The Potential Benefits of Curcumin-Enriched Diets for Adults with Colorectal Cancer: A Systematic Review
Abstract
1. Introduction
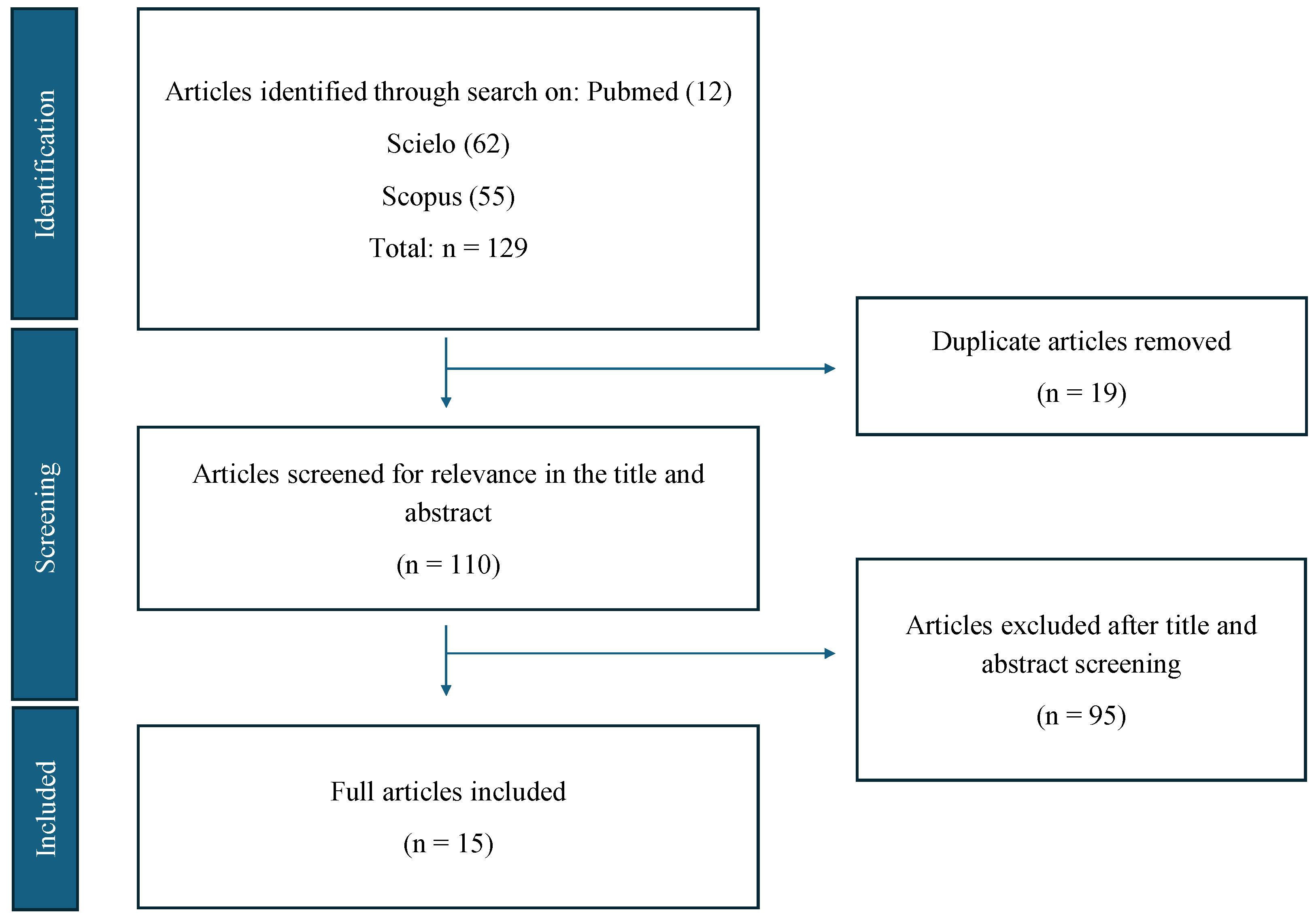
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria
2.4. Data Analysis
3. Results
4. Discussion
4.1. The Positive Impact of Curcumin-Enriched Diets on CRC Patients
4.1.1. Survival Improvement
4.1.2. Tumor Reduction
4.1.3. Anti-Inflammatory and Antioxidant Effects
4.1.4. Support to Conventional Treatment
4.1.5. Quality of Life
4.2. Mechanisms of Action of Curcumin-Enriched Diets in CRC
4.3. Adverse Events of a Curcumin Diet in Adult Patients with CRC
4.4. Limitations and Challenges
4.5. Current State of Knowledge
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| EMT | Epithelial–mesenchymal transition |
| FOLFIRI | Folinic acid, fluorouracil, and irinotecan hydrochloride |
| FOLFOX | Folinic acid, fluorouracil, and oxaliplatin |
| IL | Interleukin |
| OS | Overall survival |
| HR | Hazard ratio |
| PFS | Progression-free survival |
References
- Duan, B.; Zhao, Y.; Bai, J.; Wang, J.; Duan, X.; Luo, X.; Zhang, R.; Pu, Y.; Kou, M.; Lei, J.; et al. Colorectal Cancer: An Overview. In Gastrointestinal Cancers; Exon Publications: Brisbane City, Australia, 2022; pp. 1–12. [Google Scholar]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Colon Cancer Treatment (PDQ®): Patient Version. National Cancer Institute, USA. 2002. Available online: https://www.cancer.gov/types/colorectal/patient/colon-treatment-pdq (accessed on 2 December 2024).
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and Biological Hallmarks of Colorectal Cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- Zhou, R.W.; Harpaz, N.; Itzkowitz, S.H.; Parsons, R.E. Molecular Mechanisms in Colitis-Associated Colorectal Cancer. Oncogenesis 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Detection, Diagnosis, and Staging of Colon and Rectal Cancer. American Cancer Society, USA. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagnosis-staging.html (accessed on 2 December 2024).
- Colon Cancer: Symptoms and Causes. Mayo Clinic, USA. Available online: https://www.mayoclinic.org/es/diseases-conditions/colon-cancer/symptoms-causes/syc-20353669 (accessed on 2 December 2024).
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal Cancer: Recent Advances in Management and Treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and Emerging Therapeutic Approaches for Colorectal Cancer: A Comprehensive Review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Luo, Q.; Asher, G.N. Use of Dietary Supplements at a Comprehensive Cancer Center. J. Altern. Complement. Med. 2018, 24, 981–987. [Google Scholar] [CrossRef]
- Hoppe, C.; Buntzel, J.; Von Weikersthal, L.F.; Junghans, C.; Zomorodbakhsch, B.; Stoll, C.; Prott, F.-J.; Fuxius, S.; Micke, O.; Richter, A.; et al. Usage of Complementary and Alternative Methods, Lifestyle, and Psychological Variables in Cancer Care. In Vivo 2023, 37, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Samuel, S.M.; Mazurakova, A.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Curcumin, Calebin A and Chemosensitization: How Are They Linked to Colorectal Cancer? Life Sci. 2023, 318, 121504. [Google Scholar] [CrossRef] [PubMed]
- Idoudi, S.; Bedhiafi, T.; Hijji, Y.M.; Billa, N. Curcumin and Derivatives in Nanoformulations with Therapeutic Potential on Colorectal Cancer. AAPS PharmSciTech 2022, 23, 115. [Google Scholar] [CrossRef] [PubMed]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Calibasi-Kocal, G.; Pakdemirli, A.; Bayrak, S.; Ozupek, N.M.; Sever, T.; Basbinar, Y.; Ellidokuz, H.; Yigitbasi, T. Curcumin Effects on Cell Proliferation, Angiogenesis and Metastasis in Colorectal Cancer. J. BUON 2019, 24, 1482–1487. [Google Scholar]
- Villar Ruiz de la Torre, J.A.; Melo Herráiz, E. Guía de Plantas Medicinales Del Magreb; Fundación Dr. Antonio Esteve: Barcelona, España, 2010; pp. 1–110. [Google Scholar]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1040259. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive Metabolites of Curcumin and Their Therapeutic Effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and Anti-Inflammatory Effects of Curcumin/Turmeric Supplementation in Adults: A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.-C. Curcumin in Cancer Therapy: Exploring Molecular Mechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef] [PubMed]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a Novel Therapeutic Candidate for Cancer: Can This Natural Compound Revolutionize Cancer Treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef]
- Mayo, B.; Penroz, S.; Torres, K.; Simón, L. Curcumin Administration Routes in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 11492. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.-S.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Panahi, Y.; Saberi-Karimian, M.; Valizadeh, O.; Behnam, B.; Saadat, A.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids on Systemic Inflammation and Quality of Life in Patients with Colorectal Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. Adv. Exp. Med. Biol. 2021, 1328, 1–9. [Google Scholar] [CrossRef]
- Briata, I.M.; Paleari, L.; Rutigliani, M.; Petrera, M.; Caviglia, S.; Romagnoli, P.; Libera, M.D.; Oppezzi, M.; Puntoni, M.; Siri, G.; et al. A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps. Int. J. Mol. Sci. 2021, 22, 11024. [Google Scholar] [CrossRef] [PubMed]
- Gunther, J.R.; Chadha, A.S.; Guha, S.; Raju, G.S.; Maru, D.M.; Munsell, M.F.; Jiang, Y.; Yang, P.; Felix, E.; Clemons, M.; et al. A Phase II Randomized Double Blinded Trial Evaluating the Efficacy of Curcumin with Pre-Operative Chemoradiation for Rectal Cancer. J. Gastrointest. Oncol. 2022, 13, 2938–2950. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.; Dinesh, K.; Tasneef, Z.; Bhavna, S.; Rahul, S.; Kiran, B. Dietary Risk Factors in Gastrointestinal Cancers: A Case–Control Study in North India. J. Cancer Res. Ther. 2023, 19, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Briata, I.M.; D’Ecclesiis, O.; Johansson, H.; Aristarco, V.; Buttiron Webber, T.; Oppezzi, M.; Gandini, S.; Bonanni, B.; DeCensi, A. Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas. Nutrients 2023, 15, 3894. [Google Scholar] [CrossRef]
- Jeon, Y.; Sym, S.J.; Yoo, B.K.; Baek, J.-H. Long-Term Survival, Tolerability, and Safety of First-Line Bevacizumab and FOLFIRI in Combination With Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin in Patients with Unresectable Metastatic Colorectal Cancer. Integr. Cancer Ther. 2022, 21, 15347354221105498. [Google Scholar] [CrossRef]
- Li, B.; Shi, C.; Li, B.; Zhao, J.; Wang, L. The Effects of Curcumin on HCT-116 Cells Proliferation and Apoptosis via the MiR-491/PEG10 Pathway. J. Cell. Biochem. 2018, 119, 3091–3098. [Google Scholar] [CrossRef]
- Maletzki, C.; Scheinpflug, P.; Witt, A.; Klar, E.; Linnebacher, M. Targeting Immune-Related Molecules in Cancer Therapy: A Comprehensive In Vitro Analysis on Patient-Derived Tumor Models. Biomed. Res. Int. 2019, 2019, 4938285. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Islam, S.U.; Sonn, J.K.; Lee, Y.S. PRP4 Kinase Domain Loss Nullifies Drug Resistance and Epithelial-Mesenchymal Transition in Human Colorectal Carcinoma Cells. Mol. Cells 2020, 43, 662–670. [Google Scholar] [CrossRef]
- Khan, S.; Miles, G.J.; Demetriou, C.; Sidat, Z.; Foreman, N.; West, K.; Karmokar, A.; Howells, L.; Pritchard, C.; Thomas, A.L.; et al. Ex Vivo Explant Model of Adenoma and Colorectal Cancer to Explore Mechanisms of Action and Patient Response to Cancer Prevention Therapies. Mutagenesis 2022, 37, 227–237. [Google Scholar] [CrossRef]
- Biswas, P.; Dey, D.; Rahman, A.; Islam, M.A.; Susmi, T.F.; Kaium, M.A.; Hasan, M.N.; Rahman, M.H.; Mahmud, S.; Saleh, M.A.; et al. Analysis of SYK Gene as a Prognostic Biomarker and Suggested Potential Bioactive Phytochemicals as an Alternative Therapeutic Option for Colorectal Cancer: An In-Silico Pharmaco-Informatics Investigation. J. Pers Med. 2021, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, L.C.; Dörfler, J.; Hübner, J. Curcumin as a Complementary Treatment in Oncological Therapy: A Systematic Review. Eur. J. Clin. Pharmacol. 2025, 81, 1–33. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, W.; Zhang, Z.; Wang, Y.; Huang, D.; Ren, D.; Chen, H.; Wang, X.; Li, G.; Han, Z. 5-Fluorouracil/Curcumin Loaded Silk Fibroin Hydrogel for the Adjuvant Therapy in Colorectal Cancer. Biomater. Adv. 2025, 168, 214108. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-Y.; Chan, H.-W.; Shih, K.-C. Suppression of Colorectal Cancer Growth: Interplay between Curcumin and Metformin through DMT1 Downregulation and ROS-Mediated Pathways. Biofactors 2025, 51, e2137. [Google Scholar] [CrossRef]


| First Autor | Year | Country Where the Study Was Conducted | Type of Study | Curcumin-Containing Product Administration | Chemotherapy/Radiotherapy | Results | Ref. |
|---|---|---|---|---|---|---|---|
| Clinical trials: | |||||||
| Cruz-Correa | 2018 | Puerto Rico/USA | Double-blind, randomized controlled trial | 3000 mg/day pure oral curcumin | NA | No significant reduction in polyp number or size compared to the placebo; curcumin was well-tolerated | [33] |
| Greil | 2018 | Austria | Phase I trial, dose-escalation | Intravenous liposomal curcumin was administered starting at a dose of 100 mg/m2 over 8 h, with subsequent escalation to 300 mg/m2 over 6 h | NA | Liposomal curcumin was well tolerated up to 300 mg/m2; no dose-limiting toxicity was observed. Anti-tumor activity was not detected, but significant tumor marker responses and clinical benefits were noted in two patients | [34] |
| Howells | 2019 | United Kingdom | Phase IIa randomized trial | Oral curcumin-containing product (Curcumin C3 Complex ® (Sabinsa, East Windsor, NJ, USA) is 70–80% curcumin) (2 g/day) | FOLFOX/Bevacizumab | Improved overall survival with safe and tolerable combination therapy | [35] |
| Panahi | 2021 | Iran | Randomized double-blind placebo-controlled trial | 500 mg/day orally for 8 weeks of curcumin-containing product (Curcumin C3 Complex ® is 70–80% curcumin) | Chemotherapy | Significant reduction in erythrocyte sedimentation rate and C-reactive protein levels, slight decrease in IL-1α, and improvement in quality of life scores | [36] |
| Briata | 2021 | Italy | Randomized, double-blind, placebo-controlled, phase II presurgical trial | 1 g daily curcumin-containing product (Meriva ® (INDENA, Milan, Italy) is a complex of curcumin (20%) with soy phosphatidylcholine) combined with anthocyanins orally for 4–6 weeks | NA | Significant reduction in NF-κB and a trend toward Ki-67 reduction in adenoma tissues | [37] |
| Gunther | 2022 | USA | Phase II trial | 4 g orally, twice daily of curcumin-containing product (Curcumin C3 Complex ® is 70–80% curcumin) during chemoradiation therapy and for 6 weeks after | Capecitabine; radiation | Curcumin did not improve pathologic complete response rates. Bioavailability issues led to inconsistent plasma/tissue levels, limiting therapeutic efficacy | [38] |
| Nadeem | 2023 | India | Case-control study | Low and medium intake of mixed spices, including curcumin | NA | Curcumin, as part of mixed spices, may provide protective effects against gastrointestinal cancers at low–medium intake | [39] |
| Macis | 2023 | Italy | Presurgical trial | 1 g of curcumin-containing product (Meriva® is a complex of curcumin (20%) with soy phosphatidylcholine). Combined with anthocyanins orally | NA | The combined treatment of anthocyanins and curcumin did not directly alter circulating inflammatory and metabolic biomarkers. However, it exhibited a complex effect on biomarkers associated with colon cancer progression | [40] |
| Observational studies: | |||||||
| Jeon | 2022 | South Korea | Prospective, observational, single-group analysis | Daily oral administration of 200 mg of curcumin in G-NLC | Bevacizumab/FOLFIRI | Improved median overall survival and progression-free survival; good tolerability and safety | [41] |
| Hoppe | 2023 | Germany | Cross-sectional observational study | Curcumin is one of the complementary and alternative medicine methods listed in the questionnaire distributed to participants | NA | The use of complementary and alternative medicine is linked to spiritual well-being and the need for meaning, highlighting the need for education to set realistic goals and avoid side effects. Diet shows positive psychological associations in cancer patients, though it is unclear whether diet influences psychological stability or vice versa | [15] |
| In vitro studies: | |||||||
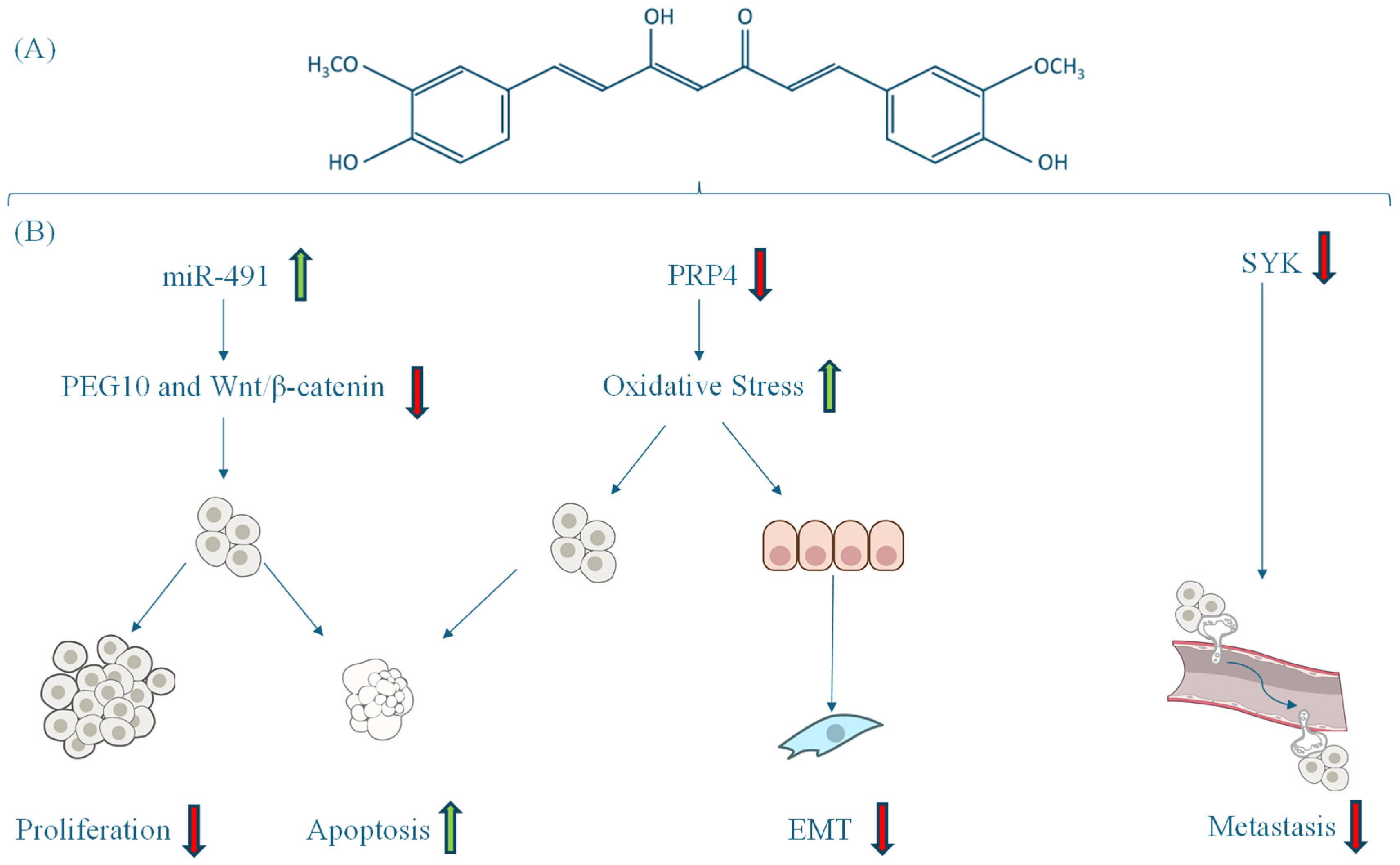
| Li | 2018 | China | In vitro | 12.5 µM curcumin | NA | Curcumin upregulates miR-491, inhibits PEG10 and Wnt/β-catenin pathways, reduces proliferation, and promotes apoptosis in HCT-116 cells | [42] |
| Maletzki | 2019 | Germany | In vitro | 20 µM curcumin for 72 h | Gemcitabine/Indoximod | Curcumin alone induced apoptosis and senescence in colorectal cancer cells. Combined with Gemcitabine, it enhanced tumor cell elimination significantly | [43] |
| Ahmed | 2020 | Republic of Korea | In vitro and in vivo study | 30 µM curcumin (Sigma-Aldrich, St. Louis, MO, USA) for 24 h | NA | PRP4 overexpression conferred resistance to curcumin-induced apoptosis and promoted epithelial–mesenchymal transition (EMT). Deletion of PRP4’s kinase domain restored sensitivity to curcumin and inhibited EMT | [44] |
| Ex vivo study: | |||||||
| Khan | 2022 | United Kingdom | Ex vivo explant model | Curcumin (Sigma, Gillingham, UK) concentrations 0–10 µM administered to tissue explants | NA | Curcumin induced apoptosis in tumor and stromal tissues, reduced immunosuppressive tumor microenvironment, increased T-cell movement toward cancer, and improved immune response | [45] |
| In silico study: | |||||||
| Biswas | 2021 | Bangladesh | In silico study | Curcumin (molecular docking as inhibitor) | NA | Curcumin exhibited strong binding to SYK (spleen tyrosine kinase), suggesting its potential as a therapeutic candidate | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neira, M.; Mena, C.; Torres, K.; Simón, L. The Potential Benefits of Curcumin-Enriched Diets for Adults with Colorectal Cancer: A Systematic Review. Antioxidants 2025, 14, 388. https://doi.org/10.3390/antiox14040388
Neira M, Mena C, Torres K, Simón L. The Potential Benefits of Curcumin-Enriched Diets for Adults with Colorectal Cancer: A Systematic Review. Antioxidants. 2025; 14(4):388. https://doi.org/10.3390/antiox14040388
Chicago/Turabian StyleNeira, María, Constanza Mena, Keila Torres, and Layla Simón. 2025. "The Potential Benefits of Curcumin-Enriched Diets for Adults with Colorectal Cancer: A Systematic Review" Antioxidants 14, no. 4: 388. https://doi.org/10.3390/antiox14040388
APA StyleNeira, M., Mena, C., Torres, K., & Simón, L. (2025). The Potential Benefits of Curcumin-Enriched Diets for Adults with Colorectal Cancer: A Systematic Review. Antioxidants, 14(4), 388. https://doi.org/10.3390/antiox14040388








