Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions
Abstract
1. Introduction
2. Hyperactivity of NADPH Oxidase in Neurodegenerative Diseases
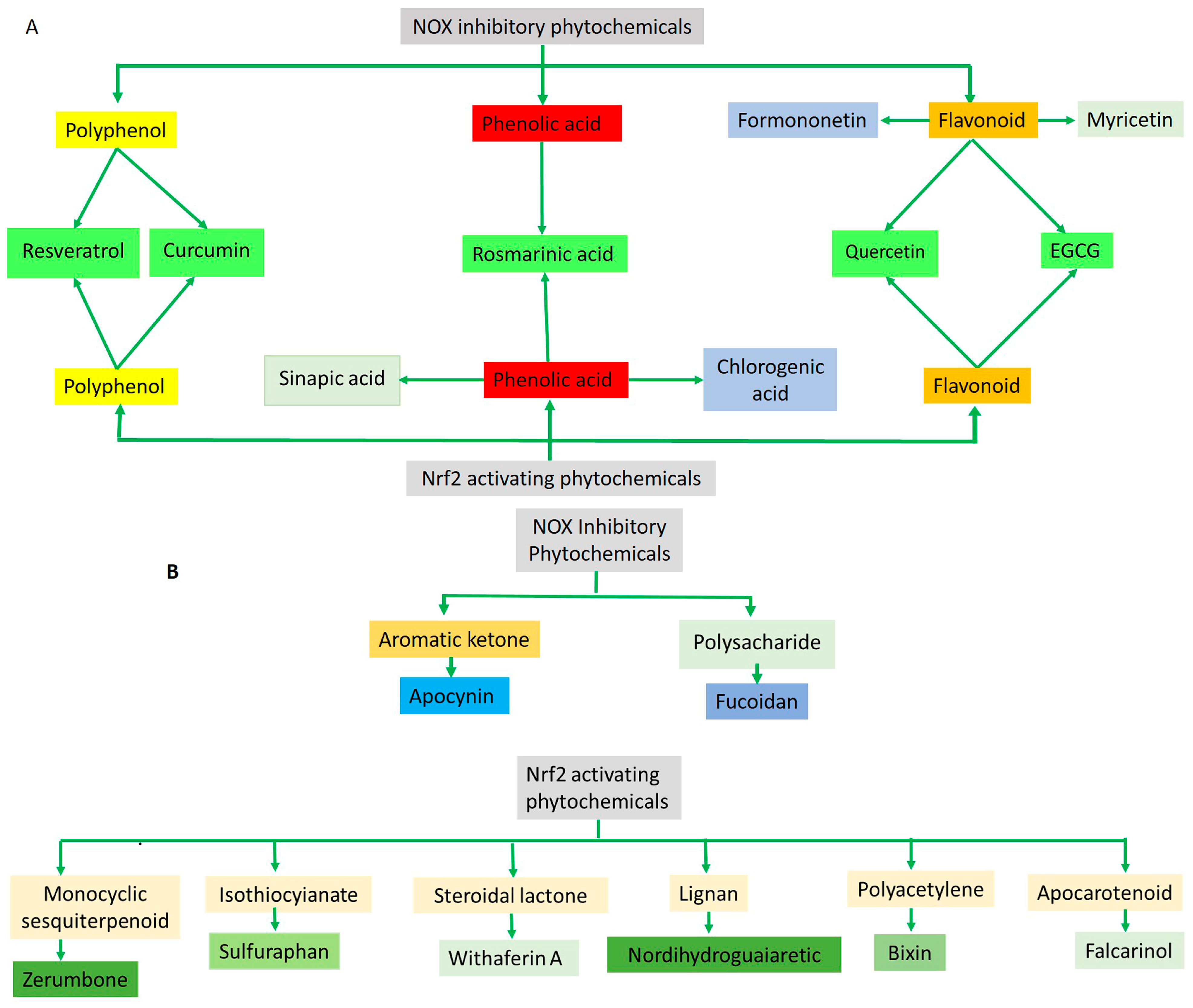
Therapeutic Effects of Phytochemicals Through NADPH Oxidase Inhibition
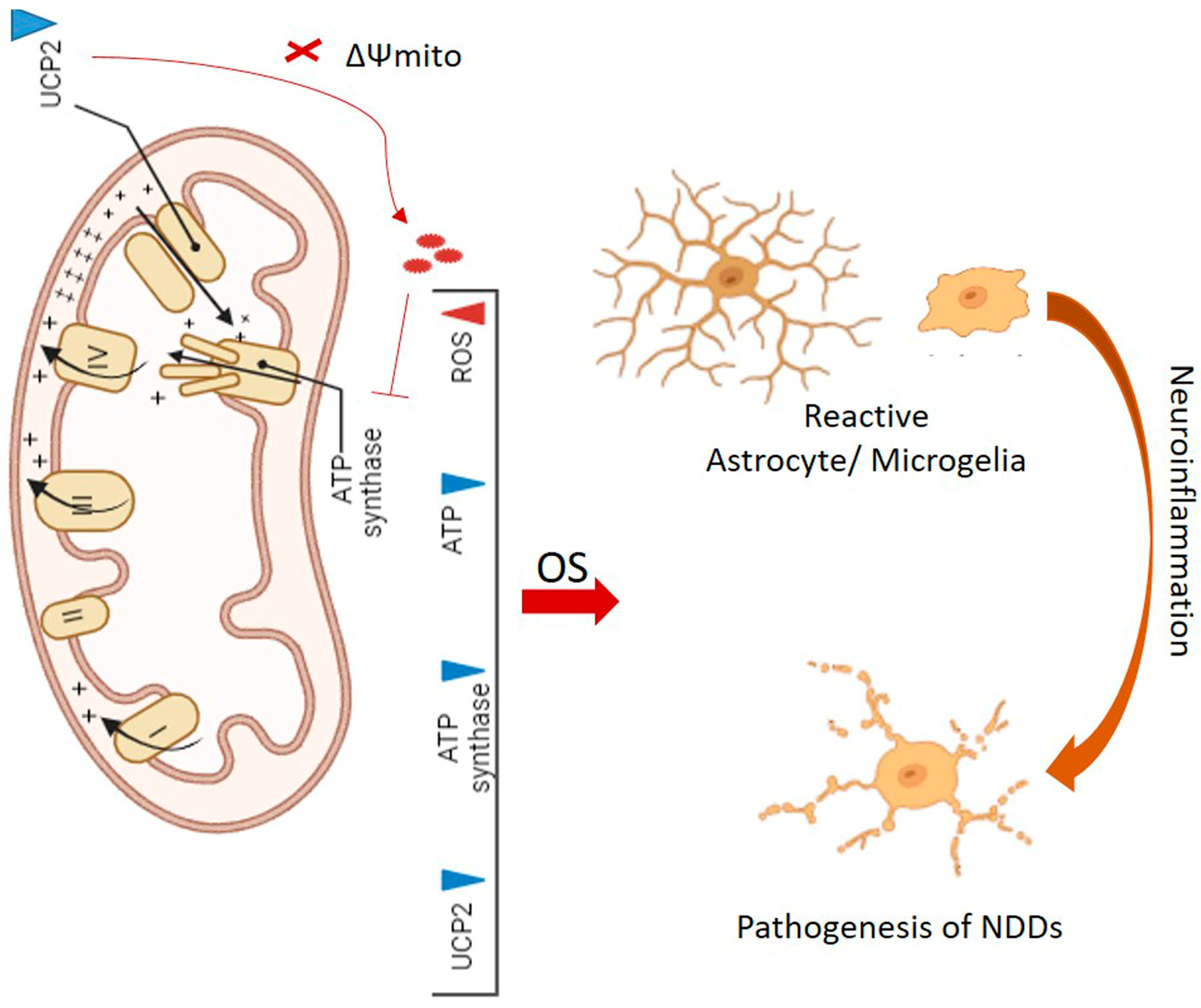
3. Downregulation of Mitochondrial UCPs in Neurodegenerative Diseases
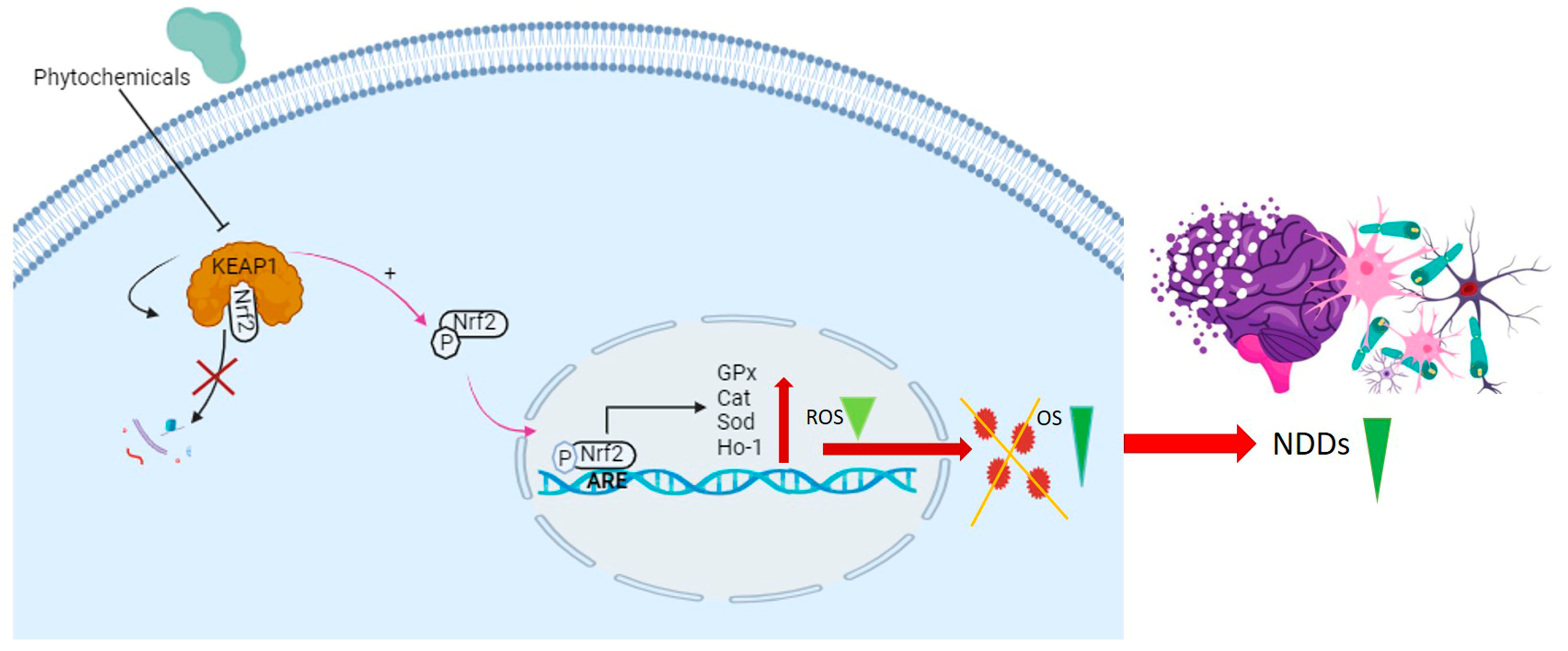
4. Cellular Antioxidant Defense Mechanisms
4.1. KEAP1/Nrf2/ARE Signaling Pathway
4.2. Therapeutic Effects of Phytochemicals Through Nrf2 Activation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 416763. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Shibata, M.; Shimizu, T.; Shibata, S.; Toriumi, H.; Ebine, T.; Kuroi, T.; Iwashita, T.; Funakubo, M.; Kayama, Y. Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. Neuroscience 2013, 248, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Li, J.; O, W.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Magi, S.; Castaldo, P.; Macrì, M.L.; Maiolino, M.; Matteucci, A.; Bastioli, G.; Gratteri, S.; Amoroso, S.; Lariccia, V. Intracellular Calcium Dysregulation: Implications for Alzheimer’s Disease. BioMed Res. Int. 2016, 2016, 6701324. [Google Scholar] [CrossRef]
- Beccano-Kelly, D.A.; Cherubini, M.; Mousba, Y.; Cramb, K.M.L.; Giussani, S.; Caiazza, M.C.; Rai, P.; Vingill, S.; Bengoa-Vergniory, N.; Ng, B.; et al. Calcium dysregulation combined with mitochondrial failure and electrophysiological maturity converge in Parkinson’s iPSC-dopamine neurons. iScience 2023, 26, 107044. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Clark, I.A.; Vissel, B. Broader insights into understanding tumor necrosis factor and neurodegenerative disease pathogenesis infer new therapeutic approaches. J. Alzheimer’s Dis. 2021, 79, 931–948. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2013, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative stress in amyotrophic lateral sclerosis: Pathophysiology and opportunities for pharmacological intervention. Oxidative Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Robberecht, W. Oxidative stress in amyotrophic lateral sclerosis. J. Neurol. 2000, 247, I1–I6. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, J.; Li, Y.; Wu, K.; Chen, Z.; Luo, Z.; Zhang, X.; Liang, Y.; Esteban, M.A.; Zhou, Y. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef]
- Wang, P.; Deng, J.; Dong, J.; Liu, J.; Bigio, E.H.; Mesulam, M.; Wang, T.; Sun, L.; Wang, L.; Lee, A.Y.-L. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 2019, 15, e1007947. [Google Scholar] [CrossRef]
- Al Ghouleh, I.; Khoo, N.K.H.; Knaus, U.G.; Griendling, K.K.; Touyz, R.M.; Thannickal, V.J.; Barchowsky, A.; Nauseef, W.M.; Kelley, E.E.; Bauer, P.M.; et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011, 51, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mucke, H.; Schmidt, H.H.H.W. NOX Inhibitors: From Bench to Naxibs to Bedside BT—Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications. In Reactive Oxygen Species. Handbook of Experimental Pharmacology; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 145–168. ISBN 978-3-030-68510-2. [Google Scholar]
- Nauseef, W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004, 122, 277–291. [Google Scholar] [CrossRef]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Angeloni, C.; Prata, C.; Hrelia, S. NADPH oxidases: Redox regulators of stem cell fate and function. Antioxidants 2021, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Yang, C.-Z.; Wang, S.-H.; Zhang, R.-H.; Lin, J.-H.; Tian, Y.-H.; Yang, Y.-Q.; Liu, J.; Ma, Y.-X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Lordén, G.; Wozniak, J.M.; Doré, K.; Dozier, L.E.; Cates-Gatto, C.; Patrick, G.N.; Gonzalez, D.J.; Roberts, A.J.; Tanzi, R.E.; Newton, A.C. Enhanced activity of Alzheimer disease-associated variant of protein kinase Cα drives cognitive decline in a mouse model. Nat. Commun. 2022, 13, 7200. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Boillée, S.; Cleveland, D.W. Revisiting oxidative damage in ALS: Microglia, Nox, and mutant SOD1. J. Clin. Investig. 2008, 118, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.E.; Patani, R. The microglial component of amyotrophic lateral sclerosis. Brain 2020, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.H.; Fathy, N.; Kortam, M.A.; Rabie, M.A.; Mohamed, A.F.; Kamel, A.S. Vildagliptin attenuates Huntington’s disease through activation of GLP-1 receptor/PI3K/Akt/BDNF pathway in 3-nitropropionic acid rat model. Neurotherapeutics 2020, 17, 252–268. [Google Scholar] [CrossRef]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A Mitochondria-Associated Oxidative Stress Perspective on Huntington’s Disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ratan, R.R. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J. Huntingtons. Dis. 2016, 5, 217–237. [Google Scholar] [CrossRef]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef]
- Distéfano-Gagné, F.; Bitarafan, S.; Lacroix, S.; Gosselin, D. Roles and regulation of microglia activity in multiple sclerosis: Insights from animal models. Nat. Rev. Neurosci. 2023, 24, 397–415. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Yenari, M.A.; Lee, J.E. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep. 2019, 7, 59–69. [Google Scholar] [CrossRef]
- ’t Hart, B.A.; Copray, S.; Philippens, I. Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed Res. Int. 2014, 2014, 298020. [Google Scholar]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, M.; Ianni, F.; Sardella, R.; Di Bona, S.; Cossignani, L.; Germani, R.; Tiecco, M.; Clementi, C. Effective and selective extraction of quercetin from onion (Allium cepa L.) skin waste using water dilutions of acid-based deep eutectic solvents. Materials 2021, 14, 6465. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Landreth, G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflammation 2006, 3, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Zielińska, D.; Setzer, W.N. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef]
- Ghasemi, F.; Bagheri, H.; Barreto, G.E.; Read, M.I.; Sahebkar, A. Effects of curcumin on microglial cells. Neurotox. Res. 2019, 36, 12–26. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Cremonini, E.; Oteiza, P.I. (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells. Arch. Biochem. Biophys. 2018, 646, 55–63. [Google Scholar] [CrossRef]
- Shaki, F.; Shayeste, Y.; Karami, M.; Akbari, E.; Rezaei, M.; Ataee, R. The effect of epicatechin on oxidative stress and mitochondrial damage induced by homocycteine using isolated rat hippocampus mitochondria. Res. Pharm. Sci. 2017, 12, 119–127. [Google Scholar]
- Wang, J.; Ferruzzi, M.G.; Ho, L.; Blount, J.; Janle, E.M.; Gong, B.; Pan, Y.; Gowda, G.A.N.; Raftery, D.; Arrieta-Cruz, I. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 2012, 32, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Wang, M.-H.; Chang, K.-C.; Soung, H.-S.; Fang, C.-H.; Lin, Y.-W.; Li, K.-Y.; Yang, C.-C.; Tsai, C.-C. Protective Effect of (−)Epigallocatechin-3-gallate on Rotenone-Induced Parkinsonism-like Symptoms in Rats. Neurotox. Res. 2020, 37, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kühne, B.A.; Barenys, M.; Sánchez-López, E.; Souto, E.B.; García, M.L. Epigallocatechin-3-gallate PEGylated poly (lactic-co-glycolic) acid nanoparticles mitigate striatal pathology and motor deficits in 3-nitropropionic acid intoxicated mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. In Proceedings of the E3S Web of Conferences; EDP Sciences, Sanya, China, 18–29 August 2021; Volume 247, p. 1063. [Google Scholar]
- Tsai, M.-H.; Hsu, L.-F.; Lee, C.-W.; Chiang, Y.-C.; Lee, M.-H.; How, J.-M.; Wu, C.-M.; Huang, C.-L.; Lee, I.-T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 2021, 88, 108552. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Zhang, F.-L.; He, Y.; Zheng, Y.; Zhang, W.-J.; Wang, Q.; Jia, Y.-J.; Song, H.-L.; An, H.-T.; Zhang, H.-B.; Qian, Y.-J.; et al. Therapeutic Effects of Fucoidan in 6-Hydroxydopamine-Lesioned Rat Model of Parkinson’s disease: Role of NADPH oxidase-1. CNS Neurosci. Ther. 2014, 20, 1036–1044. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Han, X.; Ma, Y.; Zhang, Z.; Zhao, L.; Guan, F.; Ma, S. Fucoidan: A promising agent for brain injury and neurodegenerative disease intervention. Food Funct. 2021, 12, 3820–3830. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of oxidative stress and misfolded proteins in the mechanism of neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Thadathil, N.; Hori, R.; Xiao, J.; Khan, M.M. DNA double-strand breaks: A potential therapeutic target for neurodegenerative diseases. Chromosom. Res. 2019, 27, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.H.; Frank, D.D.; Özdinler, P.H. Could Dysregulation of UPS be a Common Underlying Mechanism for Cancer and Neurodegeneration? Lessons from UCHL1. Cell Biochem. Biophys. 2013, 67, 45–53. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.-E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Monteiro, B.S.; Freire-Brito, L.; Carrageta, D.F.; Oliveira, P.F.; Alves, M.G. Mitochondrial uncoupling proteins (UCPs) as key modulators of ROS homeostasis: A crosstalk between diabesity and male infertility? Antioxidants 2021, 10, 1746. [Google Scholar] [CrossRef]
- Ho, P.W.L.; Ho, J.W.M.; Liu, H.-F.; So, D.H.F.; Tse, Z.H.M.; Chan, K.-H.; Ramsden, D.B.; Ho, S.-L. Mitochondrial neuronal uncoupling proteins: A target for potential disease-modification in Parkinson’s disease. Transl. Neurodegener. 2012, 1, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Amruthanjali, T.; Singothu, S.; Singh, S.B.; Bhandari, V. Uncoupling proteins as a therapeutic target for the development of new era drugs against neurodegenerative disorder. Biomed. Pharmacother. 2022, 147, 112656. [Google Scholar] [CrossRef]
- Derdak, Z.; Mark, N.M.; Beldi, G.; Robson, S.C.; Wands, J.R.; Baffy, G. The Mitochondrial Uncoupling Protein-2 Promotes Chemoresistance in Cancer Cells. Cancer Res. 2008, 68, 2813–2819. [Google Scholar] [CrossRef]
- Tian, X.Y.; Ma, S.; Tse, G.; Wong, W.T.; Huang, Y. Uncoupling protein 2 in cardiovascular health and disease. Front. Physiol. 2018, 9, 388072. [Google Scholar] [CrossRef]
- Laskowski, K.R.; Russell, R.R., III. Uncoupling proteins in heart failure. Curr. Heart Fail. Rep. 2008, 5, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.; Zhao, Y. New aspects of mitochondrial uncoupling proteins (UCPs) and their roles in tumorigenesis. Int. J. Mol. Sci. 2011, 12, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Uncoupling protein-2 and cancer. Mitochondrion 2010, 10, 243–252. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling protein 2 in the glial response to stress: Implications for neuroprotection. Neural Regen. Res. 2016, 11, 1197–1200. [Google Scholar] [PubMed]
- So, S.W.; Fleming, K.M.; Duffy, C.M.; Nixon, J.P.; Bernlohr, D.A.; Butterick, T.A. Microglial FABP4-UCP2 axis modulates neuroinflammation and cognitive decline in obese mice. Int. J. Mol. Sci. 2022, 23, 4354. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, S.; Wen, H.; Liu, T.; Cai, J.; Du, D.; Zhu, D.; Chen, F.; Xia, C. Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLoS ONE 2019, 14, e0212138. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Steinert, J.R.; Amal, H. The contribution of an imbalanced redox signalling to neurological and neurodegenerative conditions. Free Radic. Biol. Med. 2023, 194, 71–83. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO signaling pathways as therapeutic targets in cancer. Int. J. Biol. Sci. 2017, 13, 815. [Google Scholar] [CrossRef]
- Oli, V.; Gupta, R.; Kumar, P. FOXO and related transcription factors binding elements in the regulation of neurodegenerative disorders. J. Chem. Neuroanat. 2021, 116, 102012. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in NRF2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Collinson, E.J.; Wimmer-Kleikamp, S.; Gerega, S.K.; Yang, Y.H.; Parish, C.R.; Dawes, I.W.; Stocker, R. The Yeast Homolog of Heme Oxygenase-1 Affords Cellular Antioxidant Protection via the Transcriptional Regulation of Known Antioxidant Genes*. J. Biol. Chem. 2011, 286, 2205–2214. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Miriyala, S.; Holley, A.K.; St Clair, D.K. Mitochondrial superoxide dismutase-signals of distinction. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2011, 11, 181–190. [Google Scholar] [CrossRef]
- Cristalli, D.O.; Arnal, N.; Marra, F.A.; de Alaniz, M.J.T.; Marra, C.A. Peripheral markers in neurodegenerative patients and their first-degree relatives. J. Neurol. Sci. 2012, 314, 48–56. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. Catalase Activity Assays. Curr. Protoc. Toxicol. 2006, 27, 7.7.1–7.7.16. [Google Scholar] [CrossRef]
- Nazıroğlu, M. Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. 2012, 32, 134–141. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Conrad, M. Oxidative stress, selenium redox systems including GPX/TXNRD families. In Selenium; Michalke, B., Ed.; Springer: Cham, Switzerland, 2018; pp. 111–135. [Google Scholar]
- Mason, R.P.; Casu, M.; Butler, N.; Breda, C.; Campesan, S.; Clapp, J.; Green, E.W.; Dhulkhed, D.; Kyriacou, C.P.; Giorgini, F. Glutathione peroxidase activity is neuroprotective in models of Huntington’s disease. Nat. Genet. 2013, 45, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Pong, K. Oxidative stress in neurodegenerative diseases: Therapeutic implications for superoxide dismutase mimetics. Expert. Opin. Biol. Ther. 2003, 3, 127–139. [Google Scholar] [CrossRef]
- Schipper, H.M. Glial HO-1 expression, iron deposition and oxidative stress in neurodegenerative diseases. Neurotox. Res. 1999, 1, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.; Albrecht, J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem. Int. 2015, 88, 66–72. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Argellati, F.; Pronzato, M.A.; Domenicotti, C. Vitamin E and neurodegenerative diseases. Mol. Asp. Med. 2007, 28, 591–606. [Google Scholar] [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 Controls Postinduction Repression of the Nrf2-Mediated Antioxidant Response by Escorting Nuclear Export of Nrf2. Mol. Cell. Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Reisman, S.A.; Yeager, R.L.; Yamamoto, M.; Klaassen, C.D. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol. Sci. 2009, 108, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Wu, K.C.; McDonald, P.R.; Liu, J.; Klaassen, C.D. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med. 2014, 80, 97–104. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Iranshahi, M.; Hasheminezhad, S.H.; Hayes, A.W.; Karimi, G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phyther. Res. 2019, 33, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Mei, X.; Cheng, Z.; Tian, X.; Hu, J.; Zang, C.; Sun, B.; Wu, J.; Deng, Y.; Ghiladi, R.A. Extraction of weak hydrophobic sulforaphane from broccoli by salting-out assisted hydrophobic deep eutectic solvent extraction. Food Chem. 2023, 405, 134817. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef]
- Stefanson, A.L.; Bakovic, M. Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients 2014, 6, 3777–3801. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 regulation by curcumin: Molecular aspects for therapeutic prospects. Molecules 2022, 27, 167. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Sánchez-Ballester, N.M.; Blázquez, M.A. Healthy zerumbone: From natural sources to strategies to improve its bioavailability and oral administration. Plants 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-L.; Huang, B.-R.; Chen, G.-W.; Charoensaensuk, V.; Tsai, C.-F.; Yang, L.-Y.; Lu, D.-Y.; Chen, M.-K.; Lin, C. Role of zerumbone, a phytochemical sesquiterpenoid from Zingiber zerumbet Smith, in maintaining macrophage polarization and redox homeostasis. Nutrients 2022, 14, 5402. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef]
- Alfurayhi, R.; Huang, L.; Brandt, K. Pathways affected by falcarinol-type polyacetylenes and implications for their anti-inflammatory function and potential in cancer chemoprevention. Foods 2023, 12, 1192. [Google Scholar] [CrossRef]
- Li, Y.; Tan, W.-L.; Guo, K.; Gao, X.-W.; Wei, J.; Yi, D.; Zhang, C.; Wang, Q. Synthesis and Biological Evaluation of Falcarinol-Type Analogues as Potential Calcium Channel Blockers. J. Nat. Prod. 2021, 84, 2138–2148. [Google Scholar] [CrossRef]
- Rahman, S.; Ansari, R.A.; Rehman, H.; Parvez, S.; Raisuddin, S. Nordihydroguaiaretic acid from creosote bush (Larrea tridentata) mitigates 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory and oxidative stress responses of tumor promotion cascade in mouse skin. Evid.-Based Complement. Altern. Med. 2011, 2011, 734785. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Beltrán, S.; Espada, S.; Orozco-Ibarra, M.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci. Lett. 2008, 447, 167–171. [Google Scholar] [CrossRef]
- Tao, S.; Park, S.L.; de la Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Shadisvaaran, S.; Chin, K.-Y.; Mohd-Said, S.; Leong, X.-F. Therapeutic potential of bixin on inflammation: A mini review. Front. Nutr. 2023, 10, 1209248. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; vel Szic, K.S.; Vander Veken, P.; Lemmens, K.J.A.; Lahtela-Kakkonen, M.; Naulaerts, S.; de Beeck, K.O.; Laukens, K. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol. 2016, 109, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Mishra, G.; İlgün, S.; Samarghandian, S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phyther. Res. 2021, 35, 3078–3112. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Green tea epigallocatechin-3-gallate (EGCG) targeting protein misfolding in drug discovery for neurodegenerative diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, H.N.T.; Huang, M.-Y.; Lin, K.-H.; Pham, D.-C.; Tran, Y.B.; Su, C.-H. Optimization of aqueous enzyme-assisted extraction of rosmarinic acid from rosemary (Rosmarinus officinalis L.) leaves and the antioxidant activity of the extract. J. Food Process. Preserv. 2021, 45, e15221. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Ravaria, P.; Saxena, P.; Laksmi BS, S.; Ranjan, V.; Abidi, S.W.F.; Saha, P.; Ramamoorthy, S.; Ahmad, F.; Rana, S.S. Molecular mechanisms of neuroprotective offerings by rosmarinic acid against neurodegenerative and other CNS pathologies. Phyther. Res. 2023, 37, 2119–2143. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Kanomata, T.; Yoshitama, K. Flavonoids in the leaves of twenty-eight polygonaceous plants. Bot. Mag. Shokubutsu Gaku Zasshi 1986, 99, 63–74. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Peng, A.; Zhang, L.; Xiang, J.; Cao, X.; Ding, H.; Yin, S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016, 7, 2624–2634. [Google Scholar] [CrossRef]
- Joshi, V.; Mishra, R.; Upadhyay, A.; Amanullah, A.; Poluri, K.M.; Singh, S.; Kumar, A.; Mishra, A. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J. Cell. Physiol. 2019, 234, 20900–20914. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N. Neuropharmacological effects of quercetin: A literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 392169. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.H.; Cho, D.; Kim, T.S. Formononetin, a phyto-oestrogen, and its metabolites up-regulate interleukin-4 production in activated T cells via increased AP-1 DNA binding activity. Immunology 2005, 116, 71–81. [Google Scholar] [CrossRef]
- Fang, Y.; Ye, J.; Zhao, B.; Sun, J.; Gu, N.; Chen, X.; Ren, L.; Chen, J.; Cai, X.; Zhang, W.; et al. Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 2020, 36, 101677. [Google Scholar] [CrossRef]
- Singh, L.; Kaur, H.; Chandra Arya, G.; Bhatti, R. Neuroprotective potential of formononetin, a naturally occurring isoflavone phytoestrogen. Chem. Biol. Drug Des. 2024, 103, e14353. [Google Scholar] [CrossRef]
- Kim, M.-S.; Shin, W.-C.; Kang, D.-K.; Sohn, H.-Y. Anti-thrombosis activity of sinapic acid isolated from the lees of bokbunja wine. J. Microbiol. Biotechnol. 2016, 26, 61–65. [Google Scholar] [CrossRef]
- Alaofi, A.L. Sinapic acid ameliorates the progression of streptozotocin (STZ)-induced diabetic nephropathy in rats via NRF2/HO-1 mediated pathways. Front. Pharmacol. 2020, 11, 540139. [Google Scholar] [CrossRef]
- Lee, I.-S.; Choi, G.-Y.; Sreelatha, I.; Yoon, J.-W.; Youn, S.-H.; Maeng, S.; Park, J.-H. Effect of sinapic acid on scopolamine-induced learning and memory impairment in SD rats. Brain Sci. 2023, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Anggreani, E.; Lee, C.Y. Neuroprotective effect of chlorogenic acids against Alzheimer’s disease. Int. J. Food Sci. Nutr. Diet. 2017, 6, 330–337. [Google Scholar]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021, 193, 113704. [Google Scholar] [CrossRef]
- Liu, X.-P.; Goldring, C.E.P.; Copple, I.M.; Wang, H.-Y.; Wei, W.; Kitteringham, N.R.; Park, B.K. Extract of Ginkgo biloba induces phase 2 genes through Keap1-Nrf2-ARE signaling pathway. Life Sci. 2007, 80, 1586–1591. [Google Scholar] [CrossRef]
- Bridi, R.; Crossetti, F.P.; Steffen, V.M.; Henriques, A.T. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phyther. Res. 2001, 15, 449–451. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.-T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Dwivedi, S.; Nagarajan, R.; Hanif, K.; Siddiqui, H.H.; Nath, C.; Shukla, R. Standardized extract of Bacopa monniera attenuates okadaic acid induced memory dysfunction in rats: Effect on Nrf2 pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 294501. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Tocharus, J.; Janyou, A.; Jittiwat, J.; Changtam, C.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS ONE 2017, 12, e0189211. [Google Scholar] [CrossRef] [PubMed]
- Holubiec, M.I.; Alloatti, M.; Bianchelli, J.; Greloni, F.; Arnaiz, C.; Prinz, M.G.; Bessone, I.F.; Devoto, V.P.; Falzone, T.L. Mitochondrial vulnerability to oxidation in human brain organoids modelling Alzheimer’s disease. Free Radic. Biol. Med. 2023, 208, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Phaneuf, D.; Julien, J.-P. Withaferin-A Treatment Alleviates TAR DNA-Binding Protein-43 Pathology and Improves Cognitive Function in a Mouse Model of FTLD. Neurotherapeutics 2021, 18, 286–296. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Abramovič, H. Antioxidant Properties of Hydroxycinnamic Acid Derivatives: A Focus on Biochemistry, Physicochemical Parameters, Reactive Species, and Biomolecular Interactions. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 843–852. ISBN 978-0-12-409517-5. [Google Scholar]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial effects of epigallocatechin-3-O-gallate, chlorogenic acid, resveratrol, and curcumin on neurodegenerative diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Simpson, T.; Pase, M.; Stough, C. Bacopa monnieri as an antioxidant therapy to reduce oxidative stress in the aging brain. Evid.-Based Complement. Altern. Med. 2015, 2015, 615384. [Google Scholar] [CrossRef]

| Name of Phytochemicals and Chemical Structures | Source of Phytochemicals | Molecular Mechanisms and Type of Study | References |
|---|---|---|---|
| Apocynin (Aromatic Ketone)  | It is found in the roots of plants of the Apocynaceae family. Commonly isolated from the roots of the Coral plant (Jatropha multifidi L.). | Inhibits the assembly of NADPH oxidase subunits; reduces ROS generation in microglial cells, thereby preventing neurodegenerative diseases (in vivo study). | [41,42] |
| Quercetin (Flavonoid) 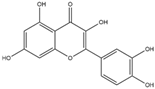 | It is distributed in various fruits and vegetables such as citrus, apples, dark berries, and onions. Discarded outer layers of onions (Allium cepa L.) are a sustainable and economically viable source for the isolation of this bioactive compound. | Inhibits NADPH oxidase through the downregulation of the P47phox subunit; mitigates reactive oxygen species (ROS) generation and mitochondrial dysfunction, thereby attenuating neuroinflammation-induced neurodegenerative diseases (in vivo and clinical studies). | [44,46] |
| Curcumin (Polyphenol)  | It is a bioactive compound naturally found in Indian spices, and can be isolated from the roots of turmeric, a staple in Indian cuisine (Curcuma longa L.) | Inhibits NADPH oxidase, suppresses microglial transformation, and reduces ROS generation, thereby preventing neurodegenerative diseases (in vivo and preclinical studies). | [49,50] |
| Epigallocatechin-3-gallate (EGCG) (Flavonoid)  | It is abundantly found in green tea and can be isolated from the leaves of the tea plant (Cammellia sinesis (L.) Kuntze) | Inhibits NADPH oxidase and attenuates mitochondrial dysfunction, thereby preventing OS-induced neurodegenerative diseases (in vivo study). | [55] |
| Resveratrol (Polyphenol)  | It is naturally present in nuts, berries, grapes, and red wine. It can be isolated from the skin of dark grapes, specifically from the variety (Vitis vinifera L.). | Inhibits NADPH oxidase and subsequent generation of reactive oxygen species (ROS); suppresses reactive microglia under neuroinflammatory conditions, contributing to the prevention of NDDs (in vivo and clinical studies). | [56,58] |
Fucoidan (Polysaccharide-Containing L-Fucos and Sulfate Ester Groups) | It is a bioactive compound that can be isolated from brown seaweed and the cell walls of brown algae belonging to the Phaeophyceae class. | Inhibits NADPH oxidase-1 and subsequent ROS generation, leading to improvement in neurodegenerative processes associated with brain injury associated in PD (in vivo study). | [59,61] |
| Name of Phytochemicals and Chemical Structure | Source of Phytochemicals | Molecular Mechanisms and Type of Study | References |
|---|---|---|---|
| Sulfuraphane (Isothiocyanates)  | It is a bioactive compound naturally found in the plant family of Brassicaceae and can be isolated from broccoli (Brassica oleracea L.). | Sulforaphane can interrupt the KEAP1-Nrf2 complex through its interaction with the KEAP1 protein, thereby facilitating the nuclear translocation of Nrf2. Consequently, this process activates the Nrf2/ARE axis, contributing to the reduction of OS-induced NDDs (in vivo and clinical studies) | [109,110] |
| Curcumin (Polyphenol)  | It is a bioactive compound naturally found in Indian spices and can be isolated from the roots of turmeric, a staple in Indian cuisine (Curcuma longa L.) | Curcumin can inhibit KEAP1, leading to the upregulation of the Nrf2/HO-1 axis, and concurrently activates the synthesis of the antioxidant peptide, glutathione. This mechanism has the potential to target α-synuclein and Aβ oligomers in PD and AD, respectively (in vitro study). | [112,113] |
| Zerumbone (Monocyclic Sesquiterpenoid)  | It is a bioactive compound found in perennial herbs and can be isolated from wild ginger (Zingiber zerumbet L.). | Zerumbone can activate the Nrf2/ARE axis, inducing antioxidant genes such as HO-1, GCLC, GCLM, and NQO1. This activation contributes to the alleviation of oxidative stress and neuroinflammation-induced NDDs (in vitro and in vivo studies) | [114,115] |
| Falcarinol (Polyacetylene)  | It is a bioactive compound found in a large number of plants of the Apiaceae family and can be isolated from carrots (Daucus carota L.). | Falcarinol can induce the Nrf2/ARE axis, leading to the activation of SOD and HO-1 enzymes. Consequently, this activation causes a reduction in cellular toxins such as LDH and MDA (in vivo study) | [116,117,118] |
Nordihydroguaiaretic acid (Lignan) | It is a bioactive compound and can be isolated from the evergreen desert shrub named creosote bush (Larrea tridentata (DC.) Coville). | Nordihydroguaiaretic acid can induce the Nrf2/HO-1 axis, which leads to reduced OS-induced NDDs (in vitro and in vivo studies) | [119,120] |
| Bixin (Apocarotenoid)  | It is a natural pigment isolated from the seeds of achiote tree (Bixa orellana L.). | Bixin can induce the Nrf2/ARE axis, leading to the reduction of pro-inflammatory cytokines. This effect can contribute to the mitigation of neuroinflammation-induced NDDs (in vivo and clinical studies) | [121,122] |
| Withaferin A (Steroidal Lactone) Withanolides  | It is a bioactive compound with applications in herbal Indian medicine and can be isolated from the Indian winter cherry [Withania somnifera (L.) Dunal]. | Withaferin A can induce the Nrf2/ARE axis through its interaction with the cysteine residue of KEAP1, thereby disrupting the KEAP1-Nrf2 complex. This mechanism has ameliorating effects on brain injury related to ALS (in silico, in vitro, and in vivo studies) | [123,124] |
| Epigallocatechin-3-gallate (EGCG) (Flavonoid)  | It is a potent antioxidant found abundantly in green tea and can be isolated from the fresh leaves of the tea plant [Camellia sinensis (L.) Kuntze]. | EGCG can induce the Nrf2/ARE axis, leading to the upregulation of endogenous antioxidant genes such as HO-1 and GPx. Additionally, it can bind to Aβ and α-synuclein, reversing the neurodegenerative process (in vivo and pre-clinical studies). | [125,126,127] |
| Rosmarinic acid (Polyphenol)  | It is a bioactive compound found in the plant family of lamiaceae and can be isolated from rosemary (Rosmarinus officinalis L.). | Rosmarinic acid can induce the Nrf2/ARE axis, thereby eliciting a reduction in OS and subsequent cellular toxins. It can be a promising therapeutic agent in the context of NDDs (in vivo and clinical studies). | [128,129,130] |
| Myricetin (Flavonoid) 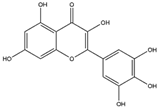 | It is a bioactive compound found in the plants of the Polygonaceae family and can be isolated from birdweed (Polygonum suffruticosum Salzm. ex Ball). | Myricetin demonstrates the capability to induce the Nrf2/ARE axis, resulting in the upregulation of serum levels of antioxidants, including CAT, SOD, and HO-1. This can contribute to the detoxification of protein aggregates in the brain associated with NDDs (in vivo and preclinical studies). | [131,132,133] |
| Quercetin (Flavonoid) 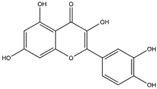 | It is a bioactive compound found in a variety of plants. It can be isolated from sustainable onion skin/peel waste (Allium cepa L.). | Quercetin exhibits the ability to induce the Nrf2/ARE axis, resulting in the upregulation of antioxidant genes such as HO-1, NQO1, and SOD2, mitigating OS. This property positions quercetin as a potential alternative therapeutic agent against NDDs (in vivo and clinical studies). | [134,135,136] |
| Resveratrol (Polyphenol) 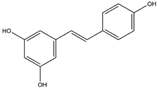 | It is a polyphenolic compound found in nuts, berries, grapes, and red wine, and can be isolated from the skin of dark grapes (Vitis vinifera L.). | Resveratrol inhibits the KEAP1 protein, facilitating the translocation of Nrf2 to the nucleus, thereby activating the Nrf2/HO-1 axis. Furthermore, the downstream cascade of Nrf2 stimulates mitochondrial biogenesis and mitigates OS-induced NDDs (in vitro and in vivo studies). | [137,138,139] |
| Formononetin (Isoflavone) 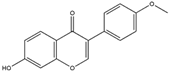 | It is a naturally occurring compound found in legumes and can be isolated from red clover (Trifolium pratense L.). | The binding of formononetin to a specific domain of KEAP1 induces a conformational change, resulting in the liberation of Nrf2 from the Nrf2-KEAP1 complex. This, in turn, leads to the induction of the Nrf2/ARE axis, promoting redox homeostasis. Additionally, formononetin demonstrates the ability to remove Aβ plaques and inhibit MAO-B activity, contributing to the preservation of dopaminergic neurons and thereby preventing NDDs (in silico and in vitro studies). | [140,141,142] |
| Sinapic Acid (Hydroxycinnamic Acid) 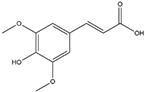 | It is a phenolic acid with antioxidant properties, and is found in coffee, tea, and wine. It can be isolated from bokunja wine, which is made from Korean bramble (Rubus coreanus Miq.). | Sinapic acid induces the Nrf2/ARE axis, leading to the upregulation of antioxidant genes such as GPx, CAT, and HO-1. Simultaneously, it inhibits the AChE enzyme and blocks cholinergic receptors, thereby mitigating NDDs (in vitro and in vivo studies). | [143,144,145] |
| Chlorogenic Acid (Cinnamate Ester) 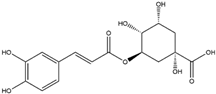 | It is a polyphenolic compound found in a variety of green and roasted coffee cultivars. It can be isolated from Coffea canephora Pierre ex A. Froehner. | Chlorogenic acid induces the Nrf2/HO-1 axis, triggering downstream cascades that stimulate the NF-κB pathway. This cascade has the potential to reverse NDDs by modulating inflammatory processes (in vitro, in vivo, and clinical studies). | [146,147,148] |
| Ginkgo Extract (Flavonoids and Terpenoids, and Lignan) 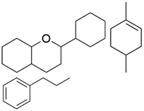 | Derived from the leaves of the Ginkgo biloba L. tree. | Ginkgo extract inhibits KEAP1, activating the Nrf2/ARE axis and upregulating antioxidant enzymes such as SOD and CAT within the hippocampus. This crucial molecular mechanism has the potential to effectively reverse NDDs (in vivo study) | [149,150,151] |
Indian Pennywort Extract (Saponin)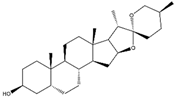 | Extracted from Indian pennywort [Bacopa monnieri (L.) Wettst.]. | Bacopa extract induces the Nrf2/ARE axis, activating antioxidant enzymes like HO-1 and GCLC. This molecular mechanism mitigates mitochondrial dysfunction and alleviates oxidative stress-induced NDDs. | [152,153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebghatollahi, Z.; Yogesh, R.; Mahato, N.; Kumar, V.; Mohanta, Y.K.; Baek, K.-H.; Mishra, A.K. Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions. Antioxidants 2025, 14, 457. https://doi.org/10.3390/antiox14040457
Sebghatollahi Z, Yogesh R, Mahato N, Kumar V, Mohanta YK, Baek K-H, Mishra AK. Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions. Antioxidants. 2025; 14(4):457. https://doi.org/10.3390/antiox14040457
Chicago/Turabian StyleSebghatollahi, Zahra, Ruchika Yogesh, Neelima Mahato, Vijay Kumar, Yugal Kishore Mohanta, Kwang-Hyun Baek, and Awdhesh Kumar Mishra. 2025. "Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions" Antioxidants 14, no. 4: 457. https://doi.org/10.3390/antiox14040457
APA StyleSebghatollahi, Z., Yogesh, R., Mahato, N., Kumar, V., Mohanta, Y. K., Baek, K.-H., & Mishra, A. K. (2025). Signaling Pathways in Oxidative Stress-Induced Neurodegenerative Diseases: A Review of Phytochemical Therapeutic Interventions. Antioxidants, 14(4), 457. https://doi.org/10.3390/antiox14040457













