Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs Through Modulation of the PERK-ATF4/MFN2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Porcine Ovarian Granulosa Cells
2.2. Cellular Viability Assay
2.3. Analysis of Intracellular ROS
2.4. qRT-PCR
2.5. Western Blotting (WB)
2.6. Measurement of Indicators of OS
2.7. Transmission Electron Microscopy
2.8. ATP Content Analysis
2.9. Measurement of the NAD+/NADH Ratio
2.10. Determination of the MMP
2.11. Analysis of Mitochondrial ROS
2.12. Measurement of Ca2+ Content
2.13. Immunofluorescence Staining
2.14. Small Interfering RNA (siRNA)
2.15. Statistical Analysis
3. Results
3.1. Modeling of OS in GCs In Vitro
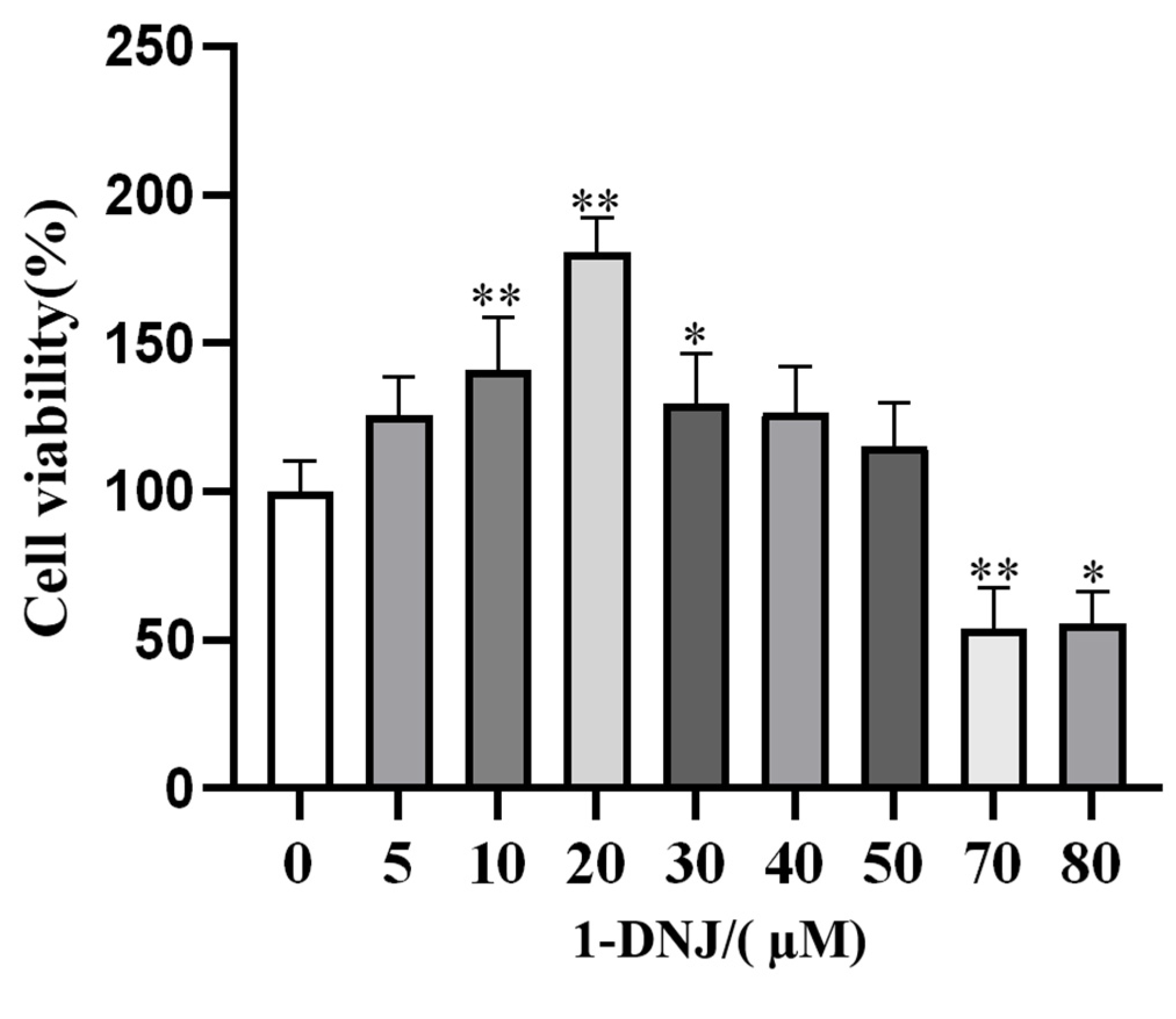
3.2. Effect of 1-DNJ on the Viability and Antioxidant Activity of GCs
3.3. Effect of 1-DNJ on H2O2-Induced OS in GCs
3.4. Effect of 1-DNJ on the Ultrastructures of Mitochondria and ER in H2O2-Treated GCs
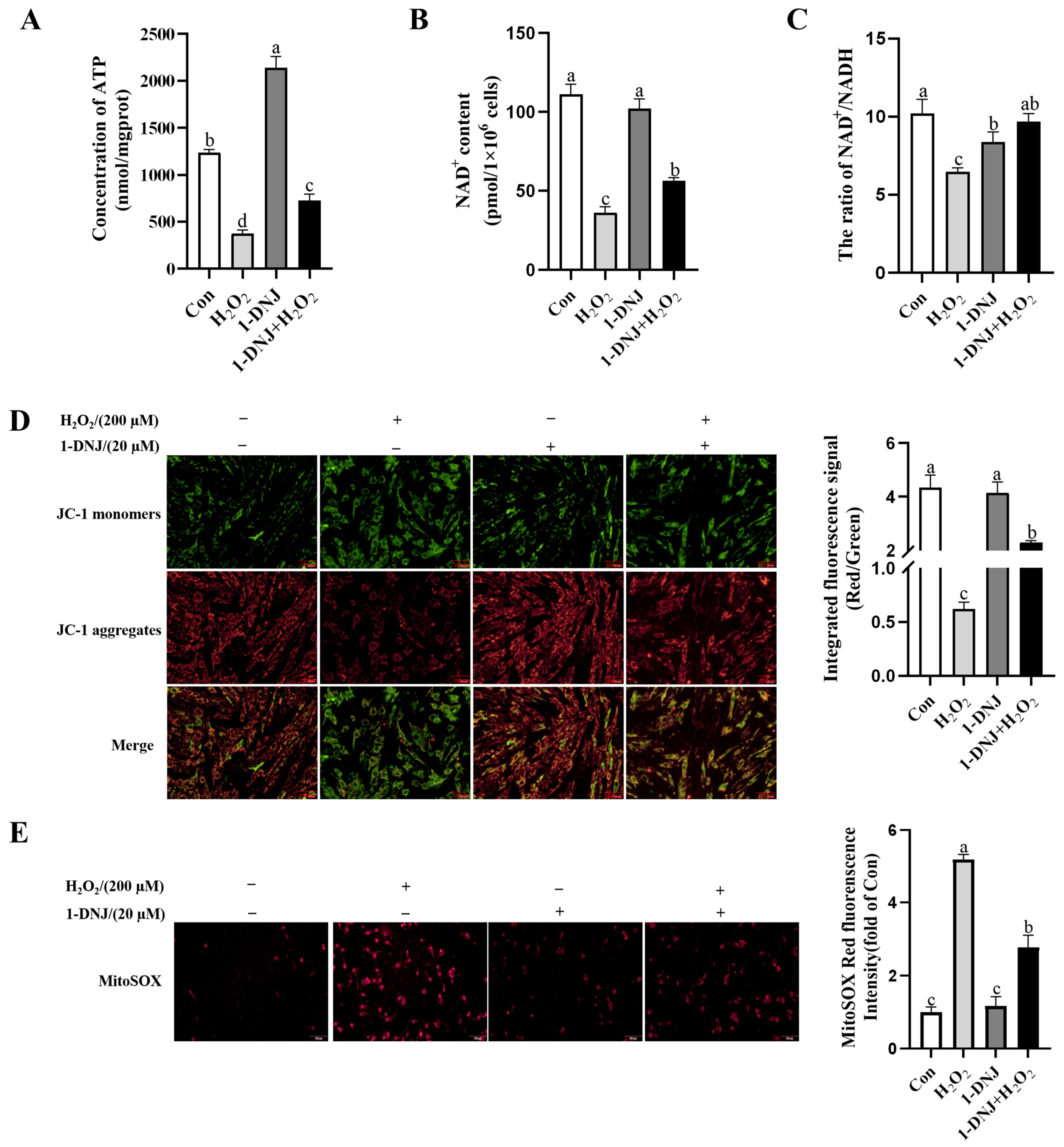
3.5. Effect of 1-DNJ on OS-Induced Mitochondrial Dysfunction in GCs
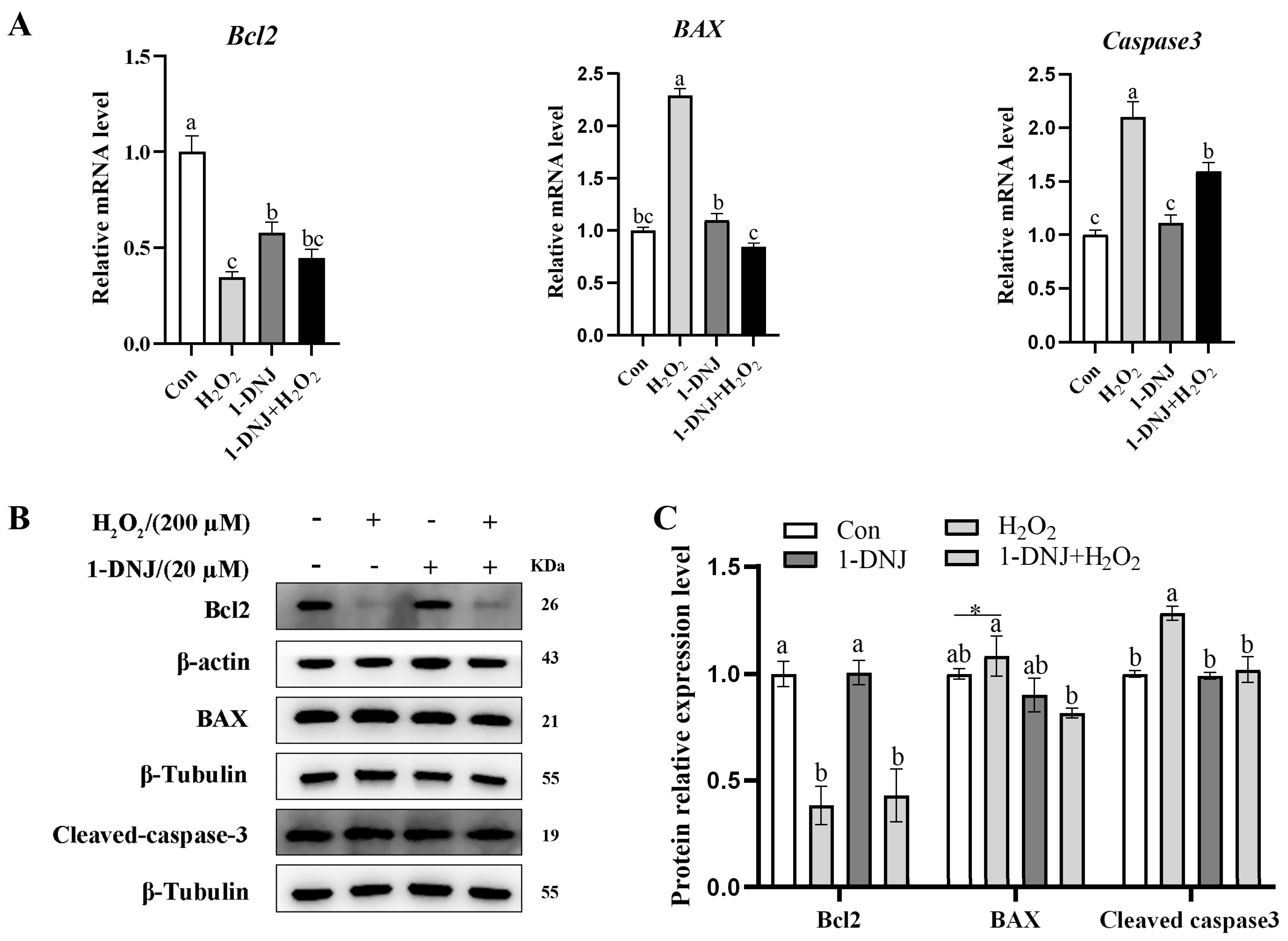
3.6. Effect of 1-DNJ on OS-Induced Apoptosis of GCs
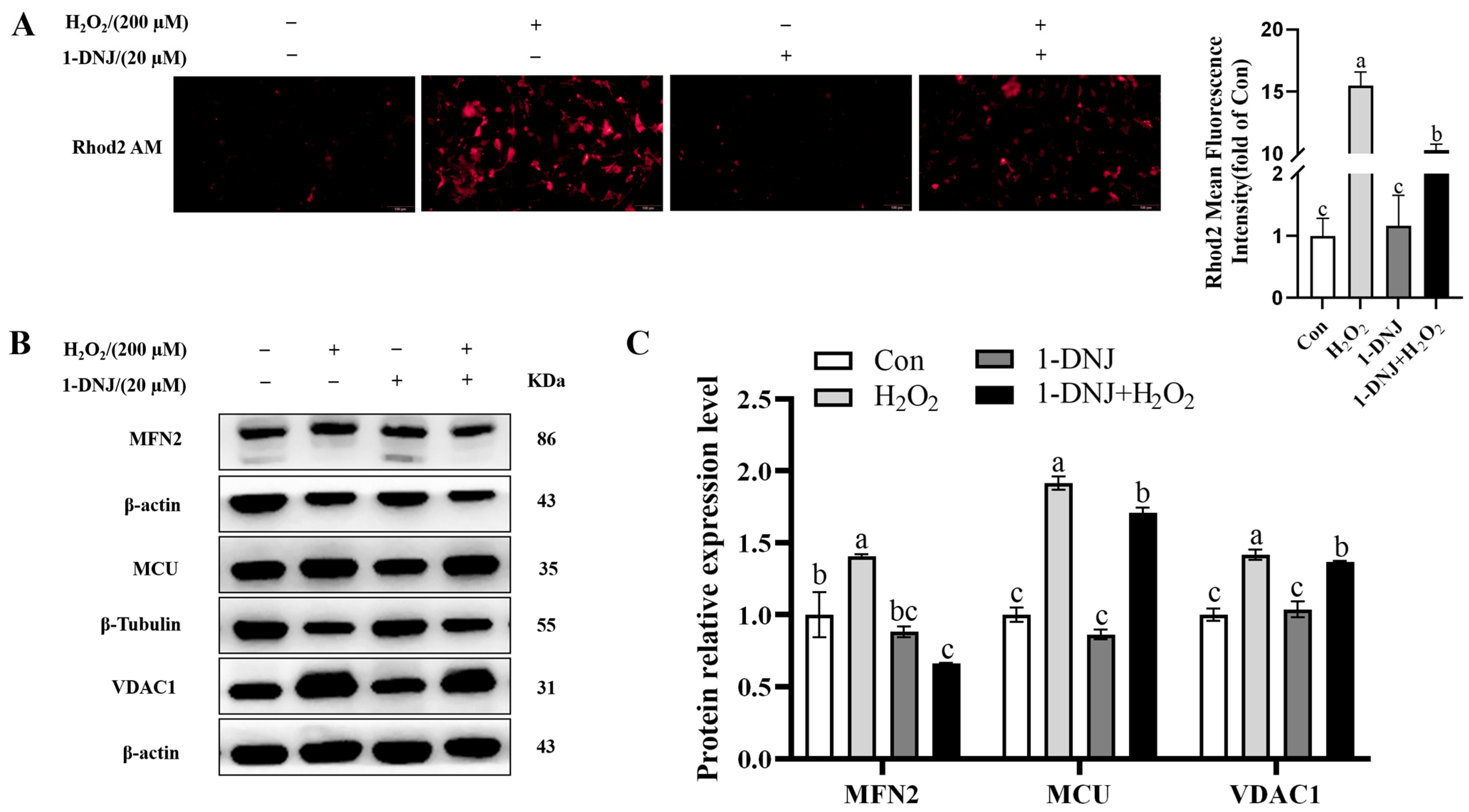
3.7. Effect of 1-DNJ on MAMs in OS-Treated GCs
3.8. Effect of 1-DNJ on OS-Induced ERS in GCs
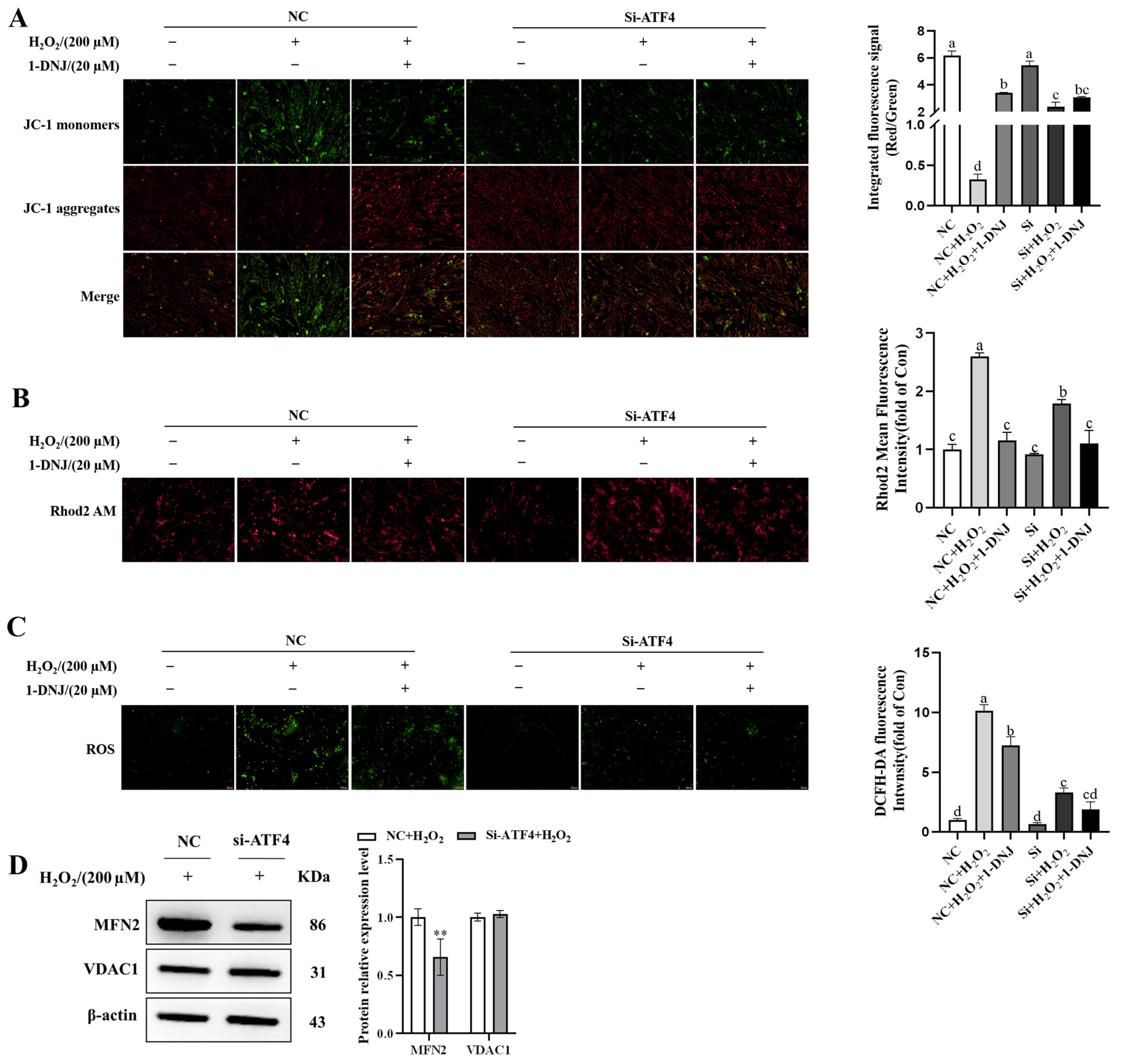
3.9. Crosstalk Between ERS, OS, and Mitochondrial Dysfunction
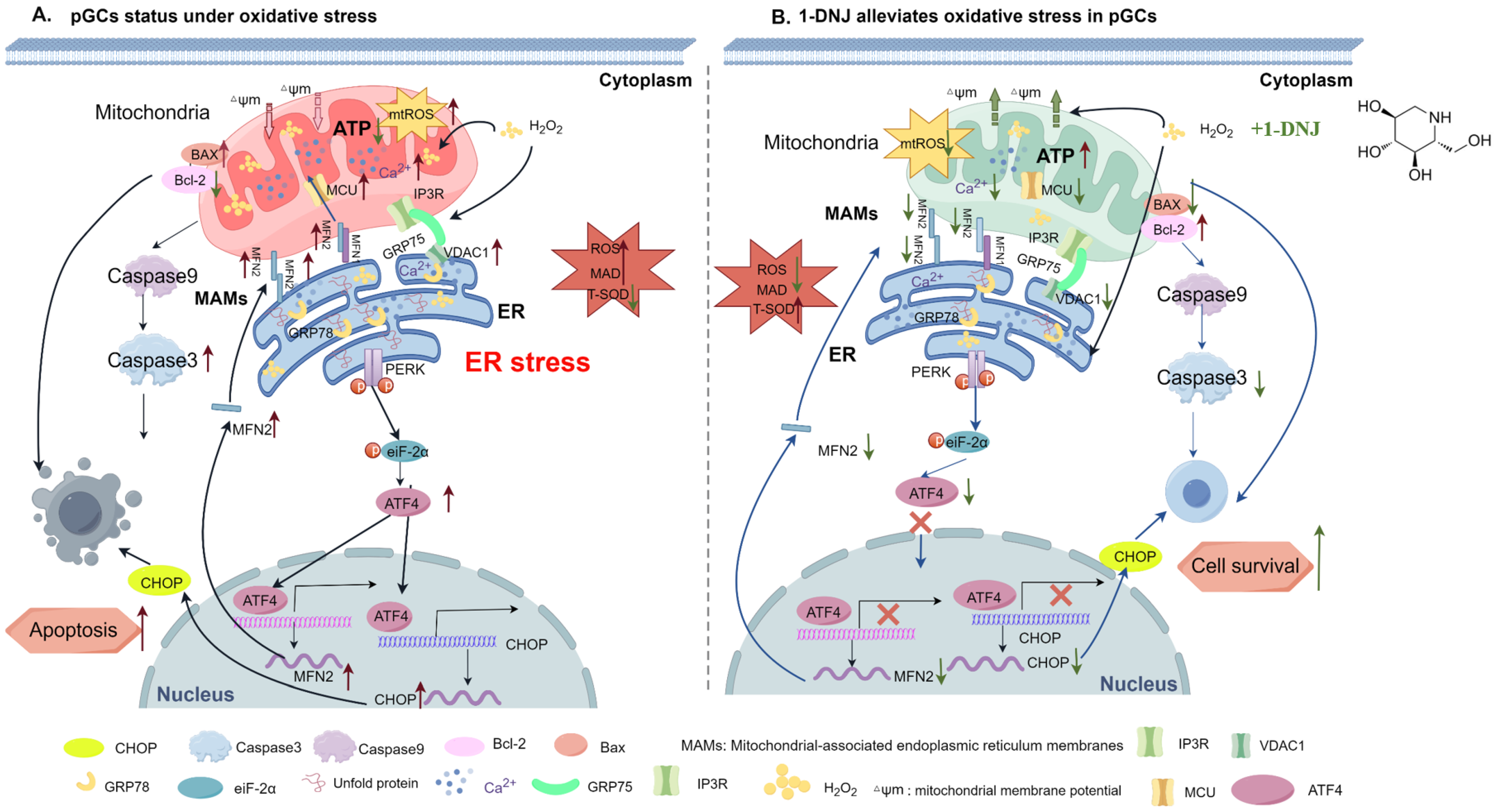
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Duan, H.; Yang, S.; Yang, S.; Zeng, J.; Yan, Z.; Zhang, L.; Ma, X.; Dong, W.; Zhang, Y.; Zhao, X.; et al. The mechanism of curcumin to protect mouse ovaries from oxidative damage by regulating AMPK/mTOR mediated autophagy. Phytomedicine 2024, 128, 155468. [Google Scholar] [PubMed]
- Wang, Y.; Pattarawat, P.; Zhang, J.; Kim, E.; Zhang, D.; Fang, M.; Jannaman, E.A.; Yuan, Y.; Chatterjee, S.; Kim, J.J.; et al. Effects of Cyanobacterial Harmful Algal Bloom Toxin Microcystin-LR on Gonadotropin-Dependent Ovarian Follicle Maturation and Ovulation in Mice. Environ. Health Perspect. 2023, 131, 67010. [Google Scholar] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar]
- Wang, X.; Yang, J.; Li, H.; Mu, H.; Zeng, L.; Cai, S.; Su, P.; Li, H.; Zhang, L.; Xiang, W. miR-484 mediates oxidative stress-induced ovarian dysfunction and promotes granulosa cell apoptosis via SESN2 downregulation. Redox Biol. 2023, 62, 102684. [Google Scholar]
- Huang, L.; Yuan, H.; Shi, S.; Song, X.; Zhang, L.; Zhou, X.; Gao, L.; Pang, W.; Yang, G.; Chu, G. CLOCK inhibits the proliferation of porcine ovarian granulosa cells by targeting ASB9. J. Anim. Sci. Biotechnol. 2023, 14, 82. [Google Scholar]
- Wu, S.; Gan, M.; Wang, Y.; Pan, Y.; He, Y.; Feng, J.; Zhao, Y.; Niu, L.; Chen, L.; Zhang, S.; et al. Copper mediated follicular atresia: Implications for granulosa cell death. J. Hazard. Mater. 2024, 477, 135391. [Google Scholar]
- Szabo, I.; Szewczyk, A. Mitochondrial Ion Channels. Annu. Rev. Biophys. 2023, 52, 229–254. [Google Scholar]
- Dorn, G.N.; Kitsis, R.N. The mitochondrial dynamism-mitophagy-cell death interactome: Multiple roles performed by members of a mitochondrial molecular ensemble. Circ. Res. 2015, 116, 167–182. [Google Scholar]
- Wang, Y.; Yang, Q.; Wang, H.; Zhu, J.; Cong, L.; Li, H.; Sun, Y. NAD+ deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndrome. Biol. Reprod. 2021, 105, 371–380. [Google Scholar]
- Chen, Y.; Zhao, Y.; Miao, C.; Yang, L.; Wang, R.; Chen, B.; Zhang, Q. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells. J. Ovarian Res. 2022, 15, 138. [Google Scholar]
- Zhu, M.; Yan, M.; Chen, J.; Li, H.; Zhang, Y. MicroRNA-129-1-3p attenuates autophagy-dependent cell death by targeting MCU in granulosa cells of laying hens under H2O2-induced oxidative stress. Poult. Sci. 2023, 102, 103006. [Google Scholar] [PubMed]
- Li, B.; Zhang, T.; Tang, M. Toxicity mechanism of nanomaterials: Focus on endoplasmic reticulum stress. Sci. Total Environ. 2022, 834, 155417. [Google Scholar] [PubMed]
- Li, H.; Jing, Y.; Qu, X.; Yang, J.; Pan, P.; Liu, X.; Gao, H.; Pei, X.; Zhang, C.; Yang, Y. The Activation of Reticulophagy by ER Stress through the ATF4-MAP1LC3A-CCPG1 Pathway in Ovarian Granulosa Cells Is Linked to Apoptosis and Necroptosis. Int. J. Mol. Sci. 2023, 24, 2749. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pei, X.; Jin, Y.; Wang, Y.; Zhang, C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res. 2016, 363, 589–597. [Google Scholar] [PubMed]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar]
- Gyongyosi, B.; Cho, Y.; Lowe, P.; Calenda, C.D.; Iracheta-Vellve, A.; Satishchandran, A.; Ambade, A.; Szabo, G. Alcohol-induced IL-17A production in Paneth cells amplifies endoplasmic reticulum stress, apoptosis, and inflammasome-IL-18 activation in the proximal small intestine in mice. Mucosal Immunol. 2019, 12, 930–944. [Google Scholar]
- Ma, L.; Chen, C.; Hai, S.; Wang, C.; Rahman, S.U.; Huang, W.; Zhao, C.; Feng, S.; Wang, X. Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells. Toxins 2023, 15, 253. [Google Scholar] [CrossRef]
- Huang, J.C.; Duan, C.C.; Jin, S.; Sheng, C.B.; Wang, Y.S.; Yue, Z.P.; Guo, B. HB-EGF induces mitochondrial dysfunction via estrogen hypersecretion in granulosa cells dependent on cAMP-PKA-JNK/ERK-Ca2+-FOXO1 pathway. Int. J. Biol. Sci. 2022, 18, 2047–2059. [Google Scholar]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar]
- Wang, M.; Feng, Y.; Li, T.; Zhao, C.; Barcenas, A.R.; Serrano, B.R.; Qu, L.; Shen, M.; Zhao, W. The Effects of 1-Deoxynojirimycin from Mulberry on Oxidative Stress and Inflammation in Laying Hens and the Direct Effects on Intestine Epithelium Cells In Vitro. Animals 2023, 13, 2830. [Google Scholar] [CrossRef]
- Ren, X.; Guo, Q.; Jiang, H.; Han, X.; He, X.; Liu, H.; Xiu, Z.; Dong, Y. Combinational application of the natural products 1-deoxynojirimycin and morin ameliorates insulin resistance and lipid accumulation in prediabetic mice. Phytomedicine 2023, 121, 155106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, L.; Yang, J.; Shi, H.; Zhu, H.; Zhai, M.; Lu, L.; Wang, X.; Li, X.Y.; Yu, S.; et al. 1-Deoxynojirimycin attenuates septic cardiomyopathy by regulating oxidative stress, apoptosis, and inflammation via the JAK2/STAT6 signaling pathway. Biomed. Pharmacother. 2022, 155, 113648. [Google Scholar]
- Zhuang, Q.; Guo, F.; Fu, L.; Dong, Y.; Xie, S.; Ding, X.; Hu, S.; Zhou, X.D.; Jiang, Y.; Zhou, H.; et al. 1-Deoxynojirimycin promotes cardiac function and rescues mitochondrial cristae in mitochondrial hypertrophic cardiomyopathy. J. Clin. Investig. 2023, 133, e164660. [Google Scholar] [PubMed]
- Mai, W.; Shang, Y.; Wang, Y.; Chen, Y.; Mu, B.; Zheng, Q.; Liu, H. 1-DNJ Alleviates Obesity-Induced Testicular Inflammation in Mice Model by Inhibiting IKKβ/ NF-kB Pathway. Reprod. Sci. 2024, 31, 2103–2113. [Google Scholar]
- Qin, L.; Huang, T.; Jing, R.; Wen, J.; Cao, M. Mulberry leaf extract reduces abdominal fat deposition via adenosine-activated protein kinase/sterol regulatory element binding protein-1c/acetyl-CoA carboxylase signaling pathway in female Arbor Acre broilers. Poult. Sci. 2023, 102, 102638. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, B.W.; Wang, J.; Ren, Q.L.; Chen, J.F.; Ma, Q.; Zhang, Z.J.; Xing, B.S. Critical Role of FoxO1 in Granulosa Cell Apoptosis Caused by Oxidative Stress and Protective Effects of Grape Seed Procyanidin B2. Oxid. Med. Cell. Longev. 2016, 2016, 6147345. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H2O2-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar]
- Li, X.; Lin, Y.; Yao, J.; Pan, B.; Zhan, X.; Chen, Z.; Bai, Y.; Zhang, H.; Wang, B.; Chen, S.; et al. Protegrin-1 inhibits porcine ovarian granulosa cell apoptosis from H2O2-induced oxidative stress via the PERK/eIF2α/CHOP signaling pathway in vitro. Theriogenology 2022, 179, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Liu, K.; Wang, Q.; Fu, R.; Si, H.; Sui, S. Periplaneta americana peptide decreases apoptosis of pig-ovary granulosa cells induced by H2O2 through FoxO1. Reprod. Domest. Anim. 2021, 56, 1413–1424. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [PubMed]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [PubMed]
- Luengo, A.; Li, Z.; Gui, D.Y.; Sullivan, L.B.; Zagorulya, M.; Do, B.T.; Ferreira, R.; Naamati, A.; Ali, A.; Lewis, C.A.; et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell 2021, 81, 691–707.e6. [Google Scholar] [CrossRef]
- Rehfeldt, S.; Laufer, S.; Goettert, M.I. A Highly Selective In Vitro JNK3 Inhibitor, FMU200, Restores Mitochondrial Membrane Potential and Reduces Oxidative Stress and Apoptosis in SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 3701. [Google Scholar] [CrossRef]
- Chang, X.; Niu, S.; Shang, M.; Li, J.; Guo, M.; Zhang, W.; Sun, Z.; Li, Y.; Zhang, R.; Shen, X.; et al. ROS-Drp1-mediated mitochondria fission contributes to hippocampal HT22 cell apoptosis induced by silver nanoparticles. Redox Biol. 2023, 63, 102739. [Google Scholar] [CrossRef]
- Abuaita, B.H.; Schultz, T.L.; O’Riordan, M.X. Mitochondria-Derived Vesicles Deliver Antimicrobial Reactive Oxygen Species to Control Phagosome-Localized Staphylococcus aureus. Cell Host Microbe 2018, 24, 625–636.e5. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, H.; Chen, B.; Liu, Z.; Wu, G.; Li, C.; Li, R.; Cao, Y.; Zhou, J.; et al. Sulforaphane Acts Through NFE2L2 to Prevent Hypoxia-Induced Apoptosis in Porcine Granulosa Cells via Activating Antioxidant Defenses and Mitophagy. J. Agric. Food Chem. 2022, 70, 8097–8110. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 2012, 81, 767–793. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Y.; Liu, L.; Pang, B.; Sun, Z.; Guan, S.; Yan, Q.; Mo, T.; Chen, R.; Xu, M.; et al. Bushen Jieyu Tiaochong Formula reduces apoptosis of granulosa cells via the PERK-ATF4-CHOP signaling pathway in a rat model of polycystic ovary syndrome with chronic stress. J. Ethnopharmacol. 2022, 292, 114923. [Google Scholar] [PubMed]
- Ridlo, M.R.; Kim, G.A.; Taweechaipaisankul, A.; Kim, E.H.; Lee, B.C. Zinc supplementation alleviates endoplasmic reticulum stress during porcine oocyte in vitro maturation by upregulating zinc transporters. J. Cell. Physiol. 2021, 236, 2869–2880. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, M.; Liang, Z.; Yang, C.; Kong, X.; Wu, Y.; Wang, S.; Fan, H.; Ning, C.; Xiao, W.; et al. PI3K/AKT/mTOR, NF-κB and ERS pathway participated in the attenuation of H2O2-induced IPEC-J2 cell injury by koumine. J. Ethnopharmacol. 2023, 304, 116028. [Google Scholar] [PubMed]
- Wortel, I.; van der Meer, L.T.; Kilberg, M.S.; van Leeuwen, F.N. Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells. Trends Endocrinol. Metab. 2017, 28, 794–806. [Google Scholar]
- Jin, H.O.; Seo, S.K.; Woo, S.H.; Kim, E.S.; Lee, H.C.; Yoo, D.H.; Choe, T.B.; Hong, S.I.; Kim, J.I.; Park, I.C. SP600125 negatively regulates the mammalian target of rapamycin via ATF4-induced Redd1 expression. FEBS Lett. 2009, 583, 123–127. [Google Scholar]






| Gene | NCBI Number | Accession Number | Primer Sequence | Primer Length | Product Length |
|---|---|---|---|---|---|
| GAPDH | NM_001206359 | AF017079 | F: TCGGAGTGAACGGATTTGGC R: TGCCGTGGGTGGAATCATAC | 20 20 | 147 |
| SOD1 | NM_001190422 | AF396674 | F: ATTCTGTGATCGCCCTCT R: AGCATTTCCCGTCTTTGT | 18 18 | 119 |
| SOD2 | NM_214127 | AF396673 | F: TCTGGACAAATCTGAGCCCTAA R: TGGACGCCGACGGATACA | 22 18 | 127 |
| GPX4 | NM_214407 | AK232479 | F: GACGACTGGCGATGTGCT R: GCTCCTGCCTCCCAAACT | 18 18 | 232 |
| CAT | XM_021081498 | AK233269 | F: CGAAGGCGAAGGTGTTTG R: CAAACCCACGAGGGTCAC | 18 18 | 114 |
| NQO1 | NM_001159613 | AK234062 | F: CCTCTGGCCAATTCAGAGTGG R: CTGGATTCGGGCATCCTCTG | 21 20 | 106 |
| GCLM | XM_001926378 | AK235653 | F: AGAAGTGCCCGTCTACACAC R: CATCTGGAAACTCCCTGACCA | 20 21 | 108 |
| Bcl2 | XM_021099593.1 | AB271960 | F: TCAGGGATGGGGTGAACT R: TCAGAGACAGCCAGGAGAAAT | 18 21 | 240 |
| BAX | XM_003127290 | AJ606301 | F: GCCGAAATGTTTGCTGAC R: GCCGATCTCGAAGGAAGT | 18 18 | 154 |
| Caspase-3 | NM_214131.1 | AB029345 | F: TTGGACTGTGGGATTGAGACG R: CGCTGCACAAAGTGACTGGA | 21 20 | 165 |
| ATF4 | XM_021090887.1 | AK233046 | F: TCAGACAACAGCAAGGAGGATG R: GCCAAAAGCTCATCTGGCAT | 23 20 | 132 |
| GRP78 | XM_001927795.7 | AK344136 | F: ACCACCTACTCGTGCGTTG R: CGTCGAAGACCGTGTTCTCA | 19 20 | 175 |
| CHOP | XM_005674378.2 | AK346637 | F: ATTGCCTTTCTCCTTCGGGAC R: GAAGGTTTTTGACTCCTCCTCAT | 21 23 | 139 |
| Target | siRNA Sequence | Sequence (5′-3′) |
|---|---|---|
| ATF4 | SiATF4-812 SiATF4-1155 SiATF4-1251 | GUCUUCCACUCCAGAUAAUTT CAGAUAAUGACAGUGGCAUTT GGAGAUUCAGUAUCUCAAATT |
| Control | NC | UUCUCCGAACGUGUCACGUTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Li, M.; Wang, B.; Huo, L.; Tian, W.; Ge, F.; Shen, M.; Sun, L.; Liu, J.; Yu, S. Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs Through Modulation of the PERK-ATF4/MFN2 Signaling Pathway. Antioxidants 2025, 14, 456. https://doi.org/10.3390/antiox14040456
Xing W, Li M, Wang B, Huo L, Tian W, Ge F, Shen M, Sun L, Liu J, Yu S. Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs Through Modulation of the PERK-ATF4/MFN2 Signaling Pathway. Antioxidants. 2025; 14(4):456. https://doi.org/10.3390/antiox14040456
Chicago/Turabian StyleXing, Wenwen, Mengxuan Li, Binbin Wang, Lele Huo, Wanru Tian, Fangcai Ge, Manman Shen, Liumei Sun, Jiying Liu, and Shali Yu. 2025. "Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs Through Modulation of the PERK-ATF4/MFN2 Signaling Pathway" Antioxidants 14, no. 4: 456. https://doi.org/10.3390/antiox14040456
APA StyleXing, W., Li, M., Wang, B., Huo, L., Tian, W., Ge, F., Shen, M., Sun, L., Liu, J., & Yu, S. (2025). Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs Through Modulation of the PERK-ATF4/MFN2 Signaling Pathway. Antioxidants, 14(4), 456. https://doi.org/10.3390/antiox14040456






