Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastics
2.2. Primary Cell Culture and Exposure
2.3. Tissue Integrity
2.4. Cytotoxicity Assay
2.5. Cilia Beat Frequency and Mucociliary Clearance
2.6. Protein Secretion
2.7. Chemotaxis
2.8. Statistics
3. Results
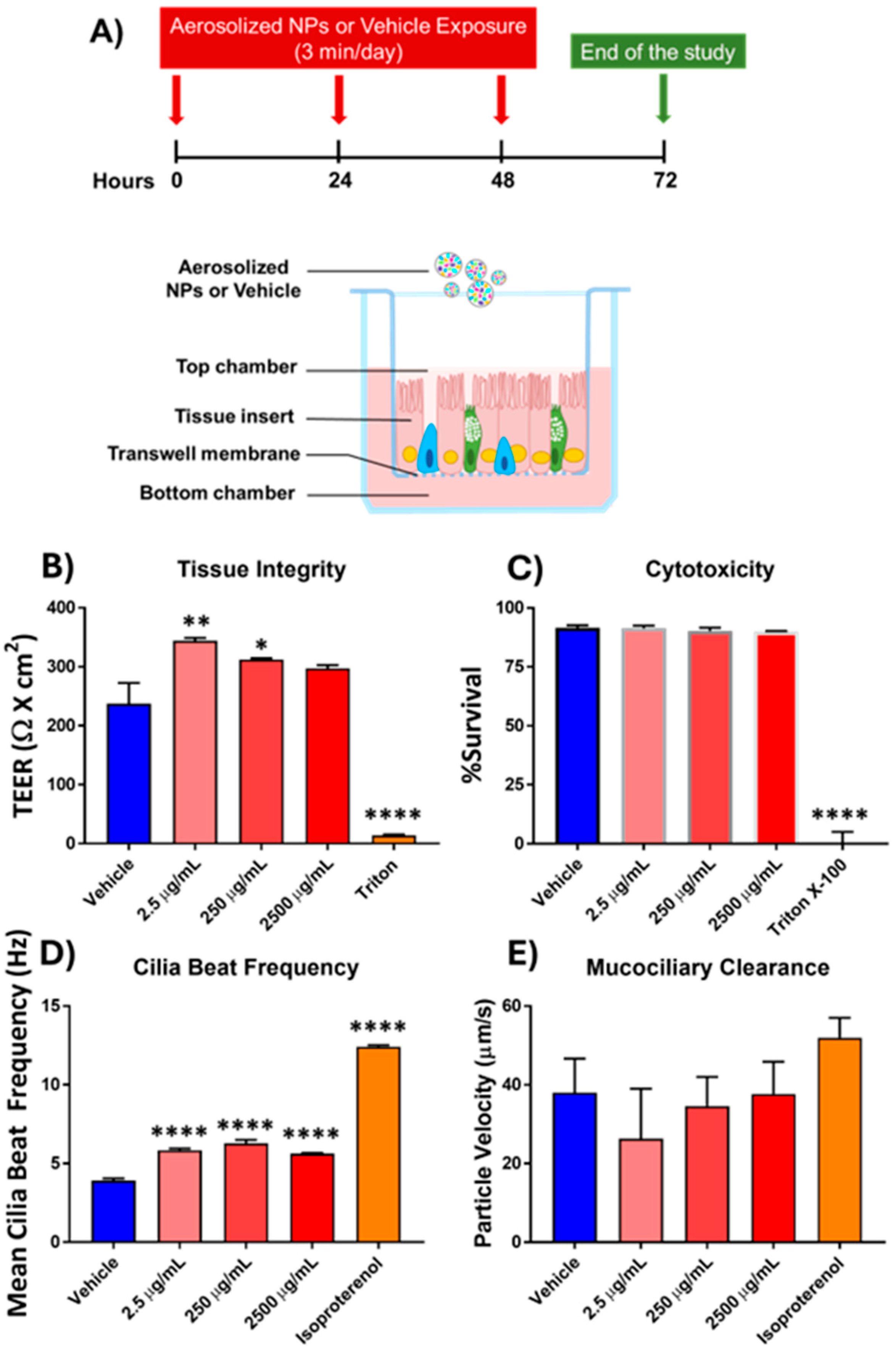
3.1. Effects of Aerosolized Nanoplastics Exposure on Functional Epithelial Measures
3.2. Differential Secretion of Cytokine and Chemokine Proteins Following Aerosolized Nanoplastics Exposure
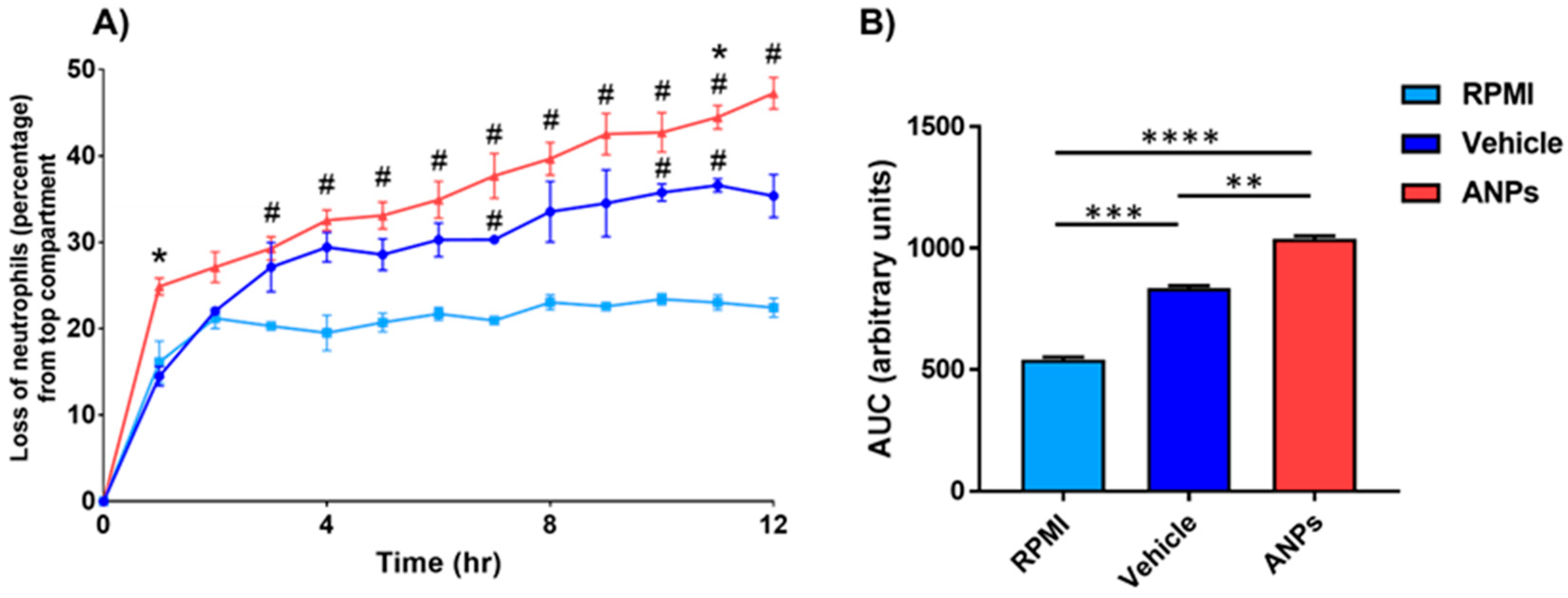
3.3. Enhanced Neutrophilic Chemotaxis Following Aerosolized Nanoplastics Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.A.; Miao, L.; Macreadie, P.I.; Adyel, T.M. Time to count plastics in climate action. Science 2025, 387, 1048. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A. Micro- and nanoplastic transfer, accumulation, and toxicity in humans. Curr. Opin. Toxicol. 2021, 28, 62–69. [Google Scholar] [CrossRef]
- Wright, S.L. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Hanke, G.; Galgani, F.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Van Franeker, J.A.; Vlachogianni, T.; et al. Joint Research Centre: Institute for Environment and Sustainability and MSFD Technical Subgroup on Marine Litter. Guidance on Monitoring of Marine Litter in European Seas, Publications Office. 2013. Available online: https://mcc.jrc.ec.europa.eu/documents/201702074014.pdf (accessed on 10 February 2025).
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Trainic, M. Airborne microplastic particles detected in the remote marine atmosphere. Commun. Earth Environ. 2021, 1, 64. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-q.; Wang, C.-c.; Ma, R.-x.; Qi, S.-q.; Fu, W.; Zhong, J.; Cao, C.; Zhang, X.-l.; Liu, G.-h.; Gao, Y.-d. Co-exposure to polyethylene microplastics and house dust mites aggravates airway epithelial barrier dysfunction and airway inflammation via CXCL1 signaling pathway in a mouse model. Int. Immunopharmacol. 2025, 146, 113921. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Kim, J.E.; Roh, Y.J.; Song, H.J.; Seol, A.; Park, J.; Lim, Y.; Seo, S.; Hwang, D.Y. Characterisation of changes in global genes expression in the lung of ICR mice in response to the inflammation and fibrosis induced by polystyrene nanoplastics inhalation. Toxicol. Res. 2023, 39, 575–599. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, Y.; Wu, Y.; Zhang, L.; Zhang, B.; Zhou, S.; Zhang, P.; Xu, T.; Wu, M.; Lv, S. Polystyrene nanoplastics of different particle sizes regulate the polarization of pro-inflammatory macrophages. Sci. Rep. 2024, 14, 16329. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Shyu, D.J.H.; Domínguez, R.; Lorenzo, J.M.; Pereira, J.A.M.; Câmara, J.S. Polystyrene microplastic particles in the food chain: Characteristics and toxicity—A review. Sci. Total Environ. 2023, 892, 164531. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hua, T.; Sang, Q.-X.A. Effects of Polystyrene Microplastics on Human Kidney and Liver Cell Morphology, Cellular Proliferation, and Metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Kwabena Danso, I.; Woo, J.-H.; Hoon Baek, S.; Kim, K.; Lee, K. Pulmonary toxicity assessment of polypropylene, polystyrene, and polyethylene microplastic fragments in mice. Toxicol. Res. 2024, 40, 313–323. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, D.; Sanderson, M.J. Effect of Isoproterenol on the Regulation of Rabbit Airway Ciliary Beat Frequency Measured with High-Speed Digital and Fluorescence Microscopy. Ann. Otol. Rhinol. Laryngol. 2005, 114, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Geary, C.A.; Davis, C.W.; Paradiso, A.M.; Boucher, R.C. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1995, 268, L1021–L1028. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Tech. Let. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Kihara, S.; Köper, I.; Mata, J.P.; McGillivray, D.J. Reviewing nanoplastic toxicology: It’s an interface problem. Adv. Colloid. Interface Sci. 2021, 288, 102337. [Google Scholar] [CrossRef]
- Lim, D.; Jeong, J.; Song, K.S.; Sung, J.H.; Oh, S.M.; Choi, J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere 2021, 262, 128330. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific Uptake and Genotoxicity induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Balogh Sivars, K.; Sivars, U.; Hornberg, E.; Zhang, H.; Brändén, L.; Bonfante, R.; Huang, S.; Constant, S.; Robinson, I.; Betts, C.J.; et al. A 3D Human Airway Model Enables Prediction of Respiratory Toxicity of Inhaled Drugs In Vitro. Toxicol. Sci. 2017, 162, 301–308. [Google Scholar] [CrossRef]
- Huang, S.; Wiszniewski, L.; Constant, S.; Roggen, E. Potential of in vitro reconstituted 3D human airway epithelia (MucilAirTM) to assess respiratory sensitizers. Toxicol. Vitr. 2013, 27, 1151–1156. [Google Scholar] [CrossRef]
- Baxter, A.; Thain, S.; Banerjee, A.; Haswell, L.; Parmar, A.; Phillips, G.; Minet, E. Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia. Toxicol. Vitr. 2015, 29, 864–875. [Google Scholar] [CrossRef]
- Gosselink, I.F.; van Schooten, F.J.; Drittij, M.J.; Höppener, E.M.; Leonhardt, P.; Moschini, E.; Serchi, T.; Gutleb, A.C.; Kooter, I.M.; Remels, A.H. Assessing toxicity of amorphous nanoplastics in airway- and lung epithelial cells using air-liquid interface models. Chemosphere 2024, 368, 143702. [Google Scholar] [CrossRef]
- Winiarska, E.; Chaszczewska-Markowska, M.; Ghete, D.; Jutel, M.; Zemelka-Wiacek, M. Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism. Int. J. Mol. Sci. 2024, 25, 4724. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Billey, L.O.; McGarvey, A.M.; Shelver, W.L. Effects of polystyrene micro/nanoplastics on liver cells based on particle size, surface functionalization, concentration and exposure period. Sci. Total Environ. 2022, 836, 155621. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.C.; Chen, J.J.; Chou, L.; Wong, B.J.F.; Chen, Z. Visualization and Detection of Ciliary Beating Pattern and Frequency in the Upper Airway using Phase Resolved Doppler Optical Coherence Tomography. Sci. Rep. 2017, 7, 8522. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Ogawara, K.-i.; Kimura, T.; Higaki, K.; Shingaki, T.; Kimura, S.; Tanaka, A.; Katsumi, H.; Sakane, T.; et al. In Vitro Evaluation of the Ciliary Beat Frequency of the Rat Nasal Epithelium Using a High-Speed Digital Imaging System. Biol. Pharm. Bull. 2013, 36, 966–973. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Brighton, L.; Hazucha, M.; Jaspers, I.; Carson, J.L. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal. Toxicol. 2009, 21, 875–881. [Google Scholar] [CrossRef]
- Workman, A.D.; Carey, R.M.; Chen, B.; Saunders, C.J.; Marambaud, P.; Mitchell, C.H.; Tordoff, M.G.; Lee, R.J.; Cohen, N.A. CALHM1-Mediated ATP Release and Ciliary Beat Frequency Modulation in Nasal Epithelial Cells. Sci. Rep. 2017, 7, 6687. [Google Scholar] [CrossRef]
- Kuek, L.E.; Lee, R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L603–l619. [Google Scholar] [CrossRef]
- Behan, L.; Rubbo, B.; Lucas, J.S.; Dunn Galvin, A. The patient’s experience of primary ciliary dyskinesia: A systematic review. Qual. Life Res. 2017, 26, 2265–2285. [Google Scholar] [CrossRef]
- Pifferi, M.; Bush, A.; Di Cicco, M.; Pradal, U.; Ragazzo, V.; Macchia, P.; Boner, A.L. Health-related quality of life and unmet needs in patients with primary ciliary dyskinesia. Eur. Respir. J. 2009, 35, 787–794. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3: As a Biomarker. General. Methods Biomark. Res. Their Appl. 2015, 1, 223–249. [Google Scholar] [CrossRef]
- Alam, R.; York, J.; Boyars, M.; Stafford, S.; Grant, J.A.; Lee, J.; Forsythe, P.; Sim, T.; Ida, N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 1996, 153, 1398–1404. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.e.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Ham, J.; Kim, J.; Ko, Y.G.; Kim, H.Y. The Dynamic Contribution of Neutrophils in the Chronic Respiratory Diseases. Allergy Asthma Immunol. Res. 2022, 14, 361–378. [Google Scholar] [PubMed]
- Wu, J.; Hillier, C.; Komenda, P.; Lobato de Faria, R.; Levin, D.; Zhang, M.; Lin, F. A Microfluidic Platform for Evaluating Neutrophil Chemotaxis Induced by Sputum from COPD Patients. PLoS ONE 2015, 10, e0126523. [Google Scholar] [CrossRef]
- Stockley, J.A.; Walton, G.M.; Lord, J.M.; Sapey, E. Aberrant neutrophil functions in stable chronic obstructive pulmonary disease: The neutrophil as an immunotherapeutic target. Int. Immunopharmacol. 2013, 17, 1211–1217. [Google Scholar] [CrossRef]
- Woo, J.H.; Seo, H.J.; Lee, J.Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part. Fibre Toxicol. 2023, 20, 2. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, L.; Song, E.; Song, Y. Polystyrene Nanoplastics Induce Neutrophil Extracellular Traps in Mice Neutrophils. Chem. Res. Toxicol. 2022, 35, 378–382. [Google Scholar] [CrossRef]
- Haegens, A.; Heeringa, P.; van Suylen, R.J.; Steele, C.; Aratani, Y.; O’Donoghue, R.J.J.; Mutsaers, S.E.; Mossman, B.T.; Wouters, E.F.M.; Vernooy, J.H.J. Myeloperoxidase Deficiency Attenuates Lipopolysaccharide-Induced Acute Lung Inflammation and Subsequent Cytokine and Chemokine Production1. J. Immunol. 2009, 182, 7990–7996. [Google Scholar] [CrossRef]
- Harbort, C.J.; Soeiro-Pereira, P.V.; von Bernuth, H.; Kaindl, A.M.; Costa-Carvalho, B.T.; Condino-Neto, A.; Reichenbach, J.; Roesler, J.; Zychlinsky, A.; Amulic, B. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood 2015, 126, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Res 2016, 5, 224. [Google Scholar] [CrossRef]
- Toews, G.B. Cytokines and the lung. Eur. Respir. J. 2001, 18, 3s–17s. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Cytokines in the balance. Nat. Immunol. 2019, 20, 1557. [CrossRef]
- Spolski, R.; Wang, L.; Wan, C.K.; Bonville, C.A.; Domachowske, J.B.; Kim, H.P.; Yu, Z.; Leonard, W.J. IL-21 promotes the pathologic immune response to pneumovirus infection. J. Immunol. 2012, 188, 1924–1932. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu. Rev. Immunol. 2008, 26, 57–79. [Google Scholar] [CrossRef]
- Verbist, K.C.; Cole, C.J.; Field, M.B.; Klonowski, K.D. A Role for IL-15 in the Migration of Effector CD8 T Cells to the Lung Airways following Influenza Infection. J. Immunol. 2011, 186, 174–182. [Google Scholar] [CrossRef]
- Booty, M.G.; Barreira-Silva, P.; Carpenter, S.M.; Nunes-Alves, C.; Jacques, M.K.; Stowell, B.L.; Jayaraman, P.; Beamer, G.; Behar, S.M. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci. Rep. 2016, 6, 36720. [Google Scholar] [CrossRef]
- Verbist, K.C.; Rose, D.L.; Cole, C.J.; Field, M.B.; Klonowski, K.D. IL-15 Participates in the Respiratory Innate Immune Response to Influenza Virus Infection. PLoS ONE 2012, 7, e37539. [Google Scholar] [CrossRef]
- Krieg, C.; Létourneau, S.; Pantaleo, G.; Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11906–11911. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Isvoranu, G.; Chiritoiu-Butnaru, M. Therapeutic potential of interleukin-21 in cancer. Front. Immunol. 2024, 15, 1369743. [Google Scholar] [CrossRef]
- Trifilo, M.J.; Montalto-Morrison, C.; Stiles, L.N.; Hurst, K.R.; Hardison, J.L.; Manning, J.E.; Masters, P.S.; Lane, T.E. CXC chemokine ligand 10 controls viral infection in the central nervous system: Evidence for a role in innate immune response through recruitment and activation of natural killer cells. J. Virol. 2004, 78, 585–594. [Google Scholar] [CrossRef]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef]
- Bartram, U.; Speer, C.P. The Role of Transforming Growth Factor in Lung Development and Disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Branchett, W.J.; Lloyd, C.M. Regulatory cytokine function in the respiratory tract. Mucosal Immunol. 2019, 12, 589–600. [Google Scholar] [CrossRef]
- Komai, T.; Inoue, M.; Okamura, T.; Morita, K.; Iwasaki, Y.; Sumitomo, S.; Shoda, H.; Yamamoto, K.; Fujio, K. Transforming Growth Factor-β and Interleukin-10 Synergistically Regulate Humoral Immunity via Modulating Metabolic Signals. Front. Immunol. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Ojiaku, C.A.; Yoo, E.J.; Panettieri, R.A., Jr. Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link? Am. J. Respir. Cell Mol. Biol. 2017, 56, 432–442. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Chuang, K.-J.; Chan, C.-C.; Su, T.-C.; Lee, C.-T.; Tang, C.-S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Dysfunction in Young Adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Kim, G.-D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lad, A.; Hunyadi, J.; Connolly, J.; Breidenbach, J.D.; Khalaf, F.K.; Dube, P.; Zhang, S.; Kleinhenz, A.L.; Baliu-Rodriguez, D.; Isailovic, D.; et al. Antioxidant Therapy Significantly Attenuates Hepatotoxicity following Low Dose Exposure to Microcystin-LR in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Asp. Med. 2009, 30, 60–76. [Google Scholar] [CrossRef]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As We Drink and Breathe: Adverse Health Effects of Microcystins and Other Harmful Algal Bloom Toxins in the Liver, Gut, Lungs and Beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breidenbach, J.D.; French, B.W.; Shrestha, U.; Adya, Z.K.; Wooten, R.M.; Fribley, A.M.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium. Antioxidants 2025, 14, 424. https://doi.org/10.3390/antiox14040424
Breidenbach JD, French BW, Shrestha U, Adya ZK, Wooten RM, Fribley AM, Malhotra D, Haller ST, Kennedy DJ. Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium. Antioxidants. 2025; 14(4):424. https://doi.org/10.3390/antiox14040424
Chicago/Turabian StyleBreidenbach, Joshua D., Benjamin W. French, Upasana Shrestha, Zaneh K. Adya, R. Mark Wooten, Andrew M. Fribley, Deepak Malhotra, Steven T. Haller, and David J. Kennedy. 2025. "Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium" Antioxidants 14, no. 4: 424. https://doi.org/10.3390/antiox14040424
APA StyleBreidenbach, J. D., French, B. W., Shrestha, U., Adya, Z. K., Wooten, R. M., Fribley, A. M., Malhotra, D., Haller, S. T., & Kennedy, D. J. (2025). Acute Exposure to Aerosolized Nanoplastics Modulates Redox-Linked Immune Responses in Human Airway Epithelium. Antioxidants, 14(4), 424. https://doi.org/10.3390/antiox14040424





