Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. R. damascena Petals
2.2. Chemicals
2.3. Extraction of R. damascena Petals
2.3.1. Infusion (INF)
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.3.3. Micellar Extraction (MCE)
2.3.4. Microwave-Assisted Extraction (MAE)
2.3.5. Pulsed Electric Field Extraction (PEF)
2.4. Chemical Composition Determination of R. damascena Petal Extracts
2.5. Determination of Biological Activities Related to Cosmetic Applications
2.5.1. Antioxidant Activities
- 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
- 2,2’-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
- Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.2. Anti-Tyrosinase Activities
2.5.3. Anti-Skin Wrinkles Activities
- Collagenase Inhibitory Activities
- Elastase Inhibitory Activities
- Hyaluronidase Inhibitory Activities
2.6. Determination of Physical, Chemical, and Biological Stability of R. damascena Petal Extracts
2.6.1. Storage Conditions
2.6.2. Physical Stability Evaluation
2.6.3. Chemical Stability Evaluation
2.6.4. Biological Stability Evaluation
2.7. Statistical Analysis
3. Results and Discussion
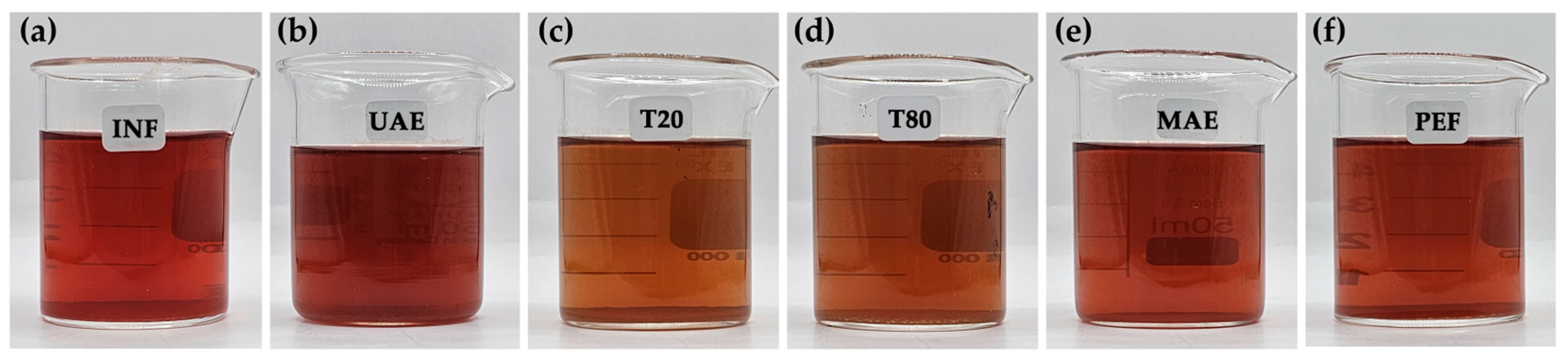
3.1. R. damascena Petal Extracts
3.2. Chemical Compositions of R. damascena Petal Extracts
3.3. Antioxidant Activities of R. damascena Petal Extracts
3.4. Anti-Trosinase Activities of R. damascena Petal Extracts
3.5. Anti-Aging Activities of R. damascena Petal Extracts
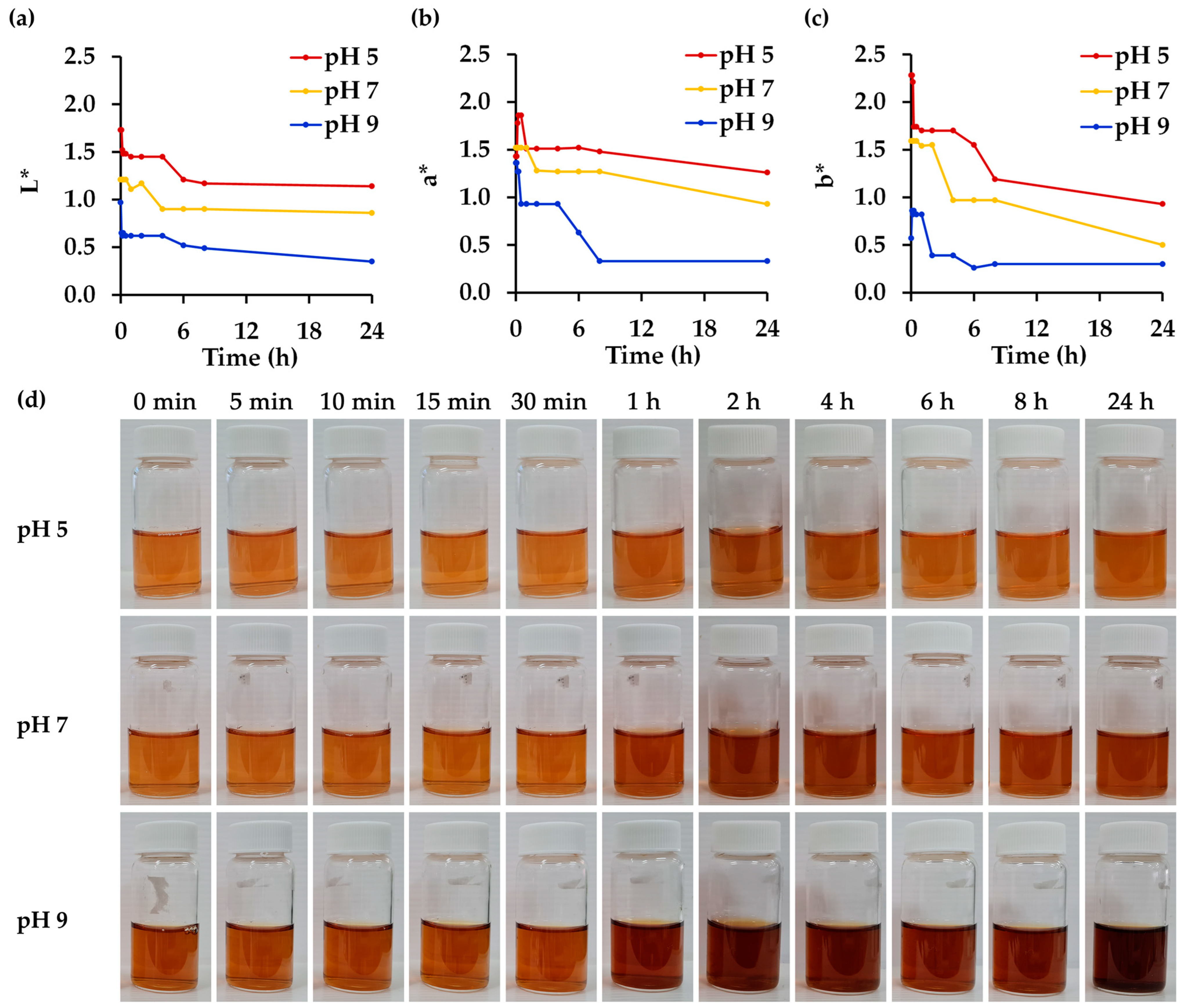
3.6. Effect of pH on Physico-Chemical and Biological Stability of R. damascena Petal Extract
3.7. Effect of Temperature on Physico-Chemical and Biological Stability of R. damascena Petal Extract
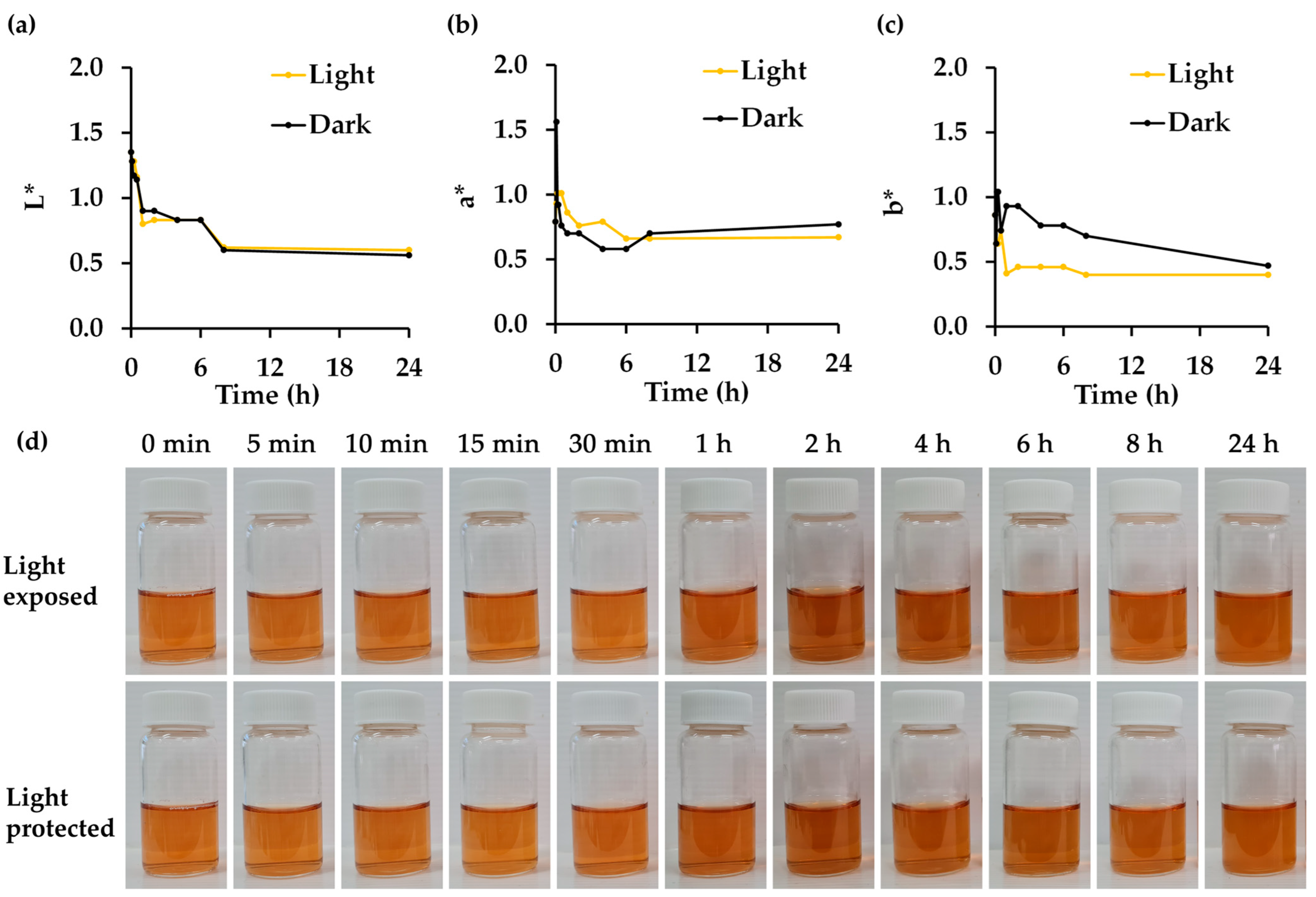
3.8. Effect of Light Exposure on Physico-Chemical and Biological Stability of R. damascena Petal Extract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAAVPN | N-Succinyl-Ala-Ala-Ala-p-nitroanilide |
| ABTS | 2,2-Azino-bis3-ethylbenzothiazoline-6-sulfonic acid |
| BSA | Bovine serum albumin |
| DI | Deionized |
| DPPH | 2,2-Diphenyl-1picrylhydrazyl |
| EC1 | Equivalent capacity |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin gallate |
| FALGPA | 2-Furanacryloyl-Leu-Gly-Pro-Ala |
| HPLC | High performance liquid chromatography |
| IC50 | Half-maximal inhibitory concentration |
| INF | Infusion |
| MAE | Microwave-assisted extraction |
| MCE | Micellar extraction |
| MCE-T20 | Micellar extraction using Tween® 20 |
| MCE-T80 | Micellar extraction using Tween® 80 |
| MMP-1 | Metalloproteinase-1 |
| NLCs | Nanostructured lipid carriers |
| PEF | Pulsed electric fields extraction |
| SLNs | Solid lipid nanoparticles |
| TEAC | Trolox equivalent antioxidant capacity |
| TPTZ | 2,4,6 Tripyridyl-s-triazine |
| UAE | Ultrasound-assisted extraction |
| UV | Ultraviolet |
References
- Jakubczyk, K.; Tuchowska, A.; Janda, M.K. Plant hydrolates–Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef] [PubMed]
- Yassa, N.; Masoomi, F.; Rankouhi, S.R.; Hadjiakhoondi, A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU J. Pharm. Sci. 2015, 17, 175–180. [Google Scholar]
- Alam, M.A.; Nyeem, M.A.B.; Awal, M.A.; Mostofa, M.; Alam, M.S.; Subhan, N.; Rahman, M.M. Antioxidant and hepatoprotective action of the crude ethanolic extract of the flowering top of Rosa damascena. Adv. Tradit. Med. 2008, 8, 164–170. [Google Scholar] [CrossRef]
- Kumar, P.; Verma, A.; Ashique, S.; Bhowmick, M.; Mohanto, S.; Singh, A.; Gupta, M.; Gupta, A.; Haider, T. Unlocking the role of herbal cosmeceutical in anti-ageing and skin ageing associated diseases. Cutan. Ocul. Toxicol. 2024, 43, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Afandi, N.; Sahudin, S. Natural active ingredients used in topical cosmetic formulations for antiageing: A systematic review. Int. J. Pharm. Nutr. Cosmet. Sci. 2022, 5, 67–78. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal extracts as potential antioxidant, anti-ageing, anti-inflammatory, and whitening cosmeceutical ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef]
- Elfitriani, E.; Raif, A.; Ginting, C.N.; Ikhtiari, R. Evaluation of antioxidant and anti-collagenase activity of Rosa damascena L. flower petal and receptacle extract. F1000 Res. 2020, 9, 716. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green extraction techniques as advanced sample preparation approaches in biological, food, and environmental matrices: A review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From herbal teabag to infusion—Impact of brewing on polyphenols and antioxidant capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. Ther. 2011, 5, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Med. Ther. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiamphun, S.; Chaiyana, W. Enhanced antioxidant, hyaluronidase, and collagenase inhibitory activities of glutinous rice husk extract by aqueous enzymatic extraction. Molecules 2022, 27, 3317. [Google Scholar] [CrossRef]
- Amnuaykan, P.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Enhanced antioxidant, anti-ageing, anti-tyrosinase, and anti-inflammatory properties of Vanda coerulea griff. Ex lindl. Protocorm through elicitations with chitosan. Plants 2024, 13, 1770. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Kalisz, A.; Włodarczyk, Z.; Bieniasz, M.; Smoleń, S.; Neugebauerová, J.; Szewczyk-Taranek, B.; Pawłowska, B. Petals of different ornamental rose cultivars as a rich source of bioactive compounds for functional foods. Sci. Hortic. 2023, 321, 112240. [Google Scholar] [CrossRef]
- Schmitzer, V.; Mikulic-Petkovsek, M.; Stampar, F. Traditional rose liqueur—A pink delight rich in phenolics. Food Chem. 2019, 272, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- Abu-Shanab, B.; ADWAN, G.M.; Jarrar, N.; Abu-Hijleh, A.; Adwan, K. Antibacterial activity of four plant extracts used in Palestine in folkloric medicine against methicillin-resistant Staphylococcus aureus. Turk. J. Biol. 2006, 30, 195–198. [Google Scholar] [CrossRef]
- Nayebi, N.; Khalili, N.; Kamalinejad, M.; Emtiazy, M. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complement. Ther. Med. 2017, 34, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Staleva, P.; Petkova, Z.; Ivanova, V.; Evstatieva, Y.; Nikolova, D.; Rasheva, I.; Atanasov, N.; Topouzova-Hristova, T.; Veleva, R.; et al. Phytochemical profile, antioxidant potential, antimicrobial activity, and cytotoxicity of dry extract from Rosa damascena Mill. Molecules 2023, 28, 7666. [Google Scholar] [CrossRef]
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites profiling of Egyptian Rosa damascena Mill. flowers as analyzed via ultra-high-performance liquid chromatography-mass spectrometry and solid-phase microextraction gas chromatography-mass spectrometry in relation to its anti-collagenase skin effect. Ind. Crops Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Vuong, Q. Maximising extraction yields of gallic acid and hesperetin from lemon myrtle (Backhousia citriodora) leaf using microwave assisted extraction. Results Chem. 2020, 2, 10080. [Google Scholar] [CrossRef]
- Romanik, G.; Gilgenast, E.; Przyjazny, A.; Kamiński, M. Techniques of preparing plant material for chromatographic separation and analysis. J. Biochem. Biophys. Methods 2007, 70, 253–261. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Villares, A.; Guillamón, E.; García-Lafuente, A.; Martínez, J.A. Sample preparation for the analysis of isoflavones from soybeans and soy foods. J. Chromatogr. A 2009, 1216, 2–29. [Google Scholar] [CrossRef]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Hacısevki, A. An overview of ascorbic acid biochemistry. J. Fac. Pharm. Ankar. 2009, 38, 233–255. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Fattahi, M. Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: With chemotyping approach concerning morphology and composition. Sci. Hortic. 2021, 288, 110341. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123. [Google Scholar] [CrossRef]
- Fan, L.; Lin, L.; Zhang, Y.; Li, S.; Tang, Z. Component characteristics and reactive oxygen species scavenging activity of anthocyanins from fruits of Lonicera caerulea L. Food Chem. 2023, 403, 134391. [Google Scholar] [CrossRef]
- Trigueros, L.; Wojdyło, A.; Sendra, E. Antioxidant activity and protein–polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014, 62, 6417–6425. [Google Scholar] [CrossRef]
- Kumari, P.; Raju, D.V.S.; Prasad, K.V.; Saha, S.; Panwar, S.; Paul, S.; Banyal, N.; Bains, A.; Chawla, P.; Fogarasi, M.; et al. Characterization of anthocyanins and their antioxidant activities in Indian rose varieties (Rosa × hybrida) using HPLC. Antioxidants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Gudarzi, A.; Soorni, A.; Golchini, M.M.; Meratian Esfahani, S. Characterization of gene expression and anthocyanin accumulation in Rosa damascena during petal development. Discover. Plants. 2024, 1, 66. [Google Scholar] [CrossRef]
- Fragou, F.; Theofanous, A.; Deligiannakis, Y.; Louloudi, M. Nanoantioxidant materials: Nanoengineering inspired by nature. Micromachines 2023, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Kavimani, S.; Saminathan, K.; Senthil Kumar, R. Antioxidant and free radical scavenging activities of Dolichandrone atrovirens using various in vitro assay models. Int. J. Phytopharm. 2014, 5, 293–300. [Google Scholar]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Saber, W.I. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef]
- Rajam, R.P.; Kannan, S.; Kajendran, D. Cosmeceuticals an emerging technology—A review. World J. Pharm. Res. 2019, 8, 664–685. [Google Scholar]
- Charoimek, N.; Sunanta, P.; Tangpao, T.; Suksathan, R.; Chanmahasathien, W.; Sirilun, S.; Hua, K.F.; Chung, H.H.; Sommano, S.R.; Junmahasathien, T. Pharmaceutical potential evaluation of Damask rose by-products from volatile oil extraction. Plants 2024, 13, 1605. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, W.C.; Han, A.; Lee, M.H.; Shin, E.J.; Lee, K.M.; Lim, T.G. Rose petal extract (Rosa gallica) exerts skin whitening and anti-skin wrinkle effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef]
- Arakaki, P.A.; Marques, M.R.; Santos, M.C.L.G. MMP-1 polymorphism and its relationship to pathological processes. J. Biosci. 2009, 34, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N.; Majid, N.M.N. Anti–elastase, anti–tyrosinase and matrix metalloproteinase–1 inhibitory activity of earthworm extracts as potential new anti–ageing agent. Asian Pac. J. Trop. Biomed. 2014, 4, S348–S352. [Google Scholar] [CrossRef]
- Porwal, M.; Rastogi, V.; Chandra, P.; Shukla, S. An Updated Review on the Role of Phytoconstituents in Modulating Signalling Pathways to Combat Skin Ageing: Nature’s Own Weapons and Approaches. J. Nat. Prod. 2024, 14, 55–71. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Klagas, I.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Papakonstantinou, E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Sil, B.K.; Jamiruddin, M.R.; Paul, P.K.; Aekwattanaphol, N.; Nakpheng, T.; Haq, M.A.; Srichana, T. Ascorbic acid as serine protease inhibitor in lung cancer cell line and human serum albumin. PLoS ONE 2024, 19, e0303706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, H.; Wu, H.; Peng, X.; Peng, X.; Yu, X.; Yuan, X. Corilagin functionalized decellularized extracellular matrix as artificial blood vessels with improved endothelialization and anti-inflammation by reactive oxygen species scavenging. Regen. Biomater. 2024, 11, rbae074. [Google Scholar] [CrossRef]
- Mallick, S.R.; Hassan, J.; Hoque, M.A.; Sultana, H.; Kayesh, E.; Ahmed, M.; Ozaki, Y.; Al-Hashimi, A.; Siddiqui, M.H. Color, proximate composition, bioactive compounds and antinutrient profiling of rose. Sci. Rep. 2024, 14, 21690. [Google Scholar] [CrossRef]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Bayram, O.; Sagdic, O.; Ekici, L. Natural food colorants and bioactive extracts from some edible flowers. J. Appl. Bot. Food Qual. 2015, 88, 170–176. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Jameson, G.B.; Singh, H. The effect of pH and sodium caseinate on the aqueous solubility, stability, and crystallinity of rutin towards concentrated colloidally stable particles for the incorporation into functional foods. Molecules 2022, 27, 534. [Google Scholar] [CrossRef] [PubMed]
- Rakić, V.P.; Skrt, M.A.; Miljković, M.N.; Kostić, D.A.; Sokolović, D.T.; Poklar-Ulrih, N.E. Effects of pH on the stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution. Hem. Ind. 2015, 69, 511–522. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [PubMed]
- Grégoire, S.; Ribaud, C.; Benech, F.; Meunier, J.R.; Garrigues-Mazert, A.; Guy, R.H. Prediction of chemical absorption into and through the skin from cosmetic and dermatological formulations. Br. J. Dermatol. 2009, 160, 80–91. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Asghar, W.; Akhtar, A.; Ayub, H.; Aslam, I.; Khalid, N.; Al-Mssallem, M.Q.; Alessa, F.M.; Ghazzawy, H.S.; Attimarad, M. Anthocyanin delivery systems: A critical review of recent research findings. Appl. Sci. 2022, 12, 12347. [Google Scholar] [CrossRef]
- Chen, B.H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S. Stability testing of pharmaceutical products in a global environment. RAJ Pharm. 2006, 5, 291–294. [Google Scholar]
- Kirkbride, L.; Humphries, L.; Kozielska, P.; Curtis, H. Designing a suitable stability protocol in the face of a changing retail landscape. Cosmetics 2021, 8, 64. [Google Scholar] [CrossRef]
- Zothanpuii, F.; Rajesh, R.; Selvakumar, K. A review on stability testing guidelines of pharmaceutical products. Asian J. Pharm. Clin. Res 2020, 13, 3–9. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Sam, J.H.; Jeevanandam, J.; Chan, Y.S.; Nandong, J. Thermal degradation of antioxidant compounds: Effects of parameters, thermal degradation kinetics, and formulation strategies. Food Bioprocess Technol. 2022, 15, 1919–1935. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Wang, Y.; Xu, Z.; Liao, X. Unveiling the influence of high hydrostatic pressure and protein interactions on the color and chemical stability of cyanidin-3-O-glucoside. Food Res. Int. 2024, 192, 114823. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Galletti, G.C.; Bocchini, P. Behaviour of catechin and ellagic acid subjected to thermally-assisted hydrolysis—Methylation/gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 1995, 9, 250–254. [Google Scholar] [CrossRef]
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum Murray and Nitraria tangutorum Bobr of Qinghai-Tibetan Plateau. Sep. Sci. Technol. 2014, 49, 2897–2906. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Ahmad, I. Stability and stabilization of ascorbic acid. Househ. Pers. Care Today 2015, 10, 22–25. [Google Scholar]
- Shad, Z.; Arsalan, A.; Bano, R.; Khan, M.F.; Ahmed, I. Physicochemical, biochemical and antioxidant properties of ascorbic acid. J. Baqai Med. Univ. 2011, 14, 33–40. [Google Scholar]
- Zhang, X.; Cardon, D.; Cabrera, J.L.; Laursen, R. The role of glycosides in the light-stabilization of 3-hydroxyflavone (flavonol) dyes as revealed by HPLC. Mikrochim. Acta 2010, 169, 327–334. [Google Scholar] [CrossRef]
- Zahri, S.; Belloncle, C.; Charrier, F.; Pardon, P.; Quideau, S.; Charrier, B. UV light impact on ellagitannins and wood surface colour of European oak (Quercus petraea and Quercus robur). Appl. Surf. Sci. 2007, 253, 4985–4989. [Google Scholar] [CrossRef]



| Compounds | RT (min) | Chemical Content (mg Per g R. damascena Petal Extract) | |||||
|---|---|---|---|---|---|---|---|
| INF | UAE | MCE-T20 | MCE-T80 | MAE | PEF | ||
| L-ascorbic acid | 2.60 | 3.59 ± 0.00 e | 3.97 ± 0.00 d | 5.50 ± 0.01 c | 5.62 ± 0.01 b | 9.97 ± 0.01 a | 3.98 ± 0.00 d |
| Gallic acid | 3.62 | 7.05 ± 0.00 d | 8.20 ± 0.01 c | 8.77 ± 0.01 b | 8.75 ± 0.01 b | 72.56 ± 0.03 a | 6.41 ± 0.01 e |
| Cyanidin-3,5-O-diglucoside | 5.24 | 14.11 ± 0.01 c | 14.29 ± 0.01 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 82.92 ± 0.02 a | 15.30 ± 0.01 b |
| Corilagin | 8.46 | 34.30 ± 0.01 c | 34.90 ± 0.02 b | 23.29 ± 0.01 e | 14.68 ± 0.01 f | 213.59 ± 0.01 a | 25.16 ± 0.01 d |
| Rutin | 13.21 | 4.30 ± 0.01 d | 3.81 ± 0.01 e | 4.74 ± 0.01 c | 5.21 ± 0.01 b | 6.70 ± 0.01 a | 3.62 ± 0.00 f |
| Ellagic acid | 14.74 | 22.97 ± 0.01 d | 22.37 ± 0.01 e | 23.74 ± 0.01 c | 24.67 ± 0.01 b | 48.49 ± 0.01 a | 17.45 ± 0.01 f |
| Samples | DPPH Inhibition IC50 (µg/mL) | TEAC (mg Trolox/g) | EC1 (mM FeSO4/g) |
|---|---|---|---|
| L-ascorbic acid | 4.8 ± 0.2 c | 17.0 ± 0.3 b | 13.4 ± 0.6 b |
| Gallic acid | 1.0 ± 0.1 b | 14.2 ± 1.8 c | 14.2 ± 1.8 b |
| Cyanidin-3,5-O-diglucoside | 33.4 ± 8.2 d | 1.9 ± 0.2 d | 0.7 ± 0.1 d |
| Corilagin | 31.4 ± 0.2 d | 16.7 ± 0.1 b | 5.0 ± 0.1 c |
| Rutin | 43.8 ± 5.1 e | 19.4 ± 0.2 a | 5.6 ± 0.0 c |
| Ellagic acid | 4.8 ± 0.2 c | 3.9 ± 0.2 d | 14.7 ± 0.6 b |
| R. damascena petal extract | 0.5 ± 0.1 a | 21.2 ± 0.3 a | 53.1 ± 1.7 a |
| Samples | Anti-Tyrosinase Activity (%) | |
|---|---|---|
| Tyrosine | L-Dopa | |
| Kojic acid | 93.1 ± 0.2 a | 96.2 ± 0.9 a |
| L-ascorbic acid | 44.1 ± 4.9 b | 42.6 ± 1.8 b |
| Gallic acid | 19.3 ± 1.7 d | 29.7 ± 1.0 c |
| Cyanidin-3,5-O-diglucoside | 32.3 ± 1.6 c | 18.9 ± 1.5 d |
| Corilagin | 20.6 ± 1.3 d | 28.0 ± 5.7 c |
| Rutin | 6.3 ± 1.7 e | 0.7 ± 4.1 e |
| Ellagic acid | 19.0 ± 0.3 d | 17.9 ± 1.0 d |
| R. damascena extract | 93.9 ± 1.8 a | 95.6 ± 0.0 a |
| Samples | Anti-Collagenase Activity (%) | Anti-Elastase Activity (%) | Anti-Hyaluronidase Activity (%) |
|---|---|---|---|
| EGCG | 74.7 ± 2.6 a | 46.0 ± 8.2 c | 80.6 ± 1.0 b |
| Oleanolic acid | 81.6 ± 0.8 a | 71.8 ± 0.5 a | 78.4 ± 2.0 b |
| L-ascorbic acid | 54.4 ± 2.0 c | 67.5 ± 10.6 a,b | 7.6 ± 3.6 d |
| Gallic acid | 66.4 ± 1.4 b | 68.4 ± 8.3 b | 0.9 ± 4.5 d |
| Cyanidin-3,5-O-diglucoside | 10.6 ± 0.9 d | 0.0 ± 1.4 d | 17.0 ± 5.3 c |
| Corilagin | 16.7 ± 0.5 d | 61.1 ± 2.5 a,b,c | 8.3 ± 2.8 c,d |
| Rutin | 10.8 ± 5.1 d | 0.0 ± 4.1 d | 1.6 ± 1.8 d |
| Ellagic acid | 0.0 ± 5.8 e | 0.0 ± 9.3 d | 19.6 ± 3.0 c |
| R. damascena extract | 69.5 ± 0.8 a | 75.3 ± 4.5 a,b | 89.6 ± 0.5 a |
| Chemical and Biological Properties | pH | ||
|---|---|---|---|
| 5 | 7 | 9 | |
| Chemical constituents | |||
| L-ascorbic acid | 31.4 ± 1.4 c | 50.4 ± 6.9 b | 99.9 ± 2.5 a |
| Gallic acid | 50.1 ± 1.8 b | 52.4 ± 1.3 b | 81.0 ± 2.0 a |
| Cyanidin-3,5-O-diglucoside | 52.2 ± 2.5 a | 53.0 ± 0.9 a | 22.4 ± 2.7 b |
| Corilagin | 83.6 ± 1.2 b | 86.4 ± 1.9 b | 110.3 ± 4.9 a |
| Rutin | 91.1 ± 1.3 b | 104.0 ± 1.8 a | 103.8 ± 1.1 a |
| Ellagic acid | 64.8 ± 2.9 b | 74.9 ± 3.1 a | 74.4 ± 0.2 a |
| Biological activities | |||
| DPPH• inhibition | 68.1 ± 9.2 b | 83.9 ± 4.9 a,b | 96.5 ± 3.7 a |
| ABTS•+ inhibition | 99.6 ± 0.2 b | 104.3 ± 0.6 a | 103.6 ± 0.2 a |
| Reducing power | 102.7 ± 1.0 b | 112.7 ± 1.0 a | 87.2 ± 0.3 c |
| Anti-tyrosinase (L-tyrosine) | 70.6 ± 5.6 b | 69.1 ± 4.6 b | 91.6 ± 8.4 a |
| Anti-tyrosinase (L-DOPA) | 72.7 ± 6.2 | 60.9 ± 2.3 | 70.4 ± 6.2 |
| Collagenase inhibition | 95.8 ± 3.1 | 99.5 ± 1.9 | 101.3 ± 3.0 |
| Elastase inhibition | 93.9 ± 2.7 | 94.3 ± 4.6 | 100.1 ± 3.2 |
| Hyaluronidase inhibition | 89.7 ± 5.6 | 87.8 ± 9.8 | 82.9 ± 9.7 |
| Chemical and Biological Properties | Temperature (°C) | ||
|---|---|---|---|
| 4 | 30 | 45 | |
| Chemical constituents | |||
| L-ascorbic acid | 60.4 ± 5.9 a | 31.4 ± 1.4 b | 28.1 ± 3.8 b |
| Gallic acid | 46.4 ± 2.0 | 50.1 ± 1.8 | 47.3 ± 0.2 |
| Cyanidin-3,5-O-diglucoside | 61.3 ± 3.6 a | 52.2 ± 2.5 a,b | 50.9 ± 5.2 b |
| Corilagin | 87.7 ± 2.2 | 83.6 ± 1.2 | 85.0 ± 5.2 |
| Rutin | 90.7 ± 2.1 | 91.1 ± 1.3 | 88.6 ± 1.6 |
| Ellagic acid | 106.6 ± 2.2 a | 64.8 ± 2.9 b | 28.5 ± 9.5 c |
| Biological activities | |||
| DPPH• inhibition | 82.3 ± 5.7 | 68.1 ± 9.2 | 68.1 ± 9.2 |
| ABTS•+ inhibition | 99.4 ± 0.2 | 99.6 ± 0.2 | 99.5 ± 0.1 |
| Reducing power | 100.9 ± 0.3 b | 102.7 ± 1.0 a | 97.1 ± 0.6 c |
| Anti-tyrosinase (L-tyrosine) | 74.7 ± 3.1 | 70.6 ± 5.6 | 68.6 ± 5.7 |
| Anti-tyrosinase (L-DOPA) | 85.8 ± 7.3 a | 72.7 ± 6.2 a,b | 63.8 ± 8.6 b |
| Collagenase inhibition | 98.9 ± 2.4 | 95.8 ± 3.1 | 95.2 ± 3.5 |
| Elastase inhibition | 91.9 ± 0.7 a | 93.9 ± 2.7 a | 77.9 ± 5.0 b |
| Hyaluronidase inhibition | 92.2 ± 6.0 | 89.7 ± 5.6 | 87.6 ± 6.5 |
| Chemical and Biological Properties | Condition | |
|---|---|---|
| Light Exposure (Light) | Light-Protected (Dark) | |
| Chemical constituents | ||
| L-ascorbic acid | 31.4 ± 1.4 | 58.9 ± 1.9 *** |
| Gallic acid | 50.1 ± 1.8 | 56.1 ± 1.8 * |
| Cyanidin-3,5-O-diglucoside | 52.2 ± 2.5 | 51.3 ± 5.2 |
| Corilagin | 83.6 ± 1.2 | 81.5 ± 2.7 |
| Rutin | 91.1 ± 1.3 | 96.7 ± 1.9 * |
| Ellagic acid | 64.8 ± 2.9 | 74.3 ± 2.7 * |
| Biological activities | ||
| DPPH• inhibition | 68.1 ± 9.2 | 101.4 ± 5.8 *** |
| ABTS•+ inhibition | 99.6 ± 0.2 | 99.4 ± 0.0 |
| Reducing power | 102.7 ± 1.0 | 106.9 ± 0.4 |
| Anti-tyrosinase (L-tyrosine) | 70.6 ± 5.6 | 82.1 ± 1.9 ** |
| Anti-tyrosinase (L-DOPA) | 72.7 ± 6.2 | 83.4 ± 8.0 |
| Collagenase inhibition | 95.8 ± 3.1 | 94.9 ± 3.5 |
| Elastase inhibition | 93.9 ± 2.7 | 77.9 ± 5.0 * |
| Hyaluronidase inhibition | 89.7 ± 5.6 | 87.3 ± 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopharadee, S.; Kittipitchakul, J.; Srisawas, N.; Neimkhum, W.; Yawootti, A.; Rades, T.; Chaiyana, W. Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability. Antioxidants 2025, 14, 541. https://doi.org/10.3390/antiox14050541
Sopharadee S, Kittipitchakul J, Srisawas N, Neimkhum W, Yawootti A, Rades T, Chaiyana W. Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability. Antioxidants. 2025; 14(5):541. https://doi.org/10.3390/antiox14050541
Chicago/Turabian StyleSopharadee, Sawat, Jutinat Kittipitchakul, Nutnaree Srisawas, Waranya Neimkhum, Artit Yawootti, Thomas Rades, and Wantida Chaiyana. 2025. "Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability" Antioxidants 14, no. 5: 541. https://doi.org/10.3390/antiox14050541
APA StyleSopharadee, S., Kittipitchakul, J., Srisawas, N., Neimkhum, W., Yawootti, A., Rades, T., & Chaiyana, W. (2025). Green Approach for Rosa damascena Mill. Petal Extract: Insights into Phytochemical Composition, Anti-Aging Potential, and Stability. Antioxidants, 14(5), 541. https://doi.org/10.3390/antiox14050541









