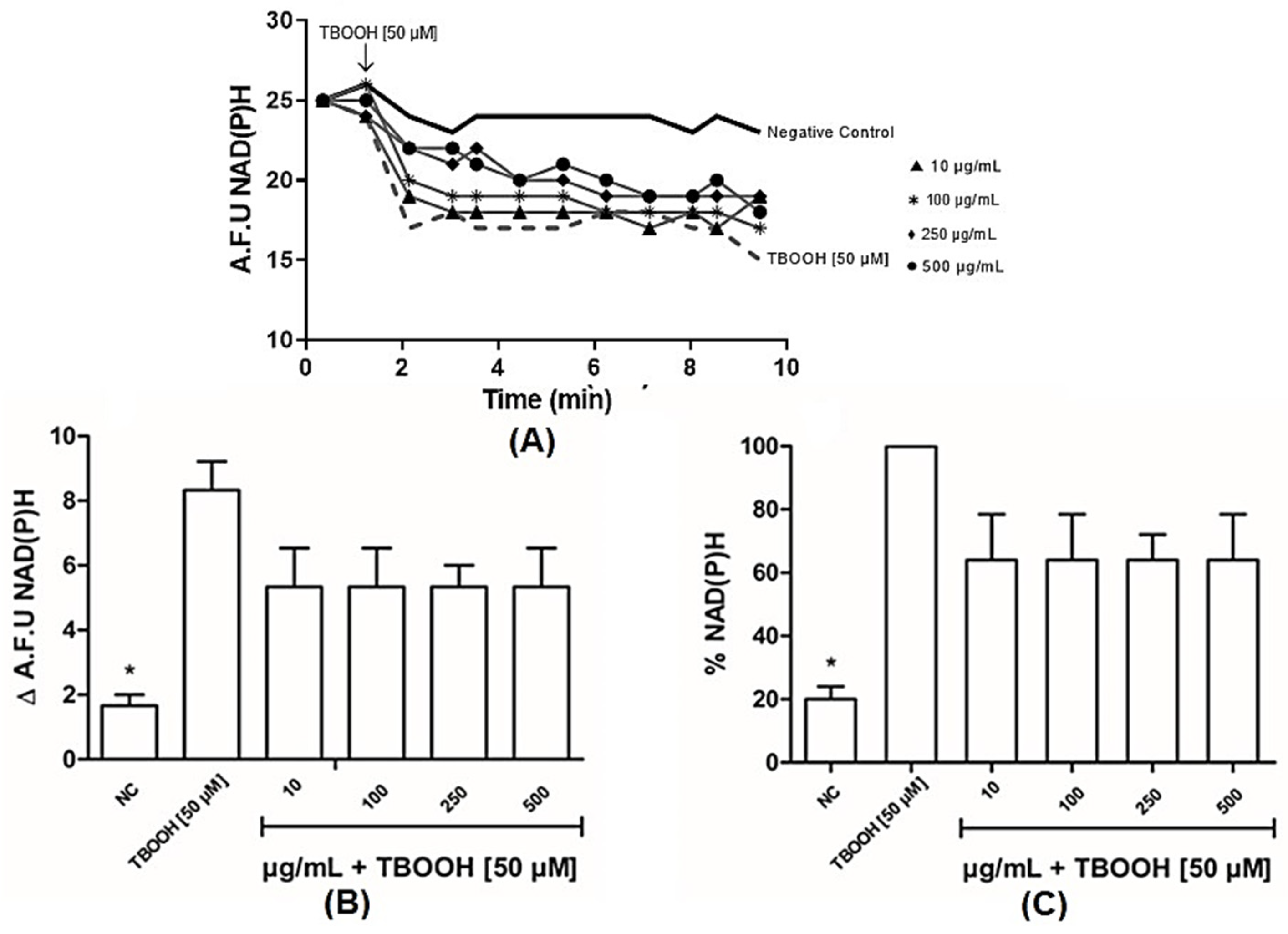
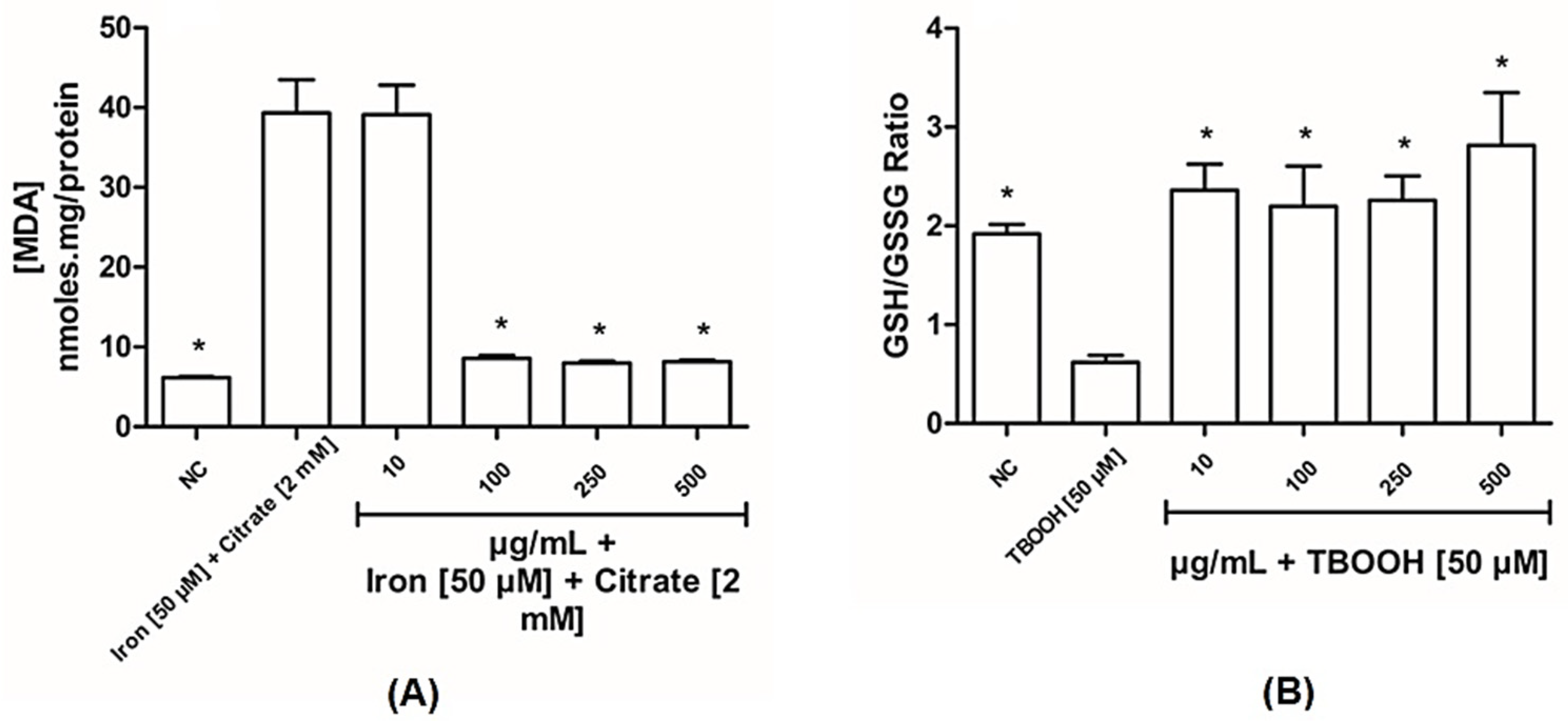
3.2.1. BLE Has Antioxidant Capacity in Liver Mitochondria
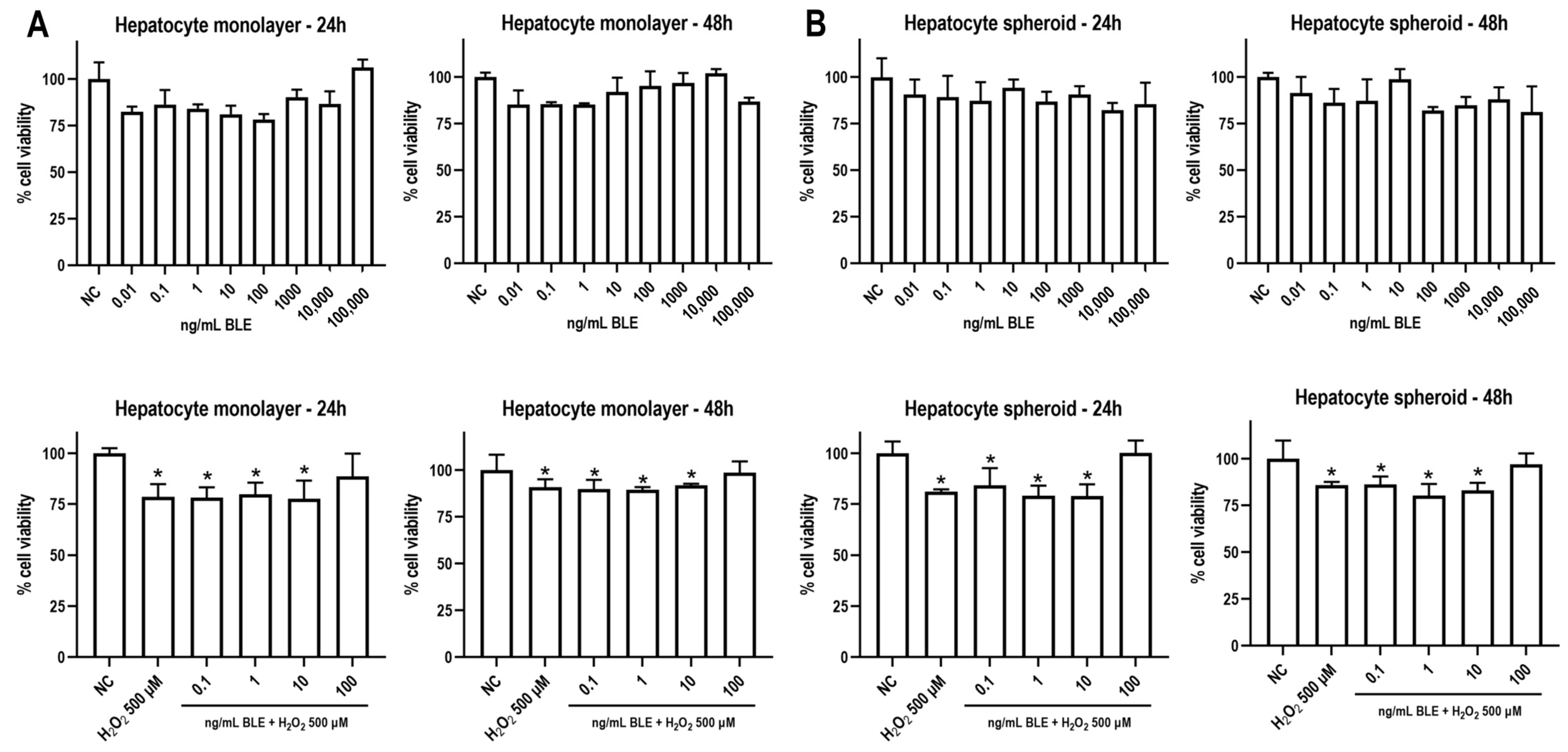
3.2.2. BLE Does Not Reduce Cell Viability and Attenuates Oxidative Stress-Induced Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLE | Bergamot leaf extract |
| C | Control diet |
| HSF | High-sugar–fat diet |
| HSF+BLE | High-sugar–fat diet + bergamot leaf extract |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| ROS | Reactive oxygen species |
| DNA | Deoxyribonucleic acid |
| GSH | Reduced glutathione |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| PUFAs | Polyunsaturated fatty acids PUFAs |
| RNS | Reactive nitrogen species |
| H&AD | Herbal & Antioxidant Derivatives S.r.l. |
| RC | Province of Reggio Calabria |
| MEM | Minimum essential medium |
| USA | United States of America |
| PBS | Phosphate-buffered saline |
| H2O2 | Hydrogen peroxide |
| EGTA | Ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| HEPES-KOH | HEPES–potassium hydroxide buffer |
| RONS | Reactive oxygen/nitrogen species |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| KCl | Potassium chloride |
| DCFDA | 2′-7′-dichlorodihydrofluorescein diacetate |
| NAD(P)H | Nicotinamide adenine dinucleotide phosphate hydrogen |
| TBA | Thiobarbituric acid |
| NaOH | Sodium hydroxide |
| GSSG | Oxidized glutathione |
| OPT | o-phthalaldehyde |
| CaCl2 | Calcium chloride |
| TCA | Trichloroacetic acid |
| NaH2PO4 | Sodium phosphate monobasic |
| NEM | N-ethyl-maleimide |
| FBS | Fetal bovine serum |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| TBOOH | Tert-butyl hydroperoxide |
| NC | Negative control |
| A.F.U | Arbitrary fluorescence unit |
| BPF | Bergamot polyphenolic fraction |
| pAECs | Porcine aortic endothelial cells |
| DOX | Doxorubicin |
| ATP | Adenosine triphosphate |
| NMDA | N-Methyl-D-aspartic acid |
| NRF-2 | Nuclear factor erythroid 2-related factor 2 |
References
- Perazzo, H.; Pacheco, A.G.; Griep, R.H.; Gracindo, R.; Goulart, A.C.; Mendes da Fonseca, J. Changing from NAFLD Trough MAFLD to MASLD: Similar Prevalence and Risk Factors in a Large Brazilian Cohort. J. Hepatol. 2024, 80, e72–e74. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Vázquez, A.L.; Juárez-Hernández, E.; Zuarth-Vázquez, J.M.; Ramos-Ostos, M.H.; Uribe, M.; Castro-Narro, G.; López-Méndez, I. Impact on Prevalence of the Application of NAFLD/MAFLD Criteria in Overweight and Normal Weight Patients. Int. J. Environ. Res. Public Health 2022, 19, 12221. [Google Scholar] [CrossRef]
- Sigala, D.M.; Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Bremer, A.A.; Cox, C.L.; Price, C.A.; Benyam, Y.; Chaudhari, A.J.; et al. Consuming Sucrose- or HFCS-Sweetened Beverages Increases Hepatic Lipid and Decreases Insulin Sensitivity in Adults. J. Clin. Endocrinol. Metab. 2021, 106, 3248–3264. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting Oxidative Stress with Polyphenols to Fight Liver Diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Lopatriello, A.; Sirignano, C.; Ramaschi, G.; Arnoldi, L.; Riva, A.; Sardone, N.; Taglialatela-Scafati, O. Detailed Phytochemical Characterization of Bergamot Polyphenolic Fraction (BPF) by UPLC-DAD-MS and LC-NMR. J. Agric. Food Chem. 2019, 67, 3159–3167. [Google Scholar] [CrossRef]
- Cappello, A.R.; Dolce, V.; Iacopetta, D.; Martello, M.; Fiorillo, M.; Curcio, R.; Muto, L.; Dhanyalayam, D. Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: An Overview. Mini-Rev. Med. Chem. 2016, 16, 619–629. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Nesci, S.; Palma, E.; Mollace, V.; Romeo, G.; Oppedisano, F. Enjoy Your Journey: The Bergamot Polyphenols from the Tree to the Cell Metabolism. J. Transl. Med. 2021, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and Hypoglycaemic Activity of Bergamot Polyphenols: From Animal Models to Human Studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Lamari, F. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Siqueira, J.S.; Vieira, T.A.; Nakandakare-Maia, E.T.; Palacio, T.L.N.; Sarzi, F.; Garcia, J.L.; de Paula, B.H.; Bazan, S.G.Z.; Baron, G.; Tucci, L.; et al. Bergamot Leaf Extract Treats Cardiorenal Metabolic Syndrome and Associated Pathophysiological Factors in Rats Fed with a High Sugar Fat Diet. Mol. Cell. Endocrinol. 2022, 556, 111721. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.S.; Garcia, J.L.; De Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Hu, Z.; Han, Y.; Yang, Y.; Li, Y. Optimized Analysis of In Vivo and In Vitro Hepatic Steatosis. J. Vis. Exp. 2017, 121, e55178. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem. Res. Int. 2016, 2016, 2645237. [Google Scholar] [CrossRef]
- Marklund, S.L. Product of Extracellular-Superoxide Dismutase Catalysis. FEBS Lett. 1985, 184, 237–239. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Chance, B.; Williams, G.R. The Respiratory Chain and Oxidative Phosphorylation. In Advances in Enzymology and Related Areas of Molecular Biology; Interscience Publishers, Inc.: New York, NY, USA, 1956; Volume 17, pp. 65–134. [Google Scholar] [CrossRef]
- Cathcart, R.; Schwiers, E.; Ames, B.N. Detection of Picomole Levels of Hydroperoxides Using a Fluorescent Dichlorofluorescein Assay. Anal. Biochem. 1983, 134, 111–116. [Google Scholar] [CrossRef]
- Oliveira, C.F.H.; Cosso, G.R.; Alberici, C.L.; Maciel, N.E.; Salerno, G.A.; Dorighello, G.G.; Velho, A.J.; De Faria, E.C.; Vercesi, A.E. Oxidative Stress in Atherosclerosis-prone Mouse Is Due to Low Antioxidant Capacity of Mitochondria. FASEB J. 2005, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Da Silva, T.C.; de Albuquerque Landi, M.F.; Morais, J.Á.; Barbisan, L.F.; Vinken, M.; Oliveira, C.P.; Cogliati, B. Sorafenib Reduces Steatosis-Induced Fibrogenesis in a Human 3D Co-Culture Model of Non-Alcoholic Fatty Liver Disease. Environ. Toxicol. 2021, 36, 168–176. [Google Scholar] [CrossRef]
- Vulchi, J.; Suryadevara, V.; Mohan, P.; Kamalanathan, S.; Sahoo, J.; Naik, D.; Selvarajan, S. Obesity and Metabolic Dysfunction-Associated Fatty Liver Disease: Understanding the Intricate Link. J. Transl. Gastroenterol. 2023, 1, 74–86. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Yang, T.; Liang, Y.; Nie, Y.; Luo, Y.; Shen, J.; Fu, X.; Tang, Y.; Luo, F. Oryzanol Modifies High Fat Diet-Induced Obesity, Liver Gene Expression Profile, and Inflammation Response in Mice. J. Agric. Food Chem. 2017, 65, 8374–8385. [Google Scholar] [CrossRef]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western Diet Causes Obesity-Induced Nonalcoholic Fatty Liver Disease Development by Differentially Compromising the Autophagic Response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Betim Cazarin, C.B.; GoisRuiz, A.L.T.; NicolauPaulino, B.; Molina, G.; MariaPastore, G. Targeting Flavonoids on Modulation of Metabolic Syndrome. J. Funct. Foods 2020, 73, 104132. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health. Am. Hear. Assoc. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.M.; Flores, M.E.J. Flavonoids: A Promising Therapy for Obesity Due to the High-Fat Diet. In Flavonoids—A Coloring Model for Cheering up Life; Badria, F.A., Ananga, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- De Leo, M.; Piragine, E.; Pirone, A.; Braca, A.; Pistelli, L.; Calderone, V.; Miragliotta, V.; Testai, L. Protective Effects of Bergamot (Citrus bergamia Risso & Poiteau) Juice in Rats Fed with High-Fat Diet. Planta Med. 2020, 86, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-T.; Chu, H.-L.; Chyau, C.-C.; Chu, C.-C.; Duh, P.-D. Protective Effects of Sweet Orange (Citrus sinensis) Peel and Their Bioactive Compounds on Oxidative Stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, K.; You, Q.; Huang, R.; Li, S.; Wu, K. Naringin Inhibits High Glucose-Induced Cardiomyocyte Apoptosis by Attenuating Mitochondrial Dysfunction and Modulating the Activation of the P38 Signaling Pathway. Int. J. Mol. Med. 2013, 32, 396–402. [Google Scholar] [CrossRef]
- Chen, J.; Guo, R.; Yan, H.; Tian, L.; You, Q.; Li, S.; Huang, R.; Wu, K. Naringin Inhibits ROS-Activated MAPK Pathway in High Glucose-Induced Injuries in H9c2 Cardiac Cells. Basic Clin. Pharmacol. Toxicol. 2014, 114, 293–304. [Google Scholar] [CrossRef]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-Inflammatory and Antioxidant Activities of Naringin Isolated from Carissa carandas L.: In Vitro and in Vivo Evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Parafati, M.; Lascala, A.; La Russa, D.; Mignogna, C.; Trimboli, F.; Morittu, V.; Riillo, C.; Macirella, R.; Mollace, V.; Brunelli, E.; et al. Bergamot Polyphenols Boost Therapeutic Effects of the Diet on Non-Alcoholic Steatohepatitis (NASH) Induced by “Junk Food”: Evidence for Anti-Inflammatory Activity. Nutrients 2018, 10, 1604. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Troisio, I.; et al. The Impairment of Cell Metabolism by Cardiovascular Toxicity of Doxorubicin Is Reversed by Bergamot Polyphenolic Fraction Treatment in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 8977. [Google Scholar] [CrossRef]
- Morrone, L.A.; Rombolà, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The Essential Oil of Bergamot Enhances the Levels of Amino Acid Neurotransmitters in the Hippocampus of Rat: Implication of Monoterpene Hydrocarbons. Pharmacol. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot Polyphenol Fraction Prevents Nonalcoholic Fatty Liver Disease via Stimulation of Lipophagy in Cafeteria Diet-Induced Rat Model of Metabolic Syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef]
- Feitosa, C.M.; da Silva Oliveira, G.L.; do Nascimento Cavalcante, A.; Morais Chaves, S.K.; Rai, M. Determination of Parameters of Oxidative Stress in Vitro Models of Neurodegenerative Diseases—A Review. Curr. Clin. Pharmacol. 2018, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kryl’skii, E.D.; Popova, T.N.; Kirilova, E.M. Activity of Glutathione Antioxidant System and NADPH-Generating Enzymes in Rats with Experimental Rheumatoid Arthritis. Bull. Exp. Biol. Med. 2015, 160, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wan, S.; An, Y.; Wu, Q.; Xing, Y.; Deng, C.; Zhang, P.; Long, Y.; Xu, B.; Jiang, Z. Targeting Cell Death in NAFLD: Mechanisms and Targeted Therapies. Cell Death Discov. 2024, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.C.; Bacil, G.P.; Riechelmann-Casarin, L.; Barbosa, G.C.; Barbisan, L.F.; Romualdo, G.R. Exploring in Vitro Modeling in Hepatocarcinogenesis Research: Morphological and Molecular Features and Similarities to the Corresponding Human Disease. Life Sci. 2024, 351, 122781. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Izyumov, D.S.; Lyamzaev, K.G.; Pashkovskaya, A.A.; Pletjushkina, O.Y.; Antonenko, Y.N.; Sakharov, D.V.; Wirtz, K.W.A.; Skulachev, V.P. Production of Reactive Oxygen Species in Mitochondria of HeLa Cells under Oxidative Stress. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 525–534. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Groups | Effect | |||||
|---|---|---|---|---|---|---|
| C | HSF | HSF+BLE | C | C | HSF | |
| vs | vs | vs | ||||
| HSF | HSF+BLE | HSF+BLE | ||||
| Final body weight (g) | 489 ± 57 | 610 ± 74 * | 562 ± 66 | 0.017 | 0.170 | 0.444 |
| Caloric intake (kcal/day) | 90.0 ± 6.0 | 104.9 ± 8.7 * | 101.7 ± 8.3 * | 0.013 | 0.049 | 0.766 |
| Adiposity index (%) | 4.18 ± 1.15 | 9.13 ± 1.38 * | 7.65 ± 1.94 * | <0.001 | 0.004 | 0.248 |
| Triglycerides (mg/dL) | 26.2 ± 4.7 | 108.4 ± 9.9 * | 60.5 ± 11.9 *,# | <0.001 | <0.001 | <0.001 |
| Glucose (mg/dL) | 81.0 ± 4.5 | 94.5 ± 3.0 * | 86.5 ± 5.0 *,# | <0.001 | 0.014 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, T.A.; Seloto, D.G.; Rizzi, J.S.; Peixoto, P.V.L.; Corrêa, G.V.B.; Siqueira, J.S.; Grandini, N.A.; Nakandakare-Maia, E.T.; Valente, L.C.; Francisqueti-Ferron, F.V.; et al. Bergamot Leaf Extract as an Agent Against Chronic Liver Diseases? In Vitro and In Vivo Findings on Oxidative Stress Modulation. Antioxidants 2025, 14, 543. https://doi.org/10.3390/antiox14050543
Vieira TA, Seloto DG, Rizzi JS, Peixoto PVL, Corrêa GVB, Siqueira JS, Grandini NA, Nakandakare-Maia ET, Valente LC, Francisqueti-Ferron FV, et al. Bergamot Leaf Extract as an Agent Against Chronic Liver Diseases? In Vitro and In Vivo Findings on Oxidative Stress Modulation. Antioxidants. 2025; 14(5):543. https://doi.org/10.3390/antiox14050543
Chicago/Turabian StyleVieira, Taynara Aparecida, Danielle Gabriel Seloto, Joyce Santana Rizzi, Paloma Vitória Lima Peixoto, Giulia Vitória Betoni Corrêa, Juliana Silva Siqueira, Nubia Alves Grandini, Erika Tiemi Nakandakare-Maia, Letícia Cardoso Valente, Fabiane Valentini Francisqueti-Ferron, and et al. 2025. "Bergamot Leaf Extract as an Agent Against Chronic Liver Diseases? In Vitro and In Vivo Findings on Oxidative Stress Modulation" Antioxidants 14, no. 5: 543. https://doi.org/10.3390/antiox14050543
APA StyleVieira, T. A., Seloto, D. G., Rizzi, J. S., Peixoto, P. V. L., Corrêa, G. V. B., Siqueira, J. S., Grandini, N. A., Nakandakare-Maia, E. T., Valente, L. C., Francisqueti-Ferron, F. V., Ferron, A. J. T., Baron, G., Aldini, G., Correa, C. R., Pereira, L. C., & Romualdo, G. R. (2025). Bergamot Leaf Extract as an Agent Against Chronic Liver Diseases? In Vitro and In Vivo Findings on Oxidative Stress Modulation. Antioxidants, 14(5), 543. https://doi.org/10.3390/antiox14050543









