Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Pilot Scale Processes
2.3.1. Equipment
2.3.2. Enzyme-Assisted Extraction Pre-Treatment (EAE)
2.3.3. Microwave-Assisted Extraction (EMAE)
2.3.4. Ultrasound-Assisted Extraction (EUAE)
2.3.5. Ultrasound–Microwave-Assisted Extraction (EUMAE)
2.3.6. Process of Phenolic Compounds Purification
2.4. Determination of the Extraction Yield
2.5. Determination and Quantification of Bioactive Compounds by HPLC-MS
2.6. Determination of Antioxidant Activity
2.6.1. Folin–Ciocalteu (F-C) Assay
2.6.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.4. DPPH Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Yield of Extraction and Total Phenolic Content of the Extracts
3.1.1. Enzyme-Assisted Extraction (EAE) Pretreatment
3.1.2. Extraction of Phenolic Compounds by MAE, UAE and UMAE
3.2. Purification of Phenolic Compounds
3.3. Determination and Quantification of Bioactive Compounds by HPLC-MS
3.4. Determination of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMAE | Enzyme–microwave-assisted extraction |
| EAE | Enzyme-assisted extraction |
| EUAE | Enzyme–ultrasound-assisted extraction |
| EUMAE | Enzyme–ultrasound–microwave-assisted extraction |
| F-C | Folin–Ciocalteu |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| HPLC | High pressure liquid chromatography |
| GAE | Gallic acid equivalents |
| TE | Trolox equivalents |
| PE | Purified extract |
| TPC | Total phenolic content |
| MS | Mass spectrometer |
| QTOF | Quadrupole time of flight |
| ESI | Electrospray ionization |
| TPTZ | 2,4,6-Tris(2-pyridyl)-s-triazine |
| Rt | Retention time |
| SD | Standard deviation |
| Rpm | Revolutions per minute |
| d.w. | Dry artichoke waste weight |
| d.e. | Dry artichoke waste extract |
| S/L | Solid to liquid |
References
- Garcia-Castello, E.M.; Mayor, L.; Calvo-Ramirez, A.; Ruiz-Melero, R.; Rodriguez-Lopez, A.D. Response Surface Optimization of Inulin and Polyphenol Extraction from Artichoke (Cynara scolymus (L.)) Solid Wastes. Appl. Sci. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Tortosa-Díaz, L.; Saura-Martínez, J.; Taboada-Rodríguez, A.; Martínez-Hernández, G.B.; López-Gómez, A.; Marín-Iniesta, F. Influence of Industrial Processing of Artichoke and By-Products on The Bioactive and Nutritional Compounds. Food Eng. Rev. 2025. [Google Scholar] [CrossRef]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive Compounds from Artichoke and Application Potential. Food Technol. Biotechnol. 2023, 61, 312–327. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Duarte, M.P.; Andrade, M.A.; Mateus, A.R.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Exploring Cynara cardunculus L. by-Products Potential: Antioxidant and Antimicrobial Properties. Ind. Crops Prod. 2024, 222, 119559. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Mezzani, I.; Alicandri, E.; Tomassi, W.; Paolacci, A.R.; Covino, S.; Vinciguerra, V.; Catalani, E.; Cervia, D.; Ciaffi, M.; et al. The Role of Extracts of Edible Parts and Production Wastes of Globe Artichoke (Cynara cardunculus L. var. scolymus (L.)) in Counteracting Oxidative Stress. Antioxidants 2025, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, A.R.; De Bellis, P.; Montemurro, M.; D’Antuono, I.; Linsalata, V.; Cardinali, A. Characterization and Functional Application of Artichoke Bracts: Enrichment of Bread with Health Promoting Compounds. LWT 2025, 215, 117256. [Google Scholar] [CrossRef]
- Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods 2022, 11, 2250. [Google Scholar] [CrossRef]
- Deng, A.; Liu, F.; Tang, X.; Wang, Y.; Xie, P.; Yang, Q.; Xiao, B. Water Extract from Artichoke Ameliorates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats. BMC Complement. Med. Ther. 2022, 22, 308. [Google Scholar] [CrossRef]
- Corrias, F.; Scano, E.; Milia, M.; Atzei, A.; Casula, M.; Arru, N.; Angioni, A. Extraction and Characterization of Artichoke (Cynara cardunculus L.) Solid Waste from the Industrial Processing of Fresh-Cut Products for Nutraceutical Use. Foods 2025, 14, 13. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Bin Mokaizh, A.A.; Baarimah, A.O.; Al-Zghoul, T. From Agro-Waste to Bioactive Wealth: Analyzing Nutraceutical Extraction and Applications. Case Stud. Chem. Environ. Eng. 2025, 11, 101066. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Salgueiro, L.; Cocco, E.; Ghiani, V.; Falconieri, D.; Maccioni, D.; Maxia, A. Agroprospecting of Biowastes: Globe Artichoke (Cynara scolymus L. Cultivar Tema, Asteraceae) as Potential Source of Bioactive Compounds. Molecules 2024, 29, 3960. [Google Scholar] [CrossRef] [PubMed]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García-Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke By-Products as Natural Source of Phenolic Food Ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) Byproducts as a Potential Source of Health-Promoting Antioxidant Phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of Artichoke Byproducts to Obtain Bioactive Extracts Enriched in Inositols and Caffeoylquinic Acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Das, R.S.; Tiwari, B.K.; Selli, S.; Kelebek, H.; Garcia-Vaquero, M. Exploring Pilot Scale Ultrasound-Microwave Assisted Extraction of Organic Acids and Phytochemicals from Brown Seaweed Alaria Esculenta. Algal Res. 2025, 86, 103896. [Google Scholar] [CrossRef]
- Cheriyan, B.V.; Karunakar, K.K.; Anandakumar, R.; Murugathirumal, A.; Senthil kumar, A. Eco-Friendly Extraction Technologies: A Comprehensive Review of Modern Green Analytical Methods. Sustain. Chem. Clim. Action 2025, 6, 100054. [Google Scholar] [CrossRef]
- El-Hadidy, G.S.; Elmeshad, W.; Abdelgaleel, M.; Ali, M. Extraction, Identification, and Quantification of Bioactive Compounds from Globe Artichoke (Cynara cardunculus var. scolymus). Sains Malays. 2022, 51, 2843–2855. [Google Scholar] [CrossRef]
- Kayahan, S.; Saloglu, D. Optimization and Kinetic Modelling of Microwave-Assisted Extraction of Phenolic Contents and Antioxidants from Turkish Artichoke. CyTA—J. Food 2020, 18, 635–643. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Comendador, F.J. Response Surface Methodology as an Experimental Strategy for Ultrasound-Assisted Extraction of Phenolic Compounds from Artichoke Heads. Antioxidants 2023, 12, 1360. [Google Scholar] [CrossRef]
- Akdogan, D.; Peksel, A. The Effectiveness of Ultrasound-Assisted Extraction on Antioxidative Properties of Bract Leaves of Globe Artichoke. Eur. J. Biol. 2023, 82, 296–305. [Google Scholar] [CrossRef]
- Cannas, M.; Conte, P.; Piga, A.; Del Caro, A. Green Recovery Optimization of Phenolic Compounds from “Spinoso Sardo” Globe Artichoke by-Products Using Response Surface Methodology. Front. Sustain. Food Syst. 2023, 7, 1215809. [Google Scholar] [CrossRef]
- Bittencourt, G.M.; Simprônio, M.d.R.; Mothé, I.R.; Ferreira, G.R.; de Oliveira, A.L. Globe Artichoke Leaf Extracts and Production of Phytotherapeutic Solid Lipid Particles Using High Pressure Technologies. J. Supercrit. Fluids 2023, 201, 106028. [Google Scholar] [CrossRef]
- Pagliari, S.; Cannavacciuolo, C.; Celano, R.; Carabetta, S.; Russo, M.; Labra, M.; Campone, L. Valorisation, Green Extraction Development, and Metabolomic Analysis of Wild Artichoke By-Product Using Pressurised Liquid Extraction UPLC–HRMS and Multivariate Data Analysis. Molecules 2022, 27, 7157. [Google Scholar] [CrossRef] [PubMed]
- Carullo, D.; Carpentieri, S.; Ferrari, G.; Pataro, G. Influence of Mechanical Comminution of Raw Materials and PEF Treatment on the Aqueous Extraction of Phenolic Compounds from Artichoke Wastes. J. Food Eng. 2024, 369, 111939. [Google Scholar] [CrossRef]
- Ozkan, G. Valorization of Artichoke Outer Petals by Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents (NADES) for the Recovery of Phenolic Compounds. J. Sci. Food Agric. 2024, 104, 2744–2749. [Google Scholar] [CrossRef]
- Foophow, T.; Roytrakul, S.; Rungsardthong, V.; Phoohinkong, W. Sequential Green Extraction, Identification, and Encapsulation of Bioactive Compound from Phellinus Linteus Fruiting Body. Results Chem. 2025, 14, 102112. [Google Scholar] [CrossRef]
- Cao, D.; Bu, F.; Cheng, X.; Zhao, C.; Yin, Y.; Liu, P. Purification of Phenolics from Plantago Depressa by Macroporous Resin: Adsorption/Desorption Characteristics, Chromatographic Process Development and UPLC-TQ-MS/MS Quantitative Analysis. LWT 2024, 203, 116405. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.J.; Cho, S. Enrichment and Purification of Marine Polyphenol Phlorotannins Using Macroporous Adsorption Resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous Separation and Purification of Total Polyphenols, Chlorogenic Acid and Phlorizin from Thinned Young Apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Carle, R.; Stanley, R.A.; Saleh, Z.S. Pilot-Scale Resin Adsorption as a Means to Recover and Fractionate Apple Polyphenols. J. Agric. Food Chem. 2010, 58, 6787–6796. [Google Scholar] [CrossRef]
- Li, J.; Chase, H.A. Development of Adsorptive (Non-Ionic) Macroporous Resins and Their Uses in the Purification of Pharmacologically-Active Natural Products from Plant Sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Ramalakshmi, K.; Hithamani, G.; Asha, K.R.; Jagan Mohan Rao, L. Separation and Characterisation of Chlorogenic Acid-Rich Conserves from Green Coffee Beans and Their Radical Scavenging Potential. Int. J. Food Sci. Technol. 2011, 46, 109–115. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Garaigordobil, E.; Martínez-Lapuente, L.; Guadalupe, Z.; Pérez-Magariño, S.; Ayestarán, B. Recovery of Polysaccharides from Red Grape Marc and White Grape Pomace by Degradation of Cell Walls by Enzymes with Different Activities. Molecules 2025, 30, 213. [Google Scholar] [CrossRef]
- Rubtsova, E.A.; Bushina, E.V.; Rozhkova, A.M.; Korotkova, O.G.; Nemashkalov, V.A.; Koshelev, A.V.; Sinitsyn, A.P. Novel Enzyme Preparations with High Pectinase and Hemicellulase Activity Based on Penicillium Canescens Strains. Appl. Biochem. Microbiol. 2015, 51, 591–599. [Google Scholar] [CrossRef]
- Shet, A.R.; Desai, S.; Achappa, S. Pectinolytic Enzymes: Classification, Production, Purification and Applications. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef]
- Thang, N.Q.; Hoa, V.T.K.; Van Tan, L.; Tho, N.T.M.; Hieu, T.Q.; Phuong, N.T.K. Extraction of Cynarine and Chlorogenic Acid from Artichoke Leaves (Cynara scolymus L.) and Evaluation of Antioxidant Activity, Antibacterial Activity of Extract. Vietnam J. Chem. 2022, 60, 571–577. [Google Scholar] [CrossRef]
- Ayuso, P.; Peñalver, R.; Quizhpe, J.; Rosell, M.d.l.Á.; Nieto, G. Broccoli, Artichoke, Carob and Apple By-Products as a Source of Soluble Fiber: How It Can Be Affected by Enzymatic Treatment with Pectinex® Ultra SP-L, Viscozyme® L and Celluclast® 1.5 L. Foods 2025, 14, 10. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic Composition in Different Parts of Some Cultivars of Globe Artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauro, R.; Mauromicale, G. Variation of Phenolic Content in Globe Artichoke in Relation to Biological, Technical and Environmental Factors. Ital. J. Agron. 2009, 4, 181–189. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sonnante, G.; Laghetti, G.; Urbano, M. Polyphenolic Compound Variation in Globe Artichoke Cultivars as Affected by Fertilization and Biostimulants Application. Plants 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Extraction of Phenolic Compounds from Lime Peel Waste Using Ultrasonic-Assisted and Microwave-Assisted Extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Eldin Awad, O.M.; El-Sohaimy, S.A.; Ghareeb, D.A.; Aboulenein, A.M.; Saleh, S.R.; Abd El-Aziz, N.M. Phytochemical Analysis and Toxicity Assessment of Artichoke By-Product Extract. Pak. J. Biol. Sci. 2020, 23, 81–91. [Google Scholar] [CrossRef]
- Ma, N.B.; Tien, N.N.T.; Vu, L.T.K.; Ton, N.M.N.; Le, N.L. Comparison on Chemical, Structural, Thermal, Functional and Antioxidant Properties of Polysaccharides and Phenolics Co-Extracted from Mangosteen (Garcinia mangostana Linn) Peels by Different Eco-Friendly Hybrid Technologies. J. Agric. Food Res. 2024, 18, 101507. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wang, C.W.; Zhang, J.; Xu, P.P.; Xue, Y.T.; Wang, Q. Effects of Different Extraction Methods on Physicochemical Characteristics and Bioactivities of Fig (Ficus carica L.) Leaves Polysaccharides. Arab. J. Chem. 2023, 16, 105319. [Google Scholar] [CrossRef]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of Standards and Interfering Compounds in the Determination of Phenolics by Folin-Ciocalteu Assay Method for Effective Bioprocessing of Biomass. Am. J. Anal. Chem. 2017, 08, 416–431. [Google Scholar] [CrossRef]
- Ren, Y.; Makhele, M.; Zhou, J.; Sun, P. Extraction, Purification, Component Analysis and Bioactivity of Polyphenols from Wampee. Process Biochem. 2025, 150, 318–327. [Google Scholar] [CrossRef]
- Conidi, C.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M.; Cassano, A. Purification of Artichoke Polyphenols by Using Membrane Filtration and Polymeric Resins. Sep. Purif. Technol. 2015, 144, 153–161. [Google Scholar] [CrossRef]
- Gore, D.D.; Mishra, N.; Kumar, D.; Jena, G.; Jachak, S.M.; Tikoo, K.; Bansal, A.K.; Singh, I.P. Anti-Inflammatory Activity, Stability, Bioavailability and Toxicity Studies on Seabuckthorn Polyphenol Enriched Fraction and Its Phospholipid Complex (Phytosomes) Preparation. Int. J. Biol. Macromol. 2025, 297, 139919. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-rabaneda, F.; Jauregui, O.; Lamuela-raventos, R.M. Identification of Phenolic Compounds in Artichoke Waste by High- Performance Liquid Chromatography—Tandem Mass Spectrometry. J. Chromatogr. A 2003, 1008, 57–72. [Google Scholar] [CrossRef]
- Cerulli, A.; Cuozzo, R.; Melis, M.P.; Serreli, G.; Deiana, M.; Masullo, M.; Piacente, S. In-Depth LC-ESI/HRMS-Guided Phytochemical Analysis and Antioxidant Activity Analysis of Eco-Sustainable Extracts of Cynara cardunculus (Carciofo Di Paestum PGI) Leaves. Plants 2024, 13, 3591. [Google Scholar] [CrossRef]
- Cuffaro, D.; Palladino, P.; Digiacomo, M.; Bertini, S.; Minunni, M.; Macchia, M. Fast, Sensitive, and Sustainable Colorimetric Detection of Chlorogenic Acid in Artichoke Waste Material. Food Chem. 2025, 463, 141505. [Google Scholar] [CrossRef]
- Cioni, E.; Di Stasi, M.; Iacono, E.; Lai, M.; Quaranta, P.; Luminare, A.G.; Gambineri, F.; De Leo, M.; Pistello, M.; Braca, A. Enhancing Antimicrobial and Antiviral Properties of Cynara Scolymus L. Waste through Enzymatic Pretreatment and Lactic Fermentation. Food Biosci. 2024, 57, 103441. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- El-Askary, H.I.; Mohamed, S.S.; El-Gohari, H.M.A.; Ezzat, S.M.; Meselhy, M.R. Quinic Acid Derivatives from Artemisia Annua L. Leaves; Biological Activities and Seasonal Variation. S. Afr. J. Bot. 2020, 128, 200–208. [Google Scholar] [CrossRef]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A Flavone with Myriads of Bioactivities and Food Applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Pereira, V.; Figueira, O.; Castilho, P.C. Hesperidin: A Flavanone with Multifaceted Applications in the Food, Animal Feed, and Environmental Fields. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A Natural Bioactive Flavone-Type Molecule with Promising Therapeutic Function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Masala, V.; Jokic, S.; Aladic, K.; Molnar, M.; Casula, M.; Tuberoso, C.I.G. Chemical Profilineg and Evaluation of Antioxidant Activity of Artichoke (Cynara cardunculus var. scolymus) Leaf By-Products’Extracts Obtained with Green Extraction Techniques. Molecules 2024, 29, 4816. [Google Scholar] [CrossRef]
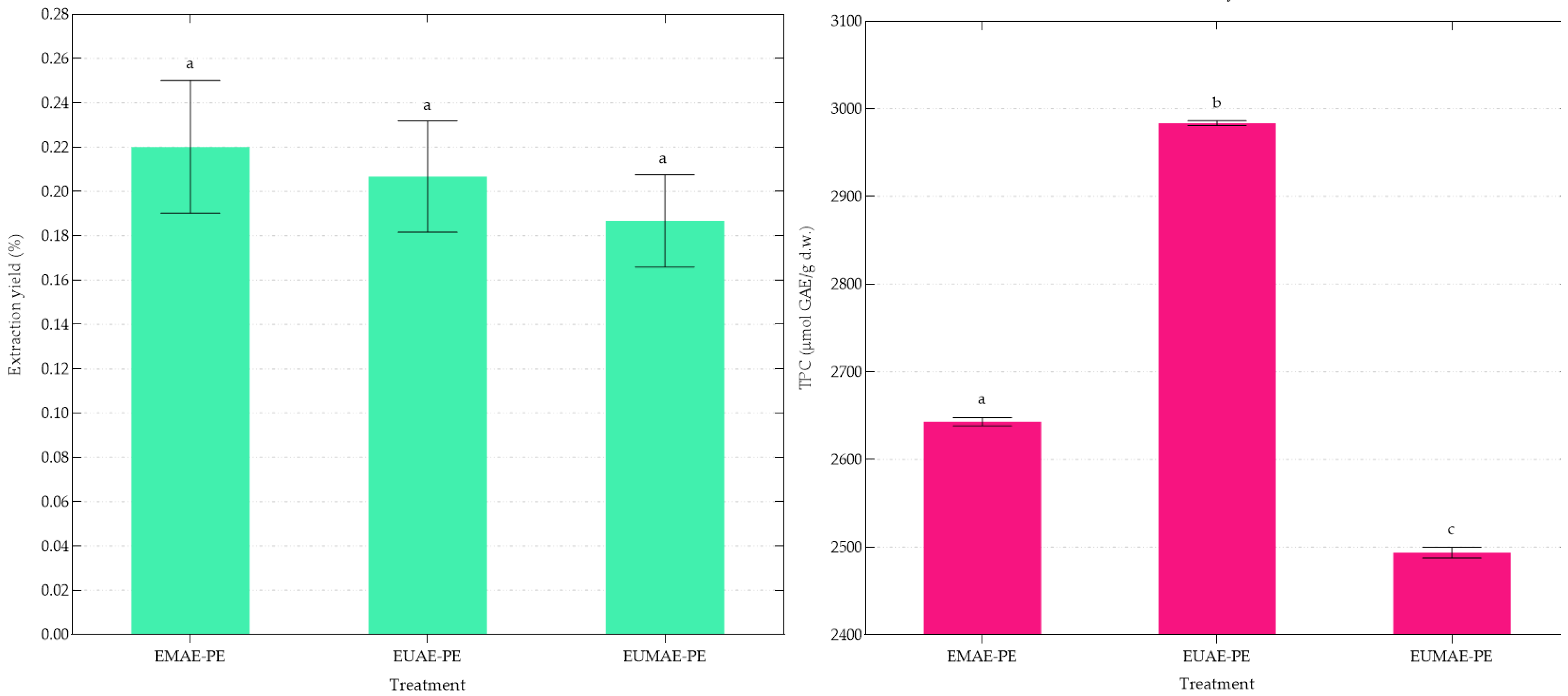


| Treatment | Extraction Yield, % (m/m) | TPC μmol GAE/g d.w. | TPC μmol GAE/g d.e. |
|---|---|---|---|
| EAE | 14.87 ± 0.92 | 44.82 ± 0.44 | 301.40 ± 2.07 |
| Treatment | Extraction Yield, % (m/m) | TPC μmol GAE/g d.w. |
|---|---|---|
| EMAE | 22.13 ± 1.34 a | 180.09 ± 1.94 a |
| EUAE | 21.38 ± 0.95 a | 210.76 ± 1.40 b |
| EUMAE | 24.68 ± 2.74 b | 211.35 ± 3.12 b |
| Peak | Rt (min) | m/z exp. | m/z calc. | Molecular Formula | Error (ppm) | Compound |
|---|---|---|---|---|---|---|
| Phenolic acids and derivatives | ||||||
| 4 | 2.78 | 353.0858 | 353.0878 | C16H18O9 | 5.31 | Chlorogenic acid isomer a |
| 5 | 5.214 | 163.0396 | 163.0401 | C9H8O3 | 2.58 | Coumaric acid isomer a |
| 6 | 5.214 | 179.0344 | 179.035 | C9H8O4 | 3.06 | Caffeic acid |
| 7 | 6.017 | 353.0855 | 353.0878 | C16H18O9 | 6.03 | Chlorogenic acid isomer b |
| 8 | 6.386 | 353.0849 | 353.0878 | C16H18O9 | 8.21 | Chlorogenic acid isomer c |
| 9 | 7.203 | 163.0396 | 163.0401 | C9H8O3 | 3.03 | Coumaric acid isomer b |
| 10 | 8.714 | 367.1014 | 367.1915 | C17H20O9 | −0.43 | Feruloylquinic acid |
| 13 | 12.223 | 515.115 | 515.1195 | C25H24O12 | 9.31 | Cynarin |
| Flavonoids and derivatives | ||||||
| 6 | 5.81 | 431.1888 | 431.0984 | C21H20O10 | −6.21 | Apigenin glucoside |
| 11 | 10.815 | 593.146 | 593.1512 | C27H30O15 | 10.45 | Luteolin rutinoside |
| 12 | 11.763 | 609.1824 | 609.1825 | C28H34O15 | 0.1 | Hesperidin |
| Other compounds | ||||||
| 1 | 0.525 | 191.0573 | 191.0561 | C7H12O6 | −5.99 | Quinic acid |
| 2 | 0.835 | 164.0712 | 164.0717 | C9H11NO2 | 2.84 | Phenylalanine |
| 3 | 1.983 | 203.0819 | 203.0829 | C11H12N2O2 | 3.32 | Tryptophan |
| FC μmol GAE/g d.e. | ABTS, μmol ET/g d.e | DPPH, μmol ET/g d.e. | FRAP, μmol ET/g d.e. | |
|---|---|---|---|---|
| EMAE | 814.93 ± 6.35 a | 91.29 ± 1.27 a | 75.68 ± 0.32 a | 178.67 ± 3.56 a |
| EUAE | 985.33 ± 4.46 b | 80.46 ± 2.39 b | 87.03 ± 1.11 b | 184.99 ± 2.52 b |
| EUMAE | 855.14 ± 5.93 c | 63.81 ± 0.97 c | 45.93 ± 0.52 c | 186.87 ± 0.52 b |
| EMAE-PE | 2642.91 ± 10.80 d | 446.69 ± 6.79 d | 531.32 ± 5.76 d | 595.39 ± 4.51 c |
| EUAE-PE | 2981.35 ± 12.16 e | 592.64 ± 5.96 e | 738.31 ± 6.78 e | 672.41 ± 2.68 d |
| EUMAE-PE | 2688.20 ± 12.54 f | 497.02 ± 10.22 f | 391.93 ± 5.87 f | 649.65 ± 4.21 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martínez, L.; de la Torre-Ramírez, J.M.; Martínez-López, S.; Ayuso-García, L.M.; Dellapina, G.; Poli, G.; Verardo, V.; Gómez-Caravaca, A.M. Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment. Antioxidants 2025, 14, 423. https://doi.org/10.3390/antiox14040423
Gil-Martínez L, de la Torre-Ramírez JM, Martínez-López S, Ayuso-García LM, Dellapina G, Poli G, Verardo V, Gómez-Caravaca AM. Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment. Antioxidants. 2025; 14(4):423. https://doi.org/10.3390/antiox14040423
Chicago/Turabian StyleGil-Martínez, Lidia, José Manuel de la Torre-Ramírez, Sofía Martínez-López, Luis Miguel Ayuso-García, Giovanna Dellapina, Giovanna Poli, Vito Verardo, and Ana María Gómez-Caravaca. 2025. "Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment" Antioxidants 14, no. 4: 423. https://doi.org/10.3390/antiox14040423
APA StyleGil-Martínez, L., de la Torre-Ramírez, J. M., Martínez-López, S., Ayuso-García, L. M., Dellapina, G., Poli, G., Verardo, V., & Gómez-Caravaca, A. M. (2025). Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment. Antioxidants, 14(4), 423. https://doi.org/10.3390/antiox14040423








