Abstract
Currently, nutraceuticals and functional food/cosmeceutical sectors are seeking natural molecules to develop various types of phytopharmaceutical products. Flavonoids have been reported in antioxidant and many medical/pharmacological activities. Monochoria angustifolia or Siam violet pearl medicinal plant is the newest species of the genus Monochoria C. Presl, which have long been consumed as food and herbal medicines. Though previous work showed that apigenin-7-O-glucoside is the most abundant antioxidant phytochemical found in this medicinal plant, the report on anti-aging activity is still lacking and needs to be filled in. The objective of this work is to explore anti-aging capacities of the most abundant antioxidant phytochemical reported in this plant using both in silico and in vitro assessments. In addition, pharmacokinetic properties were predicted. Interestingly, the results from both in silico and in vitro analysis showed a similar trend that apigenin-7-O-glucoside is a potential anti-aging agent against three enzymes. The pharmacokinetic properties, such as adsorption, distribution, metabolism, excretion and toxicity (ADMET), of this compound are also provided in this work. The current study is also the first report on anti-aging properties of this Thai medicinal plant. However, the safety and efficacy of future developed products from this compound and clinical study should be determined in the future.
1. Introduction
Flavonoids are one of the large classes of phytochemical compounds synthesized by several plants species which have been reported as having various medical/pharmacological benefits [1,2,3,4,5,6,7,8,9,10,11,12,13,14], especially anti-aging effects and in the treatment of degenerative diseases [5,15,16,17,18,19,20,21,22,23,24,25,26,27]. The antioxidant potential is one of the key pharmacological activities of flavonoids such as flavones, flavanones, flavonols, flavanonol, isoflavones, aurones, chalcones and anthocyanin, which has been extensively reported globally [1,2,3,4,5,6,7,8,9,11,12,13,14,15,16,17,18,19,20,21,22,24,25,26,27]. Currently, customers’ preference for natural products is sharply increasing. Therefore, nutraceuticals, functional food, cosmetics, cosmeceutical and other phytopharmaceutical industries are currently focusing on the natural bioactive molecules for various product developments.
Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum or the so-called Siam violet pearl is a newly discovered medicinal plant species from Thailand (Figure 1) [28,29,30]. This medicinal plant is also known by various vernacular names, i.e., Phak Lin, Phak Xi Hin or Khimuk Si Muang Haeng Siam, depending on the region/locality [28,29,30,31,32]. M. angustifolia is a species member of the genus Monochoria C. Presl (Pontederiaceae family) that has long been used in cooking as a vegetable, cosmetics and many herbal medicinal remedies [24,28,31]. Our previous study determined flavonoid phytochemical profiles of 25 M. angustifolia populations from their natural habitat, covering all of the floristic regions in Thailand, and also determined their antioxidant potential using five in vitro antioxidant assays, i.e., ABTS (2,2-azinobis(3-ethylbenzthiazoline-6-sulphonic acid)), FRAP (ferric reducing antioxidant power), ORAC (oxygen radical absorbance capacity assay) DPPH (2,2-diphenyl-1-picrylhydrazyl) and CUPRAC (cupric reducing antioxidant capacity) covering different antioxidant mechanisms as well as a cellular antioxidant assay using a yeast model [24]. The results from those various antioxidant analyses in the previous research showed that M. angustifolia extracts exhibited the antioxidant action via a hydrogen atom transfer antioxidant mechanism [24].

Figure 1.
M. angustifolia: (A) rice field, its natural habitat in Thailand; (B) floral part; (C) vegetative part. All photos were taken in Thailand by Assoc. Prof. Dr. Duangjai Tungmunnithum.
In addition, the results from our previous research work showed that Apigenin-7-O-glucoside, a flavone compound, is the most abundant antioxidant flavonoid occurring in this medicinal plant species (36.84 ± 0.49 mg/g DW) [24] and provided high antioxidant effects, determined by FRAP (503.66 ± 84.40 μmol TEAC), CUPRAC (225.45 ± 42.7 μmol TEAC), ABTS (187.95 ± 1.78 μmol TEAC), DPPH (326.19 ± 6.25 μmol TEAC) and ORAC (400.79 ± 40.41 μmol TEAC) [24]. However, there are no reports on anti-aging activity, which is an interesting pharmacological activity that can be applied in various nutraceuticals, functional foods, cosmetic/cosmeceutical and other phytopharmaceutical product development. In addition, molecular docking is a powerful computer-aided drug design (CADD) methodology that plays a crucial role in the drug discovery process, enabling researchers to predict potential drug candidates through computational algorithms. This method visualizes molecular orientation, bond distances and binding interactions between three-dimensional protein structures and chemical candidates, which serve as critical decisional factors [33,34]. CADD is also recognized as a green approach, as it reduces the chemical and biological waste commonly produced in experimental workflows [35] and provides additional benefits, such as rapid analysis, cost-efficiency and saving time by narrowing the selection of potential drug candidates [36]. Open access webservers are widely used to estimate pharmacokinetic properties—adsorption, distribution, metabolism, excretion and toxicity (ADMET)—based on quantitative structure–activity relationship (QSAR) databases [37,38] or machine learning [39], in order to gain the fundamental information to see the overview of the pharmacokinetic properties of the targeted compounds.
Thus, the current study aims to investigate the skin anti-aging potentials of the most abundant antioxidant phytochemical compounds, apigenin-7-O-glucoside, reported in Siam violet pearl medicinal plant (M. angustifolia) by using both in silico molecular docking and the three different in vitro enzymatic assessments. In addition, the pharmacokinetic properties, such as adsorption, distribution, metabolism, excretion and toxicity, of this compound were also provided as an overview of its pharmacokinetic properties.
2. Materials and Methods
2.1. Molecular Docking
Apigenin-7-O-glucoside (Api-7-O-Glc, PubChem CID: 12304093), a major flavonoid glucoside isolated from M. angustifolia [24], along with three positive control compounds—1,10-phenanthroline (PubChem CID: 1318), oleanolic acid (PubChem CID: 10494) and kojic acid (PubChem CID: 3840)—were modeled as three-dimensional structures using Chem3D Pro 12.0 software (PerkinElmer Inc., Cambridge, MA, USA). The 3D structures were then optimized to achieve minimized energy conformations using MM2 minimization [40] and CHARMm force field [41,42]. These constructed molecules were docked into three anti-aging enzymes: collagenase G from Clostridium histolyticum (PDB ID: 7ZBV, resolution 1.95 Å) [43], porcine pancreatic elastase (PDB ID: 1BRU, resolution 2.30 Å) [44] and tyrosinase from Agaricus bisporus (PDB ID: 2Y9X, resolution 2.78 Å) [45]. The crystal structures of these enzymes were downloaded from the Protein Data Bank (PDB, https://www.rcsb.org/, accessed on 14 September 2024) [46,47] and prepared by adding hydrogen atoms and removing co-crystalized ligands, cofactors and water molecules prior to docking. The GOLD software suit version 5.7.1 (Genetic Optimization for Ligand Docking, CCDC, Cambridge, UK) [48] was used to investigate the potential protein–ligand interactions within 12 angstrom (Å) radius, running 100 docking iterations per molecule with both GoldScore and ChemScore scoring functions at 100% efficiency. Api-7-O-Glc and three positive controls were docked into the central coordinates (x, y, z) of each enzyme’s binding site, based on the center of the co-crystalized ligand within the enzymatic crystal structures. The central coordinates for collagenase G, elastase and tyrosinase were (10.86, −1.76, 12.65), (23.20, 47.66, 17.09) and (−10.02, −28.82, −43.59), respectively. Discovery Studio Visualizer V.24.1.0 (BIOVIA, San Diego, CA, USA) [49] was used to visualize the three-dimensional interaction graphics of the docking results.
2.2. Prediction of Pharmacokinetic Properties and Toxicities
The pharmacokinetic properties and toxicity profiles of Api-7-O-Glc were predicted using online webservers. SwissADME (http://www.swissadme.ch/, accessed on 14 September 2024) [50] and the pKCSM (https://biosig.lab.uq.edu.au/pkcsm/, accessed on 14 September 2024) [51] webservers were utilized to evaluate partition coefficient (Log P), solubility (Log S), water solubility and skin permeability (Log Kp). The ProTox 3.0 (https://tox.charite.de/protox3/, accessed on 14 September 2024) [52] webserver was used to assess skin sensitization, hepatotoxicity, carcinogenicity and toxicity classification.
2.3. In Vitro Enzymatic Anti-Aging Assays
2.3.1. Chemical Reagents
All of the reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the standards are HPLC grade, with a purity of ≥98%, obtained from Extrasynthese (Genay Cedex, France). All solvents are analytical grade from Thermo Scientific (Waltham, MA, USA).
2.3.2. Determination of the Anti-Collagenase Assay
The collagenase from clostridium histolyticum (Sigma Aldrich) is used for this experiment, and collagenase activity was determined by the spectrophotometer (Shimadzu, Kyoto, Japan) using its substrate, N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA; Sigma Aldrich), according to the protocol suggested by the previous report of Wittenauer et al. [53] with minor modifications. The absorbance was measured to monitor the decrease in the FALGPA at 335 nm over a 20 min period using a microplate reader (BMG labtech, Victoria, Australia). The measurements were conducted in triplicate; the anti-collagenase activity was revealed as % inhibition relative to the control for every sample. The concentrations of standard compound were 100 µM. The collagenase’s specific inhibitor such as 1,10-phenantroline (100 μM) was used as the positive control of this enzyme. The % inhibition of collagenase was calculated by comparing with the initial slope during the first 10 min to the control using the following equation:
2.3.3. Determination of the Anti-Elastase Assay
The elastase assay was determined by using porcine pancreatic elastase (Sigma Aldrich) and elastase’s activity was investigated by the spectrophotometer (Shimadzu, Japan). The N-Succ-Ala-Ala-Alap-nitroanilide (AAAVPN; Sigma Aldrich) was used as its substrate and p-nitroaniline’s release was followed at 410 nm by using the microplate reader (BMG labtech, Victoria, Australia) adapted based on the method that was previously reported by Wittenauer et al. [53] with minor modifications. The measurements were conducted in triplicate; the anti-elastase activity was presented in the form of a percentage of inhibition relative to the control. The concentration of standard compound was 100 µM. The 10 μM of oleanolic acid was employed as a positive control of this enzyme. The calculation of % inhibition was performed as described previously in the case of collagenase assay using the following equation:
2.3.4. Determination of the Anti-Tyrosinase Assay
The tyrosinase assay was performed following the previous method described by Chai et al. [54] with minor modifications. In brief, the 5 mM L-DOPA (3,4-dihydroxy-L-phenylalanine; Sigma Aldrich) was used as the substrate, and then mixed in sodium phosphate buffer (50 mM, pH 6.8) with 10 μL of the sample. After that, 0.2 mg/mL of the mushroom tyrosinase solution (Sigma Aldrich) was added to the mixture, so as to reach the final volume at 200 μL. The reaction was then detected by using the microplate reader (BMG labtech, Victoria, Australia) at 475 nm. The concentrations of standard compound were 100 µM. The tyrosinase inhibitory effect was presented as the % inhibition relative to the control. The 10 μM of Kojic acid was used as the positive control of this enzyme. The calculation of % inhibition was performed as described previously in the case of collagenase assay using the following equation:
2.4. Statistical Analysis
The data contained at least 3 independent replicates, and the data were shown as means and standard deviations. The statistical analysis was determined using Student’s t-test for the statistical comparative analysis. Significant differences at p < 0.05 and 0.01 were represented by * and **. Different letters were employed to indicate significant differences at p < 0.05.
3. Results and Discussion
3.1. Binding Interactions Predicted by Molecular Docking
Flavonoids and their glycosides are valuable plant metabolites and have served extensively as active ingredients in medicines [55], supplements [56] and cosmetics [57], especially for their potential anti-aging candidates [58,59]. Api-7-O-Glc, a major component in a traditional lotus, namely M. angustifolia [24], found in Thailand, was first used to analyze the anti-aging activity through molecular docking studies, alongside in vitro assays, targeting three skin-aging-related enzymes, including collagenase, elastase and tyrosinase. The reliability of the scoring functions, GoldScore and ChemScore, and reproducibility of binding interactions were validated by self-docking 2-hydroxycyclohepta-2,4,6-trien-1-one, the co-crystallized ligand, into the binding site of tyrosinase. Due to coordination with the copper (II) ion, the postures computed by GoldScore and ChemScore showed minor deviations from the crystal structure (Figure 2), with a root-mean square deviation (RMSD) value of 2.512 and 2.336 Å, respectively. These RMSD values, both less than 3 Å, indicated acceptable and reliable docking results [60,61]. GoldScore was selected for further docking studies based on its higher fitness score relative to ChemScore.
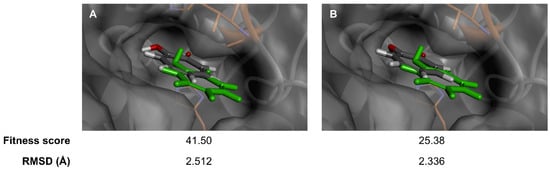
Figure 2.
Validated docking studies of 2-hydroxycyclohepta-2,4,6-trien-1-one within binding site of tyrosinase enzyme, via (A) GoldScore and (B) ChemScore scoring function, in relation to co-crystallized ligand (green).
The fitness scores of Api-7-O-Glc and positive controls are presented in Table 1, based on conformations that fulfilled three critical criteria: (i) precise alignment within the enzyme’s binding cavity, (ii) avoidance of repulsive interactions and (iii) a favorable overlay with the co-crystallized ligand. The docked molecule that maintains an optimal distance and exhibits a strong binding affinity to the enzyme’s binding cavity residues generally tends to achieve a higher fitness score [62]. Api-7-O-Glc showed potential as an anti-aging inhibitor, with its fitness scores 1.15 to 1.62 times higher than those of positive controls across all tested enzymes. Notably, Api-7-O-Glc exhibited the highest fitness score for collagenase in comparison with other two skin-aging-related enzymes, elastase and tyrosinase. This suggested that Api-7-O-Glc could be a promising candidate for targeting the skin collagenase enzyme in anti-aging treatments. Moreover, the fitness scores of other flavonoids isolated from Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum are also docked and reported in Table S1.

Table 1.
Fitness scores calculated based on GoldScore scoring function of Api-7-O-Glc and positive controls docked into collagenase, elastase and tyrosinase enzyme’s binding cavity.
Three-dimensional conformations and two-dimensional diagrams were visualized to illustrate the binding interactions between the docked molecules and the amino acid residues within the binding cavity of skin-aging-related enzymes, as summarized in Table 2. Api-7-O-Glc formed mutual interactions within the collagenase binding pocket as shown in Figure 3, including metal coordination with zinc (II) ion, pi–pi stacking with His527 and three hydrogen bonds with Gly494 (backbone carbonyl to 5-hydroxyl), Glu559 (backbone carbonyl to 4″-hydroxyl) and Arg573 (guanidine side chain to 4″-hydroxyl). Two critical factors influenced the fitness scores: (i) coordinated binding to zinc (II) ion, which played a crucial role as a cofactor in the enzyme’s catalytic activity [63], and (ii) the conventional hydrogen bonds, which were recognized as strong intermolecular electrostatic interactions. These interactions contributed to Api-7-O-Glc achieving a higher fitness score compared to 1,10-phenanthroline, which only formed pi–pi stacking with Phe515 and His523, along with van der Waal’s interactions with Glu524 and Arg573. Thus, this analysis suggested that Api-7-O-Glc may exhibit more potent anti-aging activity against collagenase enzymes than the positive control.

Table 2.
Predicted intermolecular interactions of Api-7-O-Glc within the binding site of three anti-aging enzymes, in comparison with their positive controls.
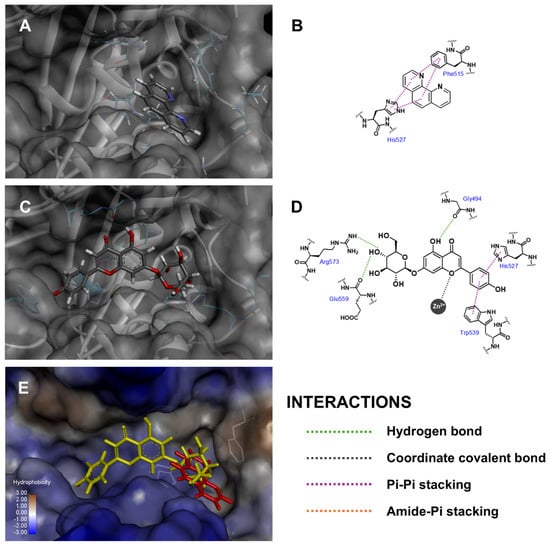
Figure 3.
Three-dimensional conformations and two-dimensional interaction diagrams of (A,B) 1,10-phenanthroline, (C,D) Api-7-O-Glc in collagenase enzyme, along with (E) superimposed view of 1,10-phenanthroline (red) and Api-7-O-Glc (yellow).
In the case of elastase enzyme binding site, Api-7-O-Glc oriented its glucoside moiety toward Ser96 and aligned similarly to oleanolic acid, the positive control, as shown in Figure 4. Api-7-O-Glc established six hydrogen bonds with Ser96 (carbonyl backbone to 6‴-hydroxyl), Ser190 (hydroxyl side chain to phenolic moiety), Gly193 (amide backbone to carbonyl), Ser195 (hydroxyl side chain to 5-hydroxyl), Gly216 (amide backbone to 1-oxygen atom) and Cys220 (carbonyl backbone to phenolic moiety). Additionally, it also formed one pi–pi stacking interaction with Phe215 and van der Waal’s interaction with Leu99. This alignment positioned the polar sugar moiety of Api-7-O-Glc next to the hydrophobic side chain of surface amino acid residues, creating some unfavorable interactions and contributing to a relatively lower fitness score. Nevertheless, Api-7-O-Glc still achieved a higher fitness score than oleanolic acid, which formed two hydrogen bonds with Ser96 (carbonyl backbone to 3-hydroxyl) and Asn192 (amide side chain to carboxyl). Accordingly, this result suggested that Api-7-O-Glc was the more effective elastase enzyme inhibitor than oleanolic acid.
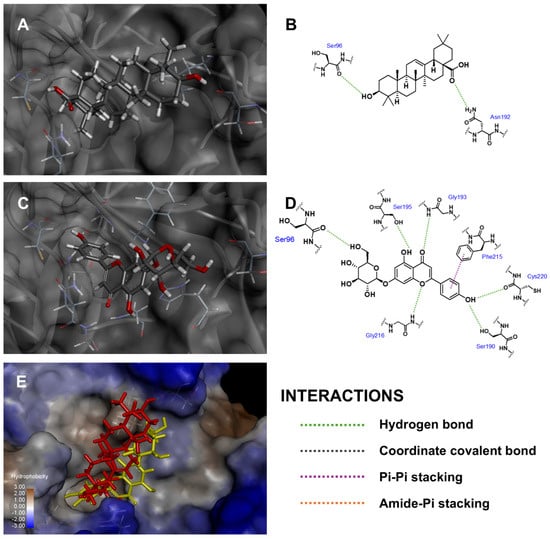
Figure 4.
Three-dimensional conformations and two-dimensional interaction diagrams of (A,B) oleanolic acid, (C,D) Api-7-O-Glc in elastase enzyme, along with (E) superimposed view of oleanolic acid (red) and Api-7-O-Glc (yellow).
Tyrosinase enzyme catalytic activity is categorized as an oxidation process, in which a monophenolic unit is transformed into an orthoquinone moiety via a binuclear copper (II) ion center [64]. According to the docking results, the phenolic ring C of Api-7-O-Glc was positioned within tyrosinase’s binding pocket, forming a coordinated covalent bond with the copper (II) ions (Figure 5), which corresponded to the enzymatic conversion of L-tyrosine to o-dopaquinone [65]. Additionally, Api-7-O-Glc formed a pi–pi stacking interaction with His263 and van der Waals interactions with His61 and His85. While no intermolecular hydrogen bonds were observed between Api-7-O-Glc and the tyrosinase enzyme, Api-7-O-Glc exhibited a silently higher fitness score than kojic acid, the positive control. Kojic acid’s interactions with tyrosinase included coordinated covalent bonds between its methanol hydroxyl group and the copper (II) ions, a hydrogen bond between the 5-hydroxyl group and the carbonyl backbone of Met280 and one pi–pi stacking interaction with His263. Although the fitness scores were not markedly different, these docking results suggested that Api-7-O-Glc exhibits competitive binding affinity and may serve as an effective tyrosinase inhibitor.
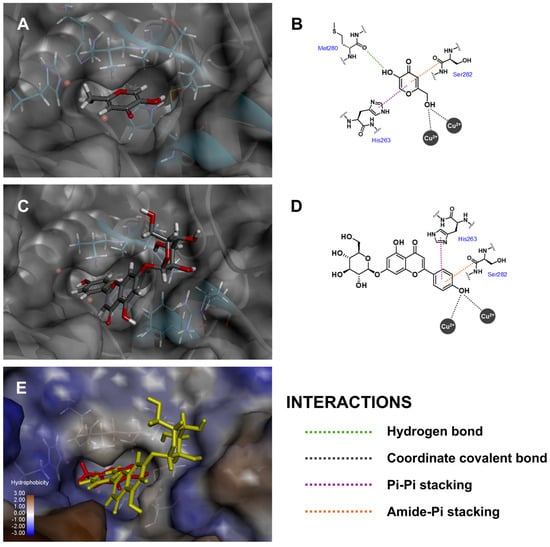
Figure 5.
Three-dimensional conformations and two-dimensional interaction diagrams of (A,B) kojic acid, (C,D) Api-7-O-Glc in tyrosinase enzyme, along with (E) superimposed view of kojic acid (red) and Api-7-O-Glc (yellow).
Although the selected crystal structures used in molecular docking experiments are not derived from human sources, they are widely utilized as initial models for evaluating anti-aging activity in both in silico and in vitro analysis [53,54,66,67,68]. Porcine pancreatic elastase has been reported to share a closely similar topology and inhibition mechanism with its human elastase [53,69,70]. While Clostridium collagenase and mushroom tyrosinase differ in domain compositions from their human equivalents, they belong to the same enzyme classification and perform similar functions. Both bacterial and human collagenase are metalloproteases with Zn2+ at their catalytic site, catalyzing the hydrolysis of collagen peptides, which contributes to reduced skin tonus, deep wrinkles and loss of resilience [71,72]. Similarly, the key enzymatic function of both fungal and human tyrosinases is melanogenesis driven by Cu2+-mediated metallooxidase activity. This process converts L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), leading to melanin formation, which causes skin darkening and the development of freckles [67,73]. Thus, these supporting pieces of evidence provide a strongly reliable basis for the preliminary study of anti-aging activity.
3.2. Pharmacokinetic Properties and Toxicities
In drug discovery, pharmacodynamic properties are the initial priority factors for evaluating drug candidates; however, pharmacokinetic properties play a critical role in determining the viability of these drug candidates in terms of efficacy, safety and distribution potential [74,75]. To assess the ADMET characteristics of Api-7-O-Glc, three predictive webservers—SwissADME, pkCSM and ProTox 3.0—were utilized, with the results summarized in Table 3. For oral drug candidates, Lipinski’s rule of five serves as a widely accepted criterion to indicate an orally bioavailability and gastrointestinal (GI) absorption potential of lead compounds [76]. Api-7-O-Glc did not fully comply with Lipinski’s rule, specifically due to an excess of hydrogen bond donors. This compound was classified as a compound with lower GI absorption potential, which may limit its oral bioavailability.

Table 3.
Predicted pharmacokinetic and toxicological properties of Api-7-O-Glc.
The skin permeability of Api-7-O-Glc, defined by the Log Kp values, was calculated to be −2.735 cm/h, which exceeds the threshold of −3.0 cm/h typically associated with high skin permeability [77,78], indicating moderate skin absorption properties. Furthermore, skin sensitization predictions classified Api-7-O-Glc as non-irritants to skin. Toxicity predictions estimated a median lethal dose (LD50) exceeding 5000 mg/kg, classifying Api-7-O-Glc in category 5 toxicity under GHS classification [79], and showed no hepatotoxic and carcinogenic risks. These predictions support the safety profile of Api-7-O-Glc as an active ingredient, emphasizing its potential suitability for nutraceuticals, functional food and/or cosmeceutical applications. Moreover, its preparation as a topical formulation is likely to be more beneficial than oral supplements, based on ADMET predictions.
3.3. In Vitro Anti-Aging Activities
The results from in vitro aging-related enzyme inhibition assay show that Api-7-O-Glc, which is the most abundant major flavonoid occurring in M. angustifolia medicinal plants, exhibits anti-aging potential against three skin-aging-related enzymes, especially anti-collagenase and anti-elastase (Table 4). Furthermore, Api-7-O-Glc plays an important role as an anti-aging agent and shows a higher percentage of enzyme inhibition than the controls for collagenase (59.18 ± 6.24% of enzyme inhibition) and elastase (56.13 ± 7.49% of enzyme inhibition) (Table 4). However, this compound plays a weak anti-aging role in inhibiting tyrosinase (54.18 ± 6.54% of enzyme inhibition) compared with the controls.

Table 4.
The in vitro skin-aging-related enzyme inhibitions of Api-7-O-Glc.
On the one hand, weak anti-tyrosinase activity was also reported in the studies of Sezen Karaoğlan and his team in 2023 [80] and the team of Nasr Bouzaiene in 2016 [81], but on the other hand, the anti-aging effects of apigenin-7-O-glucoside on collagenase and elastase were not investigated in those previous studies. In comparison with the results from in silico assessment, which showed that apigenin-7-O-glucoside is able to inhibit all three enzymes, with a higher % inhibition compared with their controls, the results from in vitro assessment revealed that this flavone is the more potent anti-aging agent to inhibit collagenase and elastase (Table 4), with a higher % inhibition compared with their controls with the statistical differences. In the case of in vitro anti-tyrosinase, this compound exhibited weak anti-aging potential, with a lower % of enzyme inhibition compared with its control (Table 4). This inconsistency between the in silico molecular model and in vitro assessment commonly occurred not only in this current study but also the other previous published works [82,83,84], especially in the case of anti-tyrosinase, which the in silico results often showed a higher % of tyrosinase inhibition [82,83] than that of in vitro analysis because the in silico results are often influenced by the molecular size of the docked molecules. Reasonable binding poses and higher levels of binding interactions between docked molecules and proteins are the key factors affecting the docked score or binding affinity. While both molecules and proteins exhibit flexibility during the in vitro testing, kojic acid, a smaller molecule, may effectively utilize this flexibility more effectively than Api-7-O-Glc, leading to stronger interactions with the tyrosinase enzyme and a greater percentage of enzyme inhibition, like the previous works in 2025 from Tahtaci et al. [82] and Jung et al. [83]. However, the results from both in silico and in vitro models in this recent study exposed a similar trend to show that apigenin-7-O-glucoside is a potential anti-collagenase and anti-elastase, respectively. It is clearly seen that comparative studies using both in silico molecular docking and in vitro models provide useful fundamental data to find the most effective anti-aging property of the studied compound, which will be helpful for future research and development of phytopharmaceutical products.
Additionally, apigenin aglycone was also reported to be able to reduce the expression of collagenase [85]. In addition, the result from this current study is also consistent with many previously published works that reported on the potential of flavonoids as the anti-aging phytochemical compounds for nutraceuticals, functional food/cosmeceutical or phytopharmaceutical applications [27,80,81,86,87,88,89,90,91,92,93]. It is worth noting that these proteins were from non-human sources due to the commercial availability of these enzymes for in vitro testing. Importantly, the collagenase from Clostridium histolyticum and pancreatic porcine elastase has typically been used for screening various compounds/plant extracts as inhibitors [53,92]. Likewise, tyrosinase from the mushroom, Agaricus bisporus, is commonly employed as an enzymatic in vitro model for developing skin-whitening substances that target human tyrosinase [54,93]. Consequently, it can be supposed that the in vitro assays performed with these enzymes are reliable experiments for the fundamental step of anti-aging agent evaluation [94]. In addition, the results from this recent study were represented by working on non-human enzymes to determine the anti-aging properties of this abundant phytochemical compound found in M. angustifolia medicinal plants. It will also be interesting to find out in future work about its anti-aging potential on human enzymes.
It is commonly known that the skin’s extracellular matrix components are degraded by these aging enzymes, e.g., collagenase, elastase, and so on, leading to the loss of skin tone, deep wrinkles and reduced resilience [20,95,96,97]. In addition, the progression of the aging process, including the function of tyrosinase enzymes, can cause malignant melanoma and also pigmentary abnormalities, e.g., freckles or melisma [86,98]. Consequently, the phytochemical compounds and/or potential extracts which are able to inhibit the activity or processes of these aging enzymes are very helpful and interesting to the cosmeceutical, cosmetics and phytopharmaceutical industries. Interestingly, it is worth noting that this study is the first report on in silico molecular docking as well as the first completed report of Apigenin-7-O-glucoside, the most abundant phytochemical from Siam violet pearl medicinal plants, and on anti-aging potentials compared between anti-tyrosinase, anti-collagenase and anti-elastase effects. Remarkably, this Thai medicinal plant has been continuously consumed as a food ingredient in various dishes, a herbal tea and a traditional medicinal remedy since the ancient periods [5,24,28,29,30,31,32], which will be helpful to ensure the safety of using this medicinal plant as an alternative raw material for nutraceuticals, cosmetic, cosmeceutical and other phytopharmaceutical product development focusing on anti-aging properties.
4. Conclusions
To recapitulate, this study is the first report to determine the anti-aging capacities of the most abundant flavonoid phytochemical, Api-7-O-Glc, found in Siam violet pearl medicinal plants from Thailand using not only in silico molecular modeling but also in vitro enzymatic assessments such as anti-collagenase, anti-elastase and anti-tyrosinase activities. Furthermore, the pharmacokinetic properties based on quantitative structure–activity relationship databases, e.g., adsorption, distribution, metabolism, excretion and toxicity, also supported the safety profile of this flavone for nutraceuticals, functional food, cosmeceutical and/or phytopharmaceutical applications. Remarkedly, the present study mainly focuses on evaluating the in silico and in vitro anti-aging potential of the most abundant flavonoid from Siam violet pearl plant; however, it is also possible to discover the interesting biological actions/medicinal potential of other structurally related flavones, as well as the other less abundant flavonoids from this medicinal plant species that will be interesting and useful as the next research question for future research works. Nevertheless, the future clinical study of the safety and efficacy of the developed products formulated from this bioactive molecule/extract should be conducted before launching those products in the marketplace.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14030272/s1, Table S1: Fitness score of phytochemical flavonoids isolated from Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum docked into collagenase, elastase and tyrosinase enzymes.
Author Contributions
Conceptualization, D.T.; methodology, D.T. and C.A.; software, C.A.; validation, D.T., C.A. and S.K.; formal analysis, D.T. and C.A.; investigation, D.T.; resources, D.T., C.A. and S.K.; data curation, D.T. and C.A.; writing—original draft preparation, D.T., C.A. and S.K.; writing—review and editing, D.T., C.A. and S.K.; visualization, D.T. and C.A.; supervision, D.T.; project administration, D.T.; funding acquisition, D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Mahidol University (Fundamental Fund: fiscal year 2024 by National Science Research and Innovation Fund (NSRF)). This research project is supported by Mahidol University (Strategic Research Fund): fiscal year 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability on request due to restrictions.
Acknowledgments
D.T. sincerely thanks MU’s Strategic Research Fund (World Class). This research project is supported by Mahidol University (MU’s Strategic Research Fund): 2023. Additionally, D.T. and S.K. gratefully acknowledge Mahidol University, Thailand. This research project has been funded by Mahidol University (Fundamental Fund: fiscal year 2024 by National Science Research and Innovation Fund (NSRF)). C.A. would like to extend their gratitude to Mahidol University and the Program Management Unit for Competitiveness (PMU-C), Office of National Higher Education Science Research and Innovation Policy Council, Thailand, under contract number C10F640366, for the financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C-Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Scognamiglio, M.; D’Abrosca, B.; Piccolella, S.; Tsafantakis, N.; Gallicchio, M.; Ricci, A.; Fiorentino, A. Spectroscopic Characterization and Antiproliferative Activity on HepG2 Human Hepatoblastoma Cells of Flavonoid C-Glycosides from Petrorhagia velutina. J. Nat. Prod. 2010, 73, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.; Gođevac, D.; Tešević, V.; Dajić-Stevanović, Z.; Ćirić, A.; Soković, M.; Novaković, M. Antibacterial and Antibiofilm Activity of Flavonoid and Saponin Derivatives from Atriplex tatarica against Pseudomonas aeruginosa. J. Nat. Prod. 2019, 82, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jin, J.; Ruan, J.; Zhu, C.; Lin, C.; Fang, W.; Cai, Y. Antioxidant Flavonoid Glycosides from Aerial Parts of the Fern Abacopteris penangiana. J. Nat. Prod. 2007, 70, 1683–1686. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Takenaka, Y.; Chen, C.-C.; Pelletier, J. Flavonoid Glycosides from Adina racemosa and Their Inhibitory Activities on Eukaryotic Protein Synthesis. J. Nat. Prod. 2004, 67, 427–431. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef]
- Nassief, S.M.; Amer, M.E.; Shawky, E.; Sishtla, K.; Mas-Claret, E.; Muniyandi, A.; Corson, T.W.; Mulholland, D.A.; El-Masry, S. Antiangiogenic Pterocarpan and Flavonoid Constituents of Erythrina lysistemon. J. Nat. Prod. 2023, 86, 759–766. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Zeng, M.; Sun, R.; Basu, S.; Ma, Y.; Ge, S.; Yin, T.; Gao, S.; Zhang, J.; Hu, M. Disposition of flavonoids via recycling: Direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol. Nutr. Food Res. 2016, 60, 1006–1019. [Google Scholar] [CrossRef]
- Hour, T.-C.; Lan Nhi, N.T.; Lai, I.J.; Chuu, C.-P.; Lin, P.-C.; Chang, H.-W.; Su, Y.-F.; Chen, C.-H.; Chen, Y.-K. Kaempferol-Enhanced Migration and Differentiation of C2C12 Myoblasts via ITG1B/FAK/Paxillin and IGF1R/AKT/mTOR Signaling Pathways. Mol. Nutr. Food Res. 2024, 68, 2300685. [Google Scholar] [CrossRef] [PubMed]
- Ozalp Unal, D.; Sel, T. Investigation of Antiproliferative Effects of Combinations of White and Black Garlic Extracts with 5-Fluorouracil (5-FU) on Caco-2 Colorectal Adenocarcinoma Cells. Mol. Nutr. Food Res. 2024, 68, 2300820. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, Y.; Zi, J.; Hu, Y.; Ma, G.; Wang, X.; Shan, S.; Cheng, G.; Xiong, J. Dietary Flavonoids Consumption and Health: An Umbrella Review. Mol. Nutr. Food Res. 2024, 68, 2300727. [Google Scholar] [CrossRef] [PubMed]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and its Dermatological Applications: A Comprehensive Review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Wang, Z.; Du, X.; Ye, G.; Wang, H.; Liu, Y.; Liu, C.; Li, F.; Ågren, H.; Zhou, Y.; Li, J.; et al. Functional Characterization, Structural Basis, and Protein Engineering of a Rare Flavonoid 2′-O-glycosyltransferase from Scutellaria baicalensis. Acta Pharm. Sin. B 2024, 14, 3746–3759. [Google Scholar] [CrossRef]
- Boligon, A.A.; de Freitas, R.B.; de Brum, T.F.; Waczuk, E.P.; Klimaczewski, C.V.; de Ávila, D.S.; Athayde, M.L.; de Freitas Bauermann, L. Antiulcerogenic Activity of Scutia buxifolia on Gastric Ulcers Induced by Ethanol in Rats. Acta Pharm. Sin. B 2014, 4, 358–367. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef]
- Luo, K.; Huang, W.; Qiao, L.; Zhang, X.; Yan, D.; Ning, Z.; Ma, C.; Dang, H.; Wang, D.; Guo, H.; et al. Dendrocalamus latiflorus and its Component Rutin Exhibit Glucose-lowering Activities by Inhibiting Hepatic Glucose Production via AKT Activation. Acta Pharm. Sin. B 2022, 12, 2239–2251. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically Modified Isoquercitrin Improves Endothelial Function in Volunteers at Risk of Cardiovascular Disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Validation of a High-Performance Liquid Chromatography with Photodiode Array Detection Method for the Separation and Quantification of Antioxidant and Skin Anti-Aging Flavonoids from Nelumbo nucifera Gaertn. Stamen Extract. Molecules 2022, 27, 1102. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Chen, R.; Shen, J.; Zhang, S.; Gu, Y.; Shi, J.; Meng, G. Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants 2023, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Effect of Traditional Cooking and In Vitro Gastrointestinal Digestion of the Ten Most Consumed Beans from the Fabaceae Family in Thailand on Their Phytochemicals, Antioxidant and Anti-Diabetic Potentials. Plants 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Segura-Carretero, A. Enhancing the Yield of Bioactive Compounds from Sclerocarya birrea Bark by Green Extraction Approaches. Molecules 2019, 24, 966. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Garros, L.; Lorenzo, J.M.; Hano, C. Flavonoid Profiles and Antioxidant Potential of Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, a New Species from the Genus Monochoria C. Presl. Antioxidants 2022, 11, 952. [Google Scholar] [CrossRef]
- Gagandeep, M.; Biplab, S.; Ashish, K.; Rahul, S.; Awanish, M. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Faysal, M.; Dehbia, Z.; Zehravi, M.; Sweilam, S.H.; Haque, M.A.; Kumar, K.P.; Chakole, R.D.; Shelke, S.P.; Sirikonda, S.; Nafady, M.H.; et al. Flavonoids as Potential Therapeutics Against Neurodegenerative Disorders: Unlocking the Prospects. Neurochem. Res. 2024, 49, 1926–1944. [Google Scholar] [CrossRef]
- Menichini, F.; Losi, L.; Bonesi, M.; Pugliese, A.; Loizzo, M.R.; Tundis, R. Chemical Profiling and In Vitro Biological Effects of Cardiospermum halicacabum L. (Sapindaceae) Aerial Parts and Seeds for Applications in Neurodegenerative Disorders. J. Enzyme Inhib. Med. Chem. 2014, 29, 677–685. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Tanaka, N.; Boonkerd, T. Monochoria angustifolia (G. X. Wang) Boonkerd & Tungmunnithum, the New Species of the Genus Monochoria from Thailand. Songklanakarin J. Sci. Technol. 2020, 42, 747–752. [Google Scholar]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Phenetic Study of the Genus Monochoria in Thailand. Songklanakarin J. Sci. Technol. 2017, 39, 49–57. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Boonkerd, T.; Zungsontiporn, S.; Tanaka, N. Morphological Variations among Populations of Monochoria vaginalis s.l. (Pontederiaceae) in Thailand. Phytotaxa 2017, 268, 57. [Google Scholar] [CrossRef]
- Chayamarit, K. Pontederiaceae. In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2005; Volume 9, pp. 51–57. [Google Scholar]
- Wu, G.; Horn, C.N. Pontederiaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 40–42. [Google Scholar]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2024, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Wołos, A.; Koszelewski, D.; Roszak, R.; Szymkuć, S.; Moskal, M.; Ostaszewski, R.; Herrera, B.T.; Maier, J.M.; Brezicki, G.; Samuel, J.; et al. Computer-designed Repurposing of Chemical Wastes into Drugs. Nature 2022, 604, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L. Grand Challenges of Computer-Aided Drug Design: The Road Ahead. Front. Drug Discov. 2021, 1, 728551. [Google Scholar] [CrossRef]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Cavasotto, C.N.; Scardino, V. Machine Learning Toxicity Prediction: Latest Advances by Toxicity End Point. ACS Omega 2022, 7, 47536–47546. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Alhayek, A.; Abdelsamie, A.S.; Schönauer, E.; Camberlein, V.; Hutterer, E.; Posselt, G.; Serwanja, J.; Blöchl, C.; Huber, C.G.; Haupenthal, J.; et al. Discovery and Characterization of Synthesized and FDA-Approved Inhibitors of Clostridial and Bacillary Collagenases. J. Med. Chem. 2022, 65, 12933–12955. [Google Scholar] [CrossRef] [PubMed]
- Jhoti, H.; Singh, O.M.P.; Wonacott, A. Structure of Porcine Pancreatic Elastase Complexed with the Elastase Inhibitor GR143783. Available online: https://www.rcsb.org/structure/1BRU (accessed on 14 September 2024).
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chao, H.; Chen, L.; Craig, P.A.; Crichlow, G.V.; Dalenberg, K.; Duarte, J.M.; et al. RCSB Protein Data Bank (RCSB.org): Delivery of Experimentally-determined PDB Structures Alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning. Nucleic Acids Res. 2023, 51, D488–D508. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Biovia Discovery Studio® Overview. Available online: https://discover.3ds.com/sites/default/files/2023-12/biovia-discovery-studio-overview-datasheet.pdf (accessed on 9 November 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Chai, W.-M.; Huang, Q.; Lin, M.-Z.; Ou-Yang, C.; Huang, W.-Y.; Wang, Y.-X.; Xu, K.-L.; Feng, H.-L. Condensed Tannins from Longan Bark as Inhibitor of Tyrosinase: Structure, Activity, and Mechanism. J. Agric. Food. Chem. 2018, 66, 908–917. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Fernie, A.R. Diversity: Current and Prospective Secondary Metabolites for Nutrition and Medicine. Curr. Opin. Biotechnol. 2022, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; Potapovich, A.; Mayer, W.; Talib, N.; De Luca, C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics 2018, 5, 32. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Garros, L.; Hano, C. Differential Flavonoid and Other Phenolic Accumulations and Antioxidant Activities of Nymphaea lotus L. Populations throughout Thailand. Molecules 2022, 27, 3590. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Gohlke, H.; Hendlich, M.; Klebe, G. Knowledge-based Scoring Function to Predict Protein-ligand Interactions. J. Mol. Biol. 2000, 295, 337–356. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Fischer, A.; Smieško, M.; Sellner, M.; Lill, M.A. Decision Making in Structure-Based Drug Discovery: Visual Inspection of Docking Results. J. Med. Chem. 2021, 64, 2489–2500. [Google Scholar] [CrossRef]
- Patel, N.Y.; Baria, D.M.; Pardhi, D.S.; Yagnik, S.M.; Panchal, R.R.; Rajput, K.N.; Raval, V.H. Chapter 16—Microbial enzymes in pharmaceutical industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 375–403. [Google Scholar]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based Specific Detection and Inhibition of Tyrosinase and Their Application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef]
- Nutho, B.; Tungmunnithum, D. Anti-Aging Potential of the Two Major Flavonoids Occurring in Asian Water Lily Using In Vitro and In Silico Molecular Modeling Assessments. Antioxidants 2024, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarc’h, N.; Hano, C. Isodon rugosus (Wall. ex Benth.) Codd In Vitro Cultures: Establishment, Phytochemical Characterization and In Vitro Antioxidant and Anti-Aging Activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Brás, N.F.; Gonçalves, R.; Fernandes, P.A.; Mateus, N.; Ramos, M.J.; de Freitas, V. Understanding the Binding of Procyanidins to Pancreatic Elastase by Experimental and Computational Methods. Biochemistry 2010, 49, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, X.; Jin, Z.; Cheng, H. Collagenases and their inhibitors: A review. Collagen Leather 2023, 5, 19. [Google Scholar] [CrossRef]
- Tohar, R.; Ansbacher, T.; Sher, I.; Afriat-Jurnou, L.; Weinberg, E.; Gal, M. Screening Collagenase Activity in Bacterial Lysate for Directed Enzyme Applications. Int. J. Mol. Sci. 2021, 22, 8552. [Google Scholar] [CrossRef]
- Nokinsee, D.; Shank, L.; Lee, V.S.; Nimmanpipug, P. Estimation of Inhibitory Effect against Tyrosinase Activity through Homology Modeling and Molecular Docking. Enzyme Res. 2015, 2015, 262364. [Google Scholar] [CrossRef]
- Le, J. Overview of Pharmacokinetics. Available online: https://www.msdmanuals.com/professional/clinical-pharmacology/pharmacokinetics/overview-of-pharmacokinetics (accessed on 9 November 2024).
- Grogan, S.; Preuss, C.V. Pharmacokinetics. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557744/ (accessed on 9 November 2024).
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, C.C.; Huang, C.W.; Chang, Y.C. Evaluating Molecular Properties Involved in Transport of Small Molecules in Stratum Corneum: A Quantitative Structure-Activity Relationship for Skin Permeability. Molecules 2018, 23, 911. [Google Scholar] [CrossRef]
- Alves, V.M.; Muratov, E.; Fourches, D.; Strickland, J.; Kleinstreuer, N.; Andrade, C.H.; Tropsha, A. Predicting Chemically-induced Skin Reactions. Part II: QSAR Models of Skin Permeability and the Relationships Between Skin Permeability and Skin Sensitization. Toxicol. Appl. Pharmacol. 2015, 284, 273–280. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 10th ed.; United Nations Publications: New York, NY, USA, 2023. [Google Scholar]
- Sezen Karaoğlan, E.; Hancı, H.; Koca, M.; Kazaz, C. Some Bioactivities of Isolated Apigenin-7-O-glucoside and Luteolin-7-O-glucoside. Appl. Sci. 2023, 13, 1503. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of Apigenin-7-glucoside, Genkwanin and Naringenin on Tyrosinase Activity and Melanin Synthesis in B16F10 Melanoma Cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tahtaci, H.; Ozcan, I.; Mirghani, A.H.; Erdogan, T.; Kisa, D.; Yıldırım, B. 1,2,4-Triazole-derived oxime ether derivatives: Synthesis, characterization, in vitro tyrosinase inhibition properties and in silico studies. J. Mol. Struct. 2025, 1323, 140722. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, H.J.; Park, H.S.; Park, H.S.; Ko, J.; Yoon, D.; Park, Y.; Chun, P.; Chung, H.Y.; Moon, H.R. Design, Synthesis, and Antioxidant and Anti-Tyrosinase Activities of (Z)-5-Benzylidene-2-(naphthalen-1-ylamino)thiazol-4(5H)-one Analogs: In Vitro and In Vivo Insights. Molecules 2025, 30, 289. [Google Scholar] [CrossRef]
- Vasilev, H.; Šmejkal, K.; Jusková, S.; Vaclavik, J.; Treml, J. Five New Tamarixetin Glycosides from Astragalus thracicus Griseb. Including Some Substituted with the Rare 3-Hydroxy-3-methylglutaric Acid and Their Collagenase Inhibitory Effects In Vitro. ACS Omega 2024, 9, 18023–18031. [Google Scholar] [CrossRef]
- Choi, S.; Youn, J.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.-S.; Kwon, S.; Youn, H.J.; et al. Apigenin Inhibits UVA-Induced Cytotoxicity In Vitro and Prevents Signs of Skin Aging In Vivo. Int. J. Mol. Med. 2016, 38, 627–634. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells 2022, 11, 599. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside Versus Apigenin: Insight into the Modes of Anticandidal and Cytotoxic Actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Wang, W.; Yue, R.-F.; Jin, Z.; He, L.-M.; Shen, R.; Du, D.; Tang, Y.-Z. Efficiency Comparison of Apigenin-7-O-glucoside and Trolox in Antioxidative Stress and Anti-inflammatory Properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef]
- Shi, Q.-Q.; Dang, J.; Wen, H.-X.; Yuan, X.; Tao, Y.-D.; Wang, Q.-L. Anti-hepatitis, Antioxidant Activities and Bioactive Compounds of Dracocephalum heterophyllum Extracts. Bot. Stud. 2016, 57, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, H.; Li, J.; Fang, X.; Wu, H.; Hu, W. Apigetrin Alleviates Intervertebral Disk Degeneration by Regulating Nucleus Pulposus Cell Autophagy. JOR SPINE 2024, 7, e1325. [Google Scholar] [CrossRef] [PubMed]
- Cetiz, M.; Isah, M.; Ak, G.; Bakar, K.; Ahamada-Himidi, A.; Mohamed, A.; Glamoclija, J.; Nikolić, F.; Gašić, U.; Cespedes-Acuña, C.L.; et al. Exploring of Chemical Profile and Biological Activities of Three Ocimum Species From Comoros Islands: A Combination of In Vitro and In Silico Insights. Cell Biochem. Funct. 2024, 42, e70000. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, U.; Schönauer, E.; Nüss, D.; Brandstetter, H. Structure of Collagenase G Reveals a Chew-and-digest Mechanism of Bacterial Collagenolysis. Nat. Struct. Mol. Biol. 2011, 18, 1109–1114. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Li, J.; Xiong, J.; Xiao, B.-L. Inhibitory Mechanism on Tyrosinase Activity of Flavonoids from Flower Buds of Sophora japonica L. Heliyon 2024, 10, e38252. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Boran, R. Investigations of anti-aging potential of Hypericum origanifolium Willd. for skincare formulations. Ind. Crop Prod. 2018, 118, 290–295. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Coricovac, D.; Soica, C.; Muntean, D.; Popovici, R.; Dehelean, C.; Hogea, E. Assessment of the effects induced by two triterpenoids on liver mitochondria respiratory function isolated from aged rats. Rev. Chim. 2015, 66, 1707–1710. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and Instrumental Approaches to Treat Hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).